Effectiveness of a Lifestyle Change Program on Insulin Resistance in Yaquis Indigenous Populations in Sonora, Mexico: PREVISY
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Intervention Program
2.4. Measurement of Outcomes
2.5. HOMA-IR and TyG Index
2.6. Anthropometry, Physical and Sociodemographic Measurements
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Basto-Abreu, A.C.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; De la Cruz-Góngora, V.V.; Rivera-Dommarco, J.; Shamah-Levy, T.; Romero-Martínez, M.; Barquera, S.; Villalpando, S.; et al. Prevalence of diabetes and glycemic control in Mexico: National results from 2018 and 2020. Salud Pública México 2021, 63, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística y Geografía (INEGI). Características de Las Defunciones Registradas en México Durante 2018. Cd. de México (MX): Instituto Nacional de Estadística y Geografía. Comunicado de Prensa 538/19. 2019. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2019/EstSociodemo/DefuncionesRegistradas2019.pdf (accessed on 24 November 2021).
- American Diabetes Association (ADA). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [CrossRef] [PubMed]
- Organización Mundial de la Salud (OMS). Obesidad y Sobrepeso. 2021. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 November 2021).
- Morimoto, A.; Tatsumi, Y.; Deura, K.; Mizuno, S.; Ohno, Y.; Miyamatsu, N.; Watanabe, S. Impact of impaired insulin secretion and insulin resistance on the incidence of type 2 diabetes mellitus in a Japanese population: The Saku study. Diabetologia 2013, 56, 1671–1679. [Google Scholar] [CrossRef]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef]
- Heise, T.; Zijlstra, E.; Nosek, L.; Heckermann, S.; Plum-Mörschel, L.; Forst, T. Euglycaemic glucose clamp: What it can and cannot do, and how to do it. Diabetes Obes. Metab. 2016, 18, 962–972. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Yamada, C.; Moriyama, K.; Takahashi, E. Optimal cut-off point for homeostasis model assessment of insulin resistance to discriminate metabolic syndrome in non-diabetic Japanese subjects. J. Diabetes Investig. 2012, 3, 384–387. [Google Scholar] [CrossRef]
- Esteghamati, A.; Ashraf, H.; Khalilzadeh, O.; Zandieh, A.; Nakhjavani, M.; Rashidi, A.; Haghazali, M.; Asgari, F. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: Third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr. Metab. 2010, 7, 26. [Google Scholar] [CrossRef]
- Almeda-Valdés, P.; Bello-Chavolla, O.Y.; Caballeros-Barragán, C.R.; Gómez-Velasco, D.V.; Viveros-Ruiz, T.; Vargas-Vázquez, A.; Aguilar-Salinas, C.A. Índices para la evaluación de la resistencia a la insulina en individuos mexicanos sin diabetes. Gac. Med. Mex. 2018, 154 (Suppl. 2), S50–S55. [Google Scholar] [CrossRef]
- Hong, S.; Han, K.; Park, C.Y. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: A population-based study. BMC Med. 2020, 18, 361. [Google Scholar] [CrossRef]
- Walker, J.; Lovett, R.; Kukutai, T.; Jones, C.; Henry, D. Indigenous health data and the path to healing. Lancet 2017, 390, 2022–2023. [Google Scholar] [CrossRef]
- Yu, C.H.; Zinman, B. Type 2 diabetes and impaired glucose tolerance in aboriginal populations: A global perspective. Diabetes Res. Clin. Pract. 2007, 78, 159–170. [Google Scholar] [CrossRef]
- Keel, S.; Foreman, J.; Xie, J.; van Wijngaarden, P.; Taylor, H.R.; Dirani, M. The Prevalence of Self-Reported Diabetes in the Australian National Eye Health Survey. PLoS ONE 2017, 12, e0169211. [Google Scholar] [CrossRef]
- Jimenez-Corona, A.; Nelson, R.G.; Jimenez-Corona, M.E.; Franks, P.W.; Aguilar-Salinas, C.A.; Graue-Hernandez, E.O.; Hernandez-Jimenez, S.; Hernandez-Avila, M. Disparities in prediabetes and type 2 diabetes prevalence between indigenous and nonindigenous populations from Southeastern Mexico: The Comitan Study. J. Clin. Transl. Endocrinol. 2019, 16, 100191. [Google Scholar] [CrossRef]
