Targeting Iron Metabolism and Ferroptosis as Novel Therapeutic Approaches in Cardiovascular Diseases
Abstract
1. Introduction
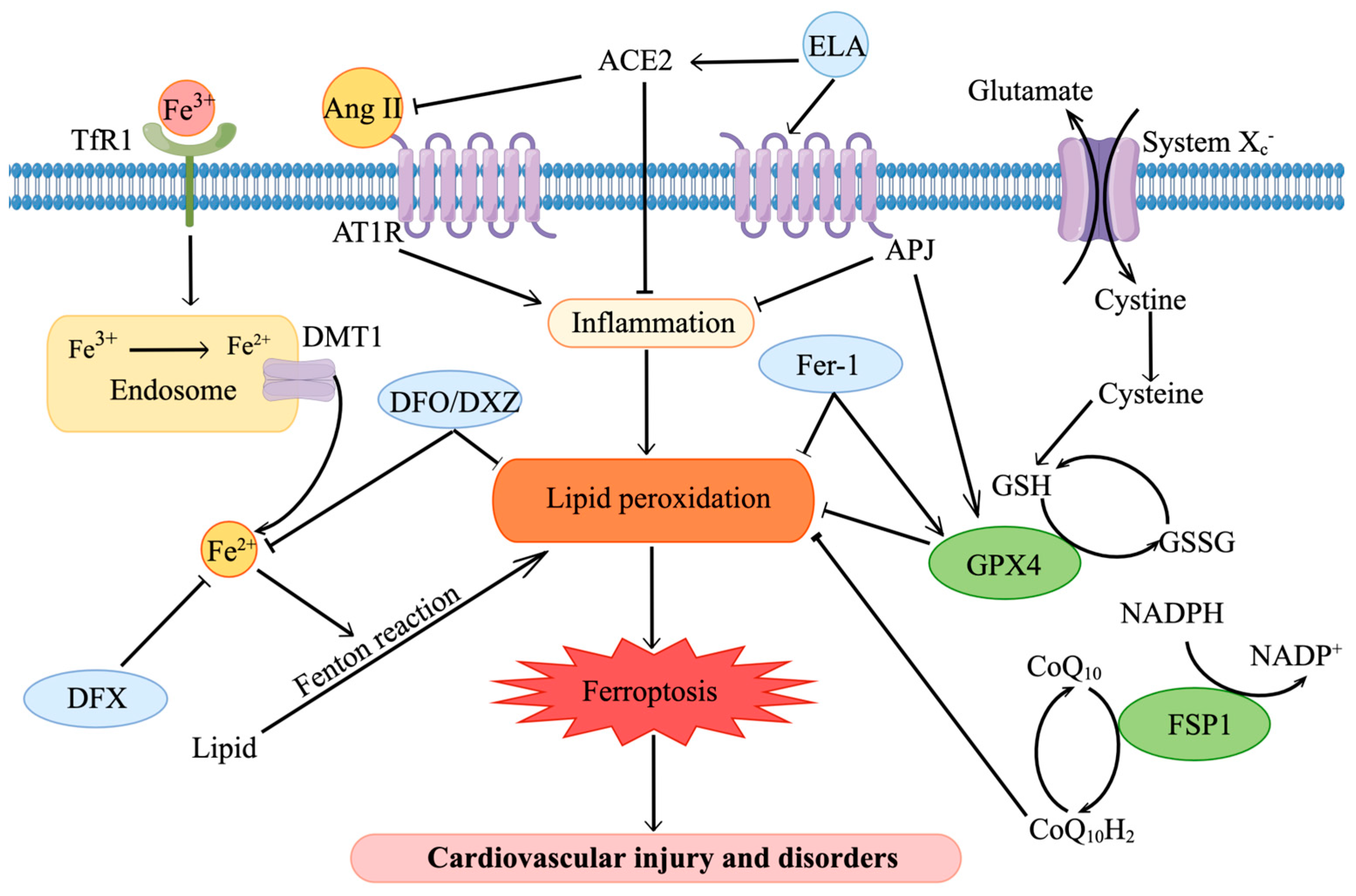
2. Iron Metabolism and Ferroptosis
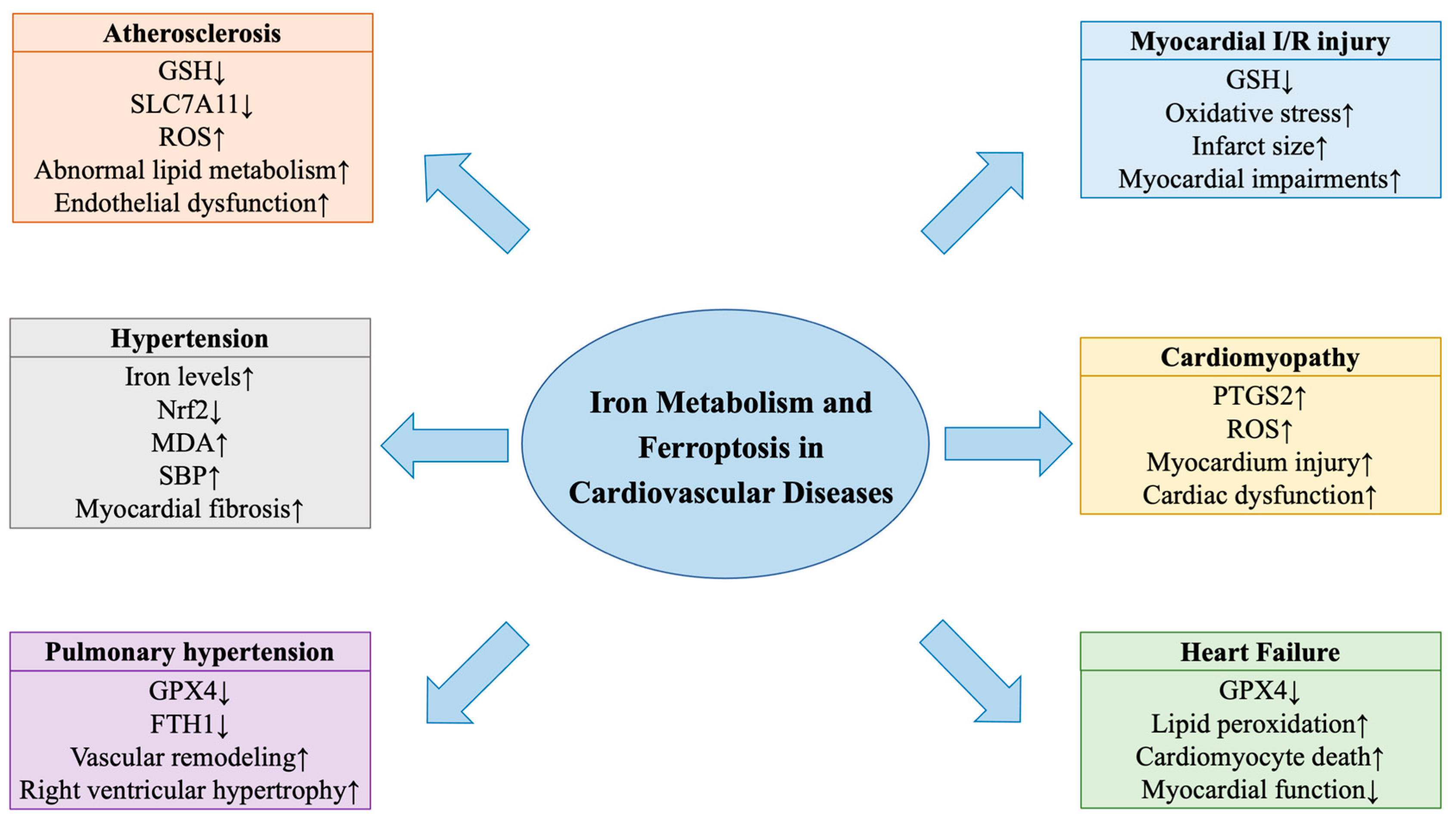
3. Iron Metabolism, Ferroptosis and Cardiovascular Diseases
3.1. Iron Metabolism and Ferroptosis in Atherosclerosis
3.2. Ferroptosis in Hypertension
3.3. Iron Metabolism and Ferroptosis in Pulmonary Hypertension
3.4. Ferroptosis in Aortic Aneurysm and Dissection
3.5. Ferroptosis in Myocardial Ischemia/Reperfusion Injury
3.6. Ferroptosis in Cardiomyopathy
3.6.1. Sepsis-Induced Cardiomyopathy
3.6.2. Doxorubicin-Induced Cardiomyopathy
3.7. Iron Metabolism and Ferroptosis in Heart Failure
4. Targeting Iron Metabolism and Ferroptosis in Cardiovascular Diseases
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Asp. Med. 2020, 75, 100864. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, A.; Yan, Z.; Geng, X.; Lian, L.; Lv, H.; Gao, D.; Zhang, J. From Iron Metabolism to Ferroptosis: Pathologic Changes in Coronary Heart Disease. Oxid. Med. Cell Longev. 2022, 2022, 6291889. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, X. Iron in Cardiovascular Disease: Challenges and Potentials. Front. Cardiovasc. Med. 2021, 8, 707138. [Google Scholar] [CrossRef] [PubMed]
- Ravingerová, T.; Kindernay, L.; Barteková, M.; Ferko, M.; Adameová, A.; Zohdi, V.; Bernátová, I.; Ferenczyová, K.; Lazou, A. The Molecular Mechanisms of Iron Metabolism and Its Role in Cardiac Dysfunction and Cardioprotection. Int. J. Mol. Sci. 2020, 21, 7889. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ye, T.; Yang, L.; Shen, Y.; Li, H. Ferroptosis Signaling and Regulators in Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 809457. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, J.; Song, J.; Xie, M.; Liu, Y.; Dong, Z.; Liu, X.; Li, X.; Zhang, M.; Chen, Y.; et al. Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling. Free Radic. Biol. Med. 2022, 181, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.S.; Deng, Y.; Guo, S.L.; Li, J.Q.; Zhou, Y.C.; Liao, J.; Wu, D.D.; Lan, W.F. Endothelial cell ferroptosis mediates monocrotaline-induced pulmonary hypertension in rats by modulating NLRP3 inflammasome activation. Sci. Rep. 2022, 12, 3056. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Xiao, Z.Z.; Ling, X.; Xu, R.N.; Zhu, P.; Zheng, S.Y. ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol. Med. 2021, 27, 14. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.D.; Ikeda, M.; Ide, T.; Tadokoro, T.; Furusawa, S.; Abe, K.; Ishimaru, K.; Enzan, N.; Sada, M.; Yamamoto, T.; et al. Iron Overload via Heme Degradation in the Endoplasmic Reticulum Triggers Ferroptosis in Myocardial Ischemia-Reperfusion Injury. JACC Basic Transl. Sci. 2022, 7, 800–819. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, B.; Fang, J.; Qin, T.; Dai, C.; Shuai, W.; Huang, H. Ferrostatin-1 alleviates lipopolysaccharide-induced cardiac dysfunction. Bioengineered 2021, 12, 9367–9376. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, W.; Zhou, H.; Wu, Q.; Duan, M.; Liu, C.; Wu, H.; Deng, W.; Shen, D.; Tang, Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 2020, 160, 303–318. [Google Scholar] [CrossRef]
- Chen, X.; Xu, S.; Zhao, C.; Liu, B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem. Biophys. Res. Commun. 2019, 516, 37–43. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2022, 20, 7–23. [Google Scholar] [CrossRef]
- Gaitán, D.; Olivares, M.; Lönnerdal, B.; Brito, A.; Pizarro, F. Non-heme iron as ferrous sulfate does not interact with heme iron absorption in humans. Biol. Trace Elem. Res. 2012, 150, 68–73. [Google Scholar] [CrossRef]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.Z. Cellular Iron Metabolism and Regulation. Adv. Exp. Med. Biol. 2019, 1173, 21–32. [Google Scholar] [PubMed]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal iron absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Rong, J.; Tao, X.; Xu, Y. The Emerging Role of Ferroptosis in Cardiovascular Diseases. Front. Pharmacol. 2022, 13, 822083. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015, 13, 533–545. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Di Paola, A.; Tortora, C.; Argenziano, M.; Marrapodi, M.M.; Rossi, F. Emerging Roles of the Iron Chelators in Inflammation. Int. J. Mol. Sci. 2022, 23, 7977. [Google Scholar] [CrossRef]
- Leng, Y.; Luo, X.; Yu, J.; Jia, H.; Yu, B. Ferroptosis: A Potential Target in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 813668. [Google Scholar] [CrossRef]
- Lakhal-Littleton, S.; Wolna, M.; Carr, C.A.; Miller, J.J.; Christian, H.C.; Ball, V.; Santos, A.; Diaz, R.; Biggs, D.; Stillion, R.; et al. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc. Natl. Acad. Sci. USA 2015, 112, 3164–3169. [Google Scholar] [CrossRef]