- Rodríguez-Morán, M.; Guerrero-Romero, F.; Brito-Zurita, O.; Rascón-Pacheco, R.A.; Pérez-Fuentes, R.; Sánchez-Guillén, M.C.; González-Ortiz, M.; Martínez-Abundis, E.; Simental-Mendía, L.E.; Madero, A.; et al. Cardiovascular risk factors and acculturation in Yaquis and Tepehuanos Indians from Mexico. Arch. Med. Res. 2008, 39, 352–357. [Google Scholar] [CrossRef]
- Escobedo, J.; Chavira, I.; Martínez, L.; Velasco, X.; Escandón, C.; Cabral, J. Diabetes and other glucose metabolism abnormalities in Mexican Zapotec and Mixe Indians. Diabet. Med. 2010, 27, 412–416. [Google Scholar] [CrossRef]
- Esparza-Romero, J.; Valencia, M.E.; Urquidez-Romero, R.; Chaudhari, L.S.; Hanson, R.L.; Knowler, W.C.; Ravussin, E.; Bennett, P.H.; Schulz, L.O. Environmentally Driven Increases in Type 2 Diabetes and Obesity in Pima Indians and Non-Pimas in Mexico Over a 15-Year Period: The Maycoba Project. Diabetes Care 2015, 38, 2075–2082. [Google Scholar] [CrossRef]
- Castro-Juarez, A.A.; Serna-Gutiérrez, A.; Lozoya-Villegas, J.F.; Toledo-Domínguez, I.; Díaz-Zavala, R.G.; Esparza-Romero, J. Prevalence of previous diagnosis of hypertension and associated factors in the Yaqui indigenous of Sonora. Rev. Mex. Card. 2018, 29, 90–97. [Google Scholar]
- Valencia, M.E.; Wong, P.; González, N.L.; Esparza, J.; Astiazarán, H.; Grijalva, M.J.; Benítez, M.; Saucedo, S.; Rodríguez, H.; Romero, D.; et al. Evaluación y diagnóstico del estado de nutrición de la tribu Yaqui. Centro de Investigación en Alimentación y Desarrollo, A.C. Boletín CIAD 1995, 4, 3–5. [Google Scholar]
- Grijalva-Haro, M.I.; Valencia, M.E.; Wong-González, P.; Esparza-Romero, J.; González-García, L.; Robles-Sardin, A.E. Alimentación tradicional de los Yaquis. Cuad. Nutr. 2020, 43, 213–218. [Google Scholar]
- Valencia, M.E.; Astiazarán, H.; Esparza, J.; González, L.; Grijalva, M.I.; Cervera, A.; Zazueta, P. Vitamin A deficiency and low prevalence of anemia in Yaqui Indian children in northwest Mexico. J. Nutr. Sci. Vitaminol. 1999, 45, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Serna-Gutiérrez, A.; Esparza-Romero, J. Adaptation and reproducibility of a questionnaire to assess physical activity in epidemiological studies among Yaqui Indians from Sonora, Mexico. Rev. Salud Publica Nutr. 2018, 17, 17–25. [Google Scholar] [CrossRef]
- Serna-Gutiérrez, A.; Esparza-Romero, J. Diseño y validación de un cuestionario de frecuencia de consumo de alimentos para evaluar la dieta en indígenas Yaquis de Sonora, México. Acta Univ. 2019, 29, e2248. [Google Scholar] [CrossRef]
- Serna-Gutiérrez, A.; Castro-Juarez, A.A.; Romero-Martínez, M.; Alemán-Mateo, H.; Díaz-Zavala, R.G.; Quihui-Cota, L.; Álvarez-Hernández, G.; Gallegos-Aguilar, A.C.; Esparza-Romero, J. Prevalence of overweight, obesity and central obesity and factors associated with BMI in indigenous yaqui people: A probabilistic cross-sectional survey. BMC Public Health 2022, 22, 308. [Google Scholar] [CrossRef]
- Castro-Juarez, A.A.; Serna-Gutiérrez, A.; Dórame-López, N.A.; Solano-Morales, M.; Gallegos-Aguilar, A.C.; Díaz-Zavala, R.G.; Alemán-Mateo, H.; Urquidez-Romero, R.; Campa-Quijada, F.; Valenzuela-Guzmán, D.M.; et al. Effectiveness of the Healthy Lifestyle Promotion Program for Yaquis with Obesity and Risk of Diabetes in the Short and Medium Term: A Translational Study. J. Diabetes Res. 2020, 2020, 6320402. [Google Scholar] [CrossRef]
- Centers of Disease Control and Prevention (CDC). National Diabetes Prevention Program. Standard and Operating Procedures. 2015. Available online: http://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf (accessed on 12 November 2021).
- Centers of Disease Control and Prevention (CDC). National Diabetes Prevention Program. 2017. Available online: https://www.cdc.gov/diabetes/spanish/prevention/index.html (accessed on 12 November 2021).
- World Health Organization (WHO). Body Mass Index-BMI. 2018. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 16 November 2021).
- Lindström, J.; Tuomilehto, J. The diabetes risk score: A practical tool to predict type 2 diabetes risk. Diabetes Care 2003, 26, 725–731. [Google Scholar] [CrossRef]
- Gallegos-Aguilar, A.C.; Esparza-Romero, J.; Hernández-Martínez, H.H.; Urquidez-Romero, R.; Rascón-Careaga, A.; Bolado-Martínez, E.; Díaz-Zavala, R.G. Agreement of HemoCue and Glucose Oxidase method for blood glucose measurement in a field work study of diabetes: The Comcaac Project. Biotecnia 2019, 21, 22–28. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Kramer, M.K.; Kriska, A.M.; Venditti, E.M.; Miller, R.G.; Brooks, M.M.; Burke, L.E.; Siminerio, L.M.; Solano, F.X.; Orchard, T.J. Translating the Diabetes Prevention Program: A comprehensive model for prevention training and program delivery. Am. J. Prev. Med. 2009, 37, 505–511. [Google Scholar] [CrossRef]
- Davis-Smith, Y.M.; Boltri, J.M.; Seale, J.P.; Shellenberger, S.; Blalock, T.; Tobin, B. Implementing a diabetes prevention program in a rural African-American church. J. Natl. Med. Assoc. 2007, 99, 440–446. [Google Scholar]
- Katula, J.A.; Vitolins, M.Z.; Rosenberger, E.L.; Blackwell, C.S.; Morgan, T.M.; Lawlor, M.S.; Goff, D.C., Jr. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care 2011, 34, 1451–1457. [Google Scholar] [CrossRef]