- Fang, J.; Kong, B.; Shuai, W.; Xiao, Z.; Dai, C.; Qin, T.; Gong, Y.; Zhu, J.; Liu, Q.; Huang, H. Ferroportin-mediated ferroptosis involved in new-onset atrial fibrillation with LPS-induced endotoxemia. Eur. J. Pharmacol. 2021, 913, 174622. [Google Scholar] [CrossRef]
- Zlatanova, I.; Pinto, C.; Bonnin, P.; Mathieu, J.R.R.; Bakker, W.; Vilar, J.; Lemitre, M.; Voehringer, D.; Vaulont, S.; Peyssonnaux, C.; et al. Iron Regulator Hepcidin Impairs Macrophage-Dependent Cardiac Repair after Injury. Circulation 2019, 139, 1530–1547. [Google Scholar] [CrossRef] [PubMed]
- Ghafourian, K.; Shapiro, J.S.; Goodman, L.; Ardehali, H. Iron and Heart Failure: Diagnosis, Therapies, and Future Directions. JACC Basic Transl. Sci. 2020, 5, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Protchenko, O.; Baratz, E.; Jadhav, S.; Li, F.; Shakoury-Elizeh, M.; Gavrilova, O.; Ghosh, M.C.; Cox, J.E.; Maschek, J.A.; Tyurin, V.A.; et al. Iron Chaperone Poly rC Binding Protein 1 Protects Mouse Liver from Lipid Peroxidation and Steatosis. Hepatology 2021, 73, 1176–1193. [Google Scholar] [CrossRef]
- Lee, J.; You, J.H.; Roh, J.L. Poly(rC)-binding protein 1 represses ferritinophagy-mediated ferroptosis in head and neck cancer. Redox Biol. 2022, 51, 102276. [Google Scholar] [CrossRef]
- Haddad, S.; Wang, Y.; Galy, B.; Korf-Klingebiel, M.; Hirsch, V.; Baru, A.M.; Rostami, F.; Reboll, M.R.; Heineke, J.; Flögel, U.; et al. Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur. Heart J. 2017, 38, 362–372. [Google Scholar] [CrossRef]
- Yang, K.; Song, H.; Yin, D. PDSS2 Inhibits the Ferroptosis of Vascular Endothelial Cells in Atherosclerosis by Activating Nrf2. J. Cardiovasc. Pharmacol. 2021, 77, 767–776. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Fefelova, N.; Pamarthi, S.H.; Gwathmey, J.K. Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease. Cells 2022, 11, 2726. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, C.; Hu, K.; Cai, C.; Wang, W. Ferroptosis in Cardiovascular Diseases: Current Status, Challenges, and Future Perspectives. Biomolecules 2022, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Qiao, Y.; Wang, D.; Tang, C.; Yan, G. Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications. Biomed. Pharmacother. 2021, 141, 111872. [Google Scholar] [CrossRef]