- Herder, C.; Peltonen, M.; Koenig, W.; Sütfels, K.; Lindström, J.; Martin, S.; Ilanne-Parikka, P.; Eriksson, J.G.; Aunola, S.; Keinänen-Kiukaanniemi, S.; et al. Finnish Diabetes Prevention Study Group. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia 2009, 52, 433–442. [Google Scholar] [CrossRef]
- Chamroonkiadtikun, P.; Ananchaisarp, T.; Wanichanon, W. The triglyceride-glucose index, a predictor of type 2 diabetes development: A retrospective cohort study. Prim. Care Diabetes 2020, 14, 161–167. [Google Scholar] [CrossRef]
- Low, S.; Khoo, K.; Irwan, B.; Sum, C.F.; Subramaniam, T.; Lim, S.C.; Wong, T. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 143, 43–49. [Google Scholar] [CrossRef]
- Navarro-González, D.; Sánchez-Íñigo, L.; Fernández-Montero, A.; Pastrana-Delgado, J.; Martinez, J.A. TyG Index Change Is More Determinant for Forecasting Type 2 Diabetes Onset Than Weight Gain. Medicine 2016, 95, e3646. [Google Scholar] [CrossRef]
- da Silva, A.; Caldas, A.; Rocha, D.; Bressan, J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: A systematic review and meta-analysis of cohort studies. Prim. Care Diabetes 2020, 14, 584–593. [Google Scholar] [CrossRef] [PubMed]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, T.; Moilanen, L.; Korpi-Hyövälti, E.; Vanhala, M.; Saltevo, J.; Niskanen, L.; Jokelainen, J.; Peltonen, M.; Oksa, H.; Tuomilehto, J.; et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: One-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010, 33, 2146–2151. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA 2003, 289, 1799–1804. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Kolb, H.; Mandrup-Poulsen, T. An immune origin of type 2 diabetes? Diabetologia 2005, 48, 1038–1050. [Google Scholar] [CrossRef]
- Lee, J.H.; Ragolia, L. AKT phosphorylation is essential for insulin-induced relaxation of rat vascular smooth muscle cells. American journal of physiology. Cell. Physiol. 2006, 291, C1355–C1365. [Google Scholar] [CrossRef]
- Gutiérrez-Rodelo, C.; Roura-Guiberna, A.; Olivares-Reyes, J.A. Mecanismos Moleculares de la Resistencia a la Insulina: Una Actualización. Gac. Med. Mex. 2017, 153, 214–228. [Google Scholar]
- Jiang, L.; Manson, S.M.; Beals, J.; Henderson, W.G.; Huang, H.; Acton, K.J.; Roubideaux, Y.; Special Diabetes Program for Indians Diabetes Prevention Demonstration Project. Translating the Diabetes Prevention Program into American Indian and Alaska Native communities: Results from the Special Diabetes Program for Indians Diabetes Prevention demonstration project. Diabetes Care 2013, 36, 2027–2034. [Google Scholar] [CrossRef]
- Boltri, J.M.; Davis-Smith, M.; Okosun, I.S.; Seale, J.P.; Foster, B. Translation of the National Institutes of Health Diabetes Prevention Program in African American churches. J. Natl. Med. Assoc. 2011, 103, 194–202. [Google Scholar] [CrossRef]
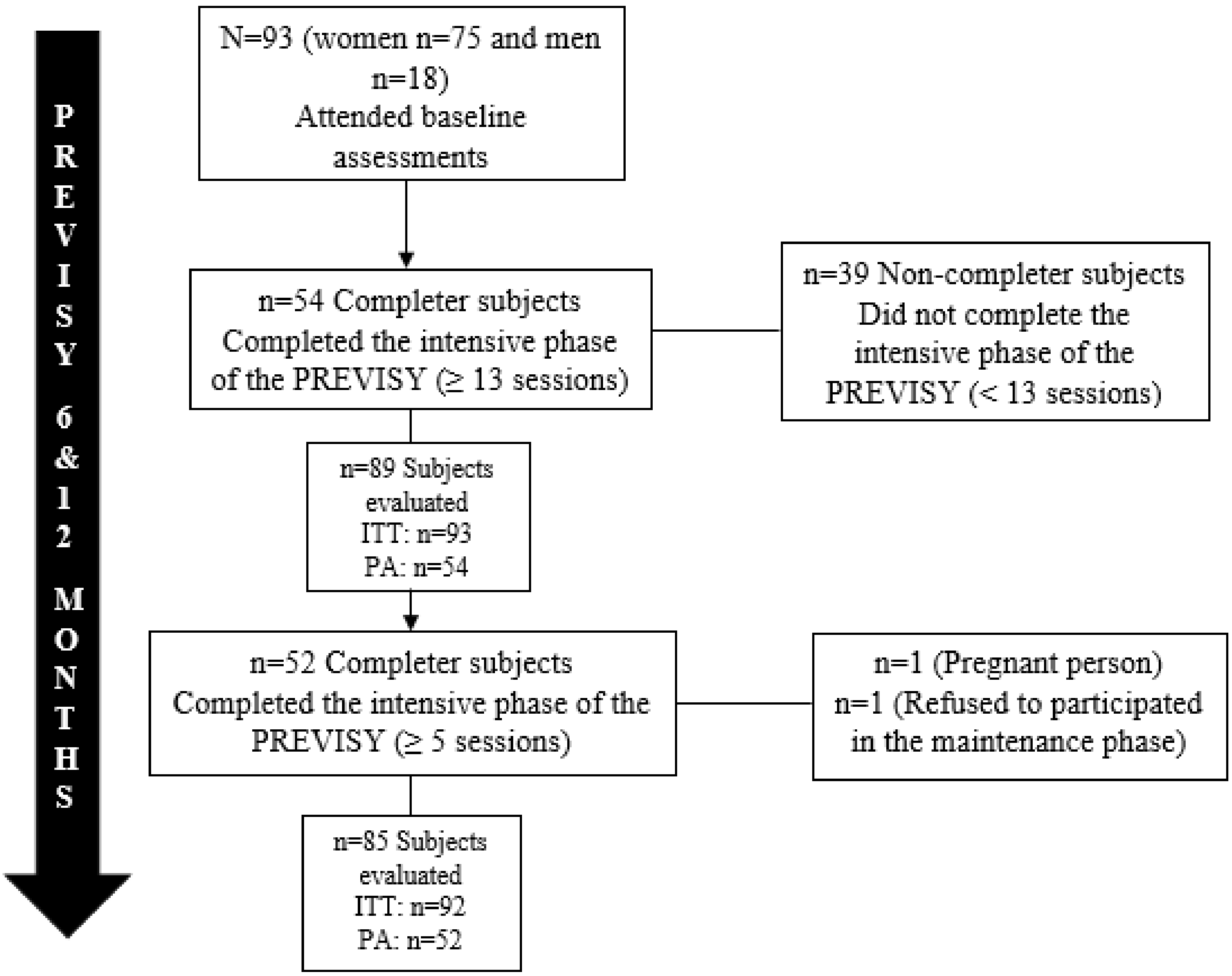
| Characteristics | Total | Completers | Non-Completers | p |
|---|---|---|---|---|
| N | 93 | 54 | 39 | |
| Gender ‡ Women Men | 1.00 | |||
| 75 (80.6) | 44 (81.5) | 31 (79.5) | ||
| 18 (19.4) | 10 (18.5) | 8 (20.5) | ||
| Age (years) † | 39.5 ± 11.2 | 40.1 ± 10.5 | 38.6 ± 12.1 | 0.54 |
| Scholarship ‡ Primary Secondary school High school University | 0.59 | |||
| 27 (29.1) | 13 (24.1) | 14 (35.9) | ||
| 36 (38.7) | 21 (38.9) | 15 (38.5) | ||
| 15 (16.1) | 10 (18.5) | 5 (12.8) | ||
| 15 (16.1) | 10 (18.5) | 5 (12.8) | ||
| Civil status ‡ Single, widower or separate Married or free union | 0.008 | |||
| 31 (33.3) | 24 (44.4) | 7 (18.0) | ||
| 62 (66.7) | 30 (55.6) | 32 (82.0) | ||
| Overweight ‡ | 28 (30.1) | 19 (35.2) | 9 (23.1) | 0.25 |
| Obesity ‡ | 65 (69.9) | 35 (64.8) | 30 (76.9) | 0.25 |
| Central obesity ‡ | 92 (98.9) | 53 (98.2) | 39 (100.0) | 1.00 |
| Previous diagnosis of HT ‡ | 13 (14.0) | 8 (14.8) | 5 (12.8) | 1.00 |
| Risk of T2D (FINDRISC) ‡ Moderate risk High and very high risk | 0.51 | |||
| 33 (35.5) | 21 (38.9) | 12 (30.8) | ||
| 60 (64.5) | 33 (61.1) | 27 (69.2) | ||
| Body weight (kg) † | 85.9 ± 14.6 | 85.0 ± 13.5 | 87.1 ± 16.1 | 0.50 |
| WC (cm) † | 104.4 ± 10.6 | 103.3 ± 10.1 | 106.0 ± 11.3 | 0.23 |
| BMI (kg/m2) † | 33.2 ± 5.2 | 32.6 ± 4.6 | 34.1 ± 6.0 | 0.17 |
| SBP (mmHg) † | 115.7 ± 13.0 | 116.6 ± 11.0 | 114.5 ± 15.5 | 0.45 |
| DBP (mmHg) † | 73.3 ± 9.4 | 73.6 ± 8.6 | 72.8 ± 10.5 | 0.67 |
| Fasting glucose (mg/dL) † | 109.6 ± 26.2 | 110 ± 28.9 | 108.7 ± 22.4 | 0.77 |
| Triglycerides (mg/dL) † | 155.7 ± 87.7 | 159.1 ± 75.2 | 151.1 ± 103.4 | 0.66 |
| Serum insulin (µU/mL) † | 23.8 ± 11.7 | 22.4 ± 10.9 | 25.8 ± 12.6 | 0.16 |
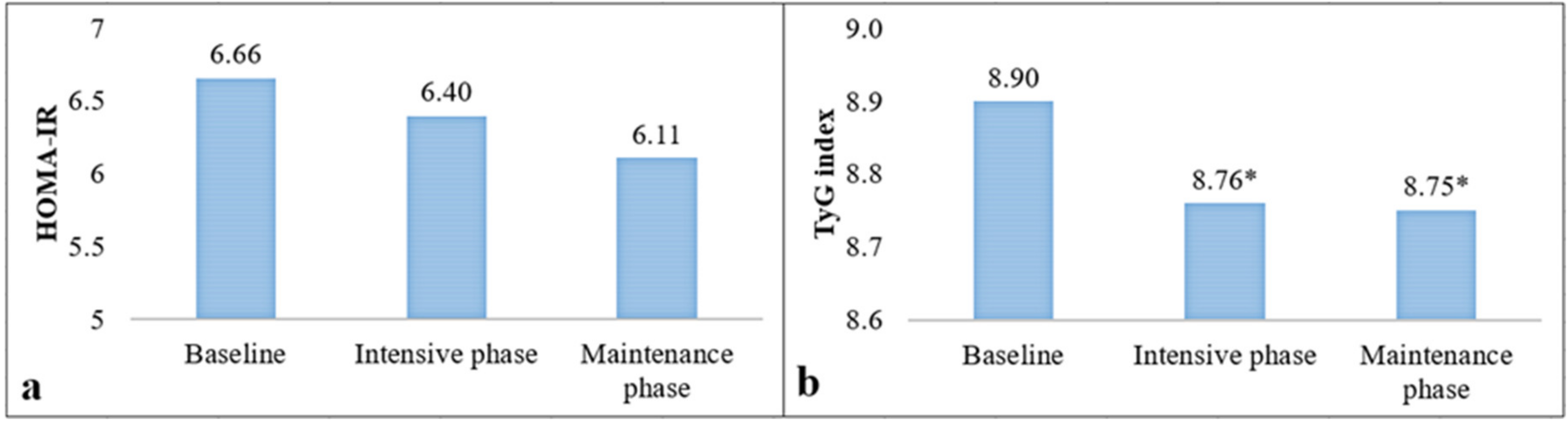
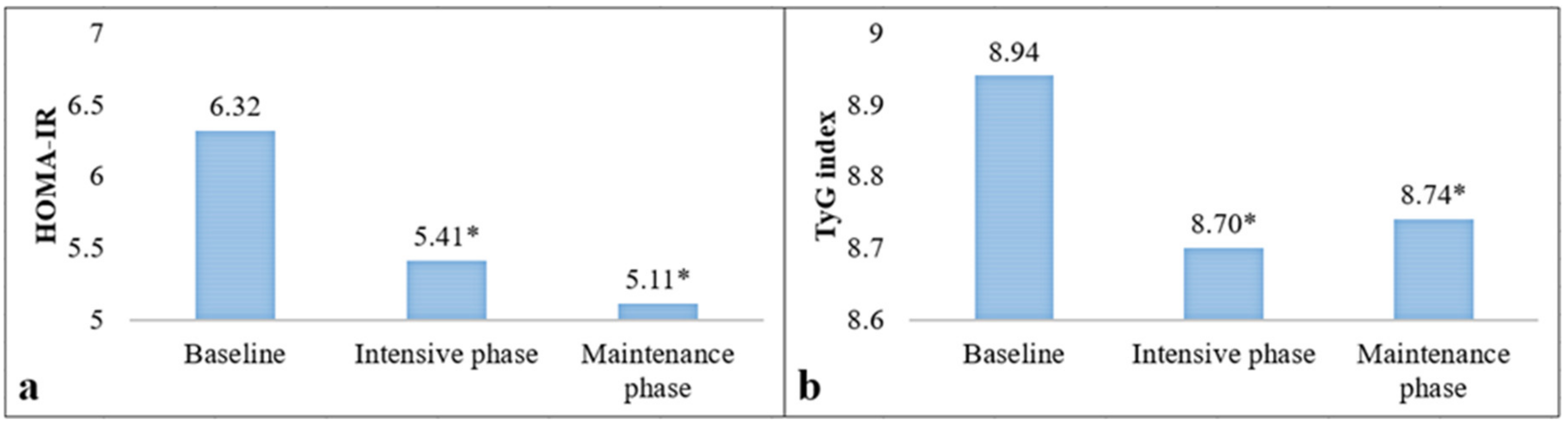
| HOMA-IR † | 6.6 ± 4.2 | 6.3 ± 3.9 | 7.1 ± 4.6 | 0.37 |
| TyG index † | 8.9 ± 0.5 | 8.9 ± 0.5 | 8.8 ± 0.5 | 0.43 |
| HOMA-IR Levels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body weight goal (%) | n | Baseline | Intensive phase | ∆ | p value | n | Baseline | Maintenance phase | ∆ | p value |
| 5 to 7 | 11 | 5.54 ± 3.45 | 4.73 ± 2.58 | −0.81 ± 2.01 | 0.21 | 11 | 8.51 ± 7.76 | 5.91 ± 4.22 | −2.60 ± 5.30 | 0.13 |
| 7 to 10 | 6 | 9.76 ± 4.98 | 5.53 ± 2.46 | −4.23 ± 4.39 | 0.06 | 4 | 8.92 ± 6.02 | 4.15 ± 1.83 | −4.77 ± 4.24 | 0.10 |
| ≥10 | 7 | 8.27 ± 2.79 | 4.94 ± 2.02 | −3.32 ± 1.82 | 0.002 | 10 | 8.98 ± 3.31 | 4.09 ± 1.59 | −4.89 ± 3.93 | 0.003 |
| TyG Index Levels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body weight goal (%) | n | Baseline | Intensive phase | ∆ | p value | n | Baseline | Maintenance phase | ∆ | p value |
| 5 to 7 | 11 | 9.13 ± 0.75 | 8.87 ± 0.59 | −0.26 ± 0.32 | 0.02 | 11 | 9.17 ± 0.57 | 9.00 ± 0.58 | −0.16 ± 0.39 | 0.20 |
| 7 to 10 | 6 | 9.15 ± 0.63 | 8.81 ± 0.37 | −0.33 ± 0.46 | 0.14 | 4 | 9.02 ± 0.54 | 8.47 ± 0.28 | −0.55 ± 0.47 | 0.10 |
| ≥10 | 7 | 9.14 ± 0.41 | 8.34 ± 0.38 | −0.80 ± 0.35 | 0.001 | 10 | 8.90 ± 0.59 | 8.30 ± 0.31 | −0.60 ± 0.65 | 0.01 |
| Outcomes | Overweight (n = 19) | Obesity (n = 35) | ∆ Overweight | ∆ Obesity |
|---|---|---|---|---|
| HOMA-IR Baseline Intensive phase Maintenance phase | ||||
| 4.77 ± 2.43 | 7.17 ± 4.37 | |||
| 4.63 ± 2.28 | 5.83 ± 2.88 | −0.14 ± 1.50 | −1.34 ± 3.62 * | |
| 4.87 ± 2.32 | 5.24 ± 3.21 | +0.16 ± 2.35 | −2.06 ± 4.49 * | |
| TyG index | ||||
| Baseline Intensive phase Maintenance phase | 8.99 ± 0.67 | 8.91 ± 0.52 | ||
| 8.81 ± 0.58 | 8.65 ± 0.48 | −0.18 ± 0.30 * | −0.26 ± 0.53 * | |
| 8.96 ± 0.60 | 8.62 ± 0.47 | −0.02 ± 0.47 | −0.29 ± 0.53 * |
| Outcomes | Moderate Risk (n = 21) | High and Very High Risk (n = 33) | ∆ Moderate Risk | ∆ High and Very High Risk |
|---|---|---|---|---|
| HOMA-IR | ||||
| Baseline Intensive phase Maintenance phase | 5.08 ± 2.78 | 7.12 ± 4.39 | ||
| 5.46 ± 2.90 | 5.37 ± 2.65 | +0.38 ± 2.48 | −1.74 ± 3.18 * | |
| 5.71 ± 3.50 | 4.74 ± 2.47 | +0.68 ± 3.65 | −2.52 ± 3.75 * | |
| TyG index | ||||
| Baseline Intensive phase Maintenance phase | 8.94 ± 0.70 | 8.94 ± 0.49 | ||
| 8.84 ± 0.62 | 8.61 ± 0.43 | −0.09 ± 0.48 | −0.32 ± 0.43 * | |
| 8.82 ± 0.68 | 8.69 ± 0.43 | −0.11 ± 0.42 | −0.25 ± 0.58 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Juarez, A.A.; Serna-Gutiérrez, A.; Alemán-Mateo, H.; Gallegos-Aguilar, A.C.; Dórame-López, N.A.; Valenzuela-Sánchez, A.; Valenzuela-Guzmán, D.M.; Díaz-Zavala, R.G.; Urquidez-Romero, R.; Esparza-Romero, J. Effectiveness of a Lifestyle Change Program on Insulin Resistance in Yaquis Indigenous Populations in Sonora, Mexico: PREVISY. Nutrients 2023, 15, 597. https://doi.org/10.3390/nu15030597
Castro-Juarez AA, Serna-Gutiérrez A, Alemán-Mateo H, Gallegos-Aguilar AC, Dórame-López NA, Valenzuela-Sánchez A, Valenzuela-Guzmán DM, Díaz-Zavala RG, Urquidez-Romero R, Esparza-Romero J. Effectiveness of a Lifestyle Change Program on Insulin Resistance in Yaquis Indigenous Populations in Sonora, Mexico: PREVISY. Nutrients. 2023; 15(3):597. https://doi.org/10.3390/nu15030597
Chicago/Turabian StyleCastro-Juarez, Alejandro Arturo, Araceli Serna-Gutiérrez, Heliodoro Alemán-Mateo, Ana Cristina Gallegos-Aguilar, Norma Alicia Dórame-López, Abraham Valenzuela-Sánchez, Diana Marcela Valenzuela-Guzmán, Rolando Giovanni Díaz-Zavala, Rene Urquidez-Romero, and Julián Esparza-Romero. 2023. "Effectiveness of a Lifestyle Change Program on Insulin Resistance in Yaquis Indigenous Populations in Sonora, Mexico: PREVISY" Nutrients 15, no. 3: 597. https://doi.org/10.3390/nu15030597
APA StyleCastro-Juarez, A. A., Serna-Gutiérrez, A., Alemán-Mateo, H., Gallegos-Aguilar, A. C., Dórame-López, N. A., Valenzuela-Sánchez, A., Valenzuela-Guzmán, D. M., Díaz-Zavala, R. G., Urquidez-Romero, R., & Esparza-Romero, J. (2023). Effectiveness of a Lifestyle Change Program on Insulin Resistance in Yaquis Indigenous Populations in Sonora, Mexico: PREVISY. Nutrients, 15(3), 597. https://doi.org/10.3390/nu15030597