- Guo, Z.; Ran, Q.; Roberts, L.J., 2nd; Zhou, L.; Richardson, A.; Sharan, C.; Wu, D.; Yang, H. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free Radic. Biol. Med. 2008, 44, 343–352. [Google Scholar] [CrossRef]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020, 5, e132747. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Li, X.-T.; Song, J.-W.; Zhang, Z.-Z.; Zhang, M.-W.; Liang, L.-R.; Miao, R.; Liu, Y.; Chen, Y.-H.; Liu, X.-Y.; Zhong, J.-C. Sirtuin 7 mitigates renal ferroptosis, fibrosis and injury in hypertensive mice by facilitating the KLF15/Nrf2 signaling. Free Radic. Biol. Med. 2022, 193, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Xu, Y.; Jiang, Y.; Huang, J.; Liu, Y.; Wang, D.; Tao, T.; Sun, Z.; Liu, Y. The mechanism of the imbalance between proliferation and ferroptosis in pulmonary artery smooth muscle cells based on the activation of SLC7A11. Eur. J. Pharmacol. 2022, 928, 175093. [Google Scholar] [CrossRef]
- Li, N.; Yi, X.; He, Y.; Huo, B.; Chen, Y.; Zhang, Z.; Wang, Q.; Li, Y.; Zhong, X.; Li, R.; et al. Targeting Ferroptosis as a Novel Approach to Alleviate Aortic Dissection. Int. J. Biol. Sci. 2022, 18, 4118–4134. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Wunderer, F.; Traeger, L.; Sigurslid, H.H.; Meybohm, P.; Bloch, D.B.; Malhotra, R. The role of hepcidin and iron homeostasis in atherosclerosis. Pharmacol. Res. 2020, 153, 104664. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, H.Q.; Hao, Y.M.; Ren, Z.; Qu, S.L.; Liu, L.S.; Wei, D.H.; Tang, Z.H.; Zhang, J.F.; Jiang, Z.S. Macrophage polarization in atherosclerosis. Clin. Chim. Acta 2020, 501, 142–146. [Google Scholar] [CrossRef]
- Vallurupalli, M.; MacFadyen, J.G.; Glynn, R.J.; Thuren, T.; Libby, P.; Berliner, N.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Incident Anemia: Exploratory Analyses from a Randomized Trial. Ann. Intern. Med. 2020, 172, 523–532. [Google Scholar] [CrossRef]
- Meng, Z.; Liang, H.; Zhao, J.; Gao, J.; Liu, C.; Ma, X.; Liu, J.; Liang, B.; Jiao, X.; Cao, J.; et al. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci. 2021, 284, 119935. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.A.M.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension. Arter. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chi, L.; Kuebler, W.M.; Goldenberg, N.M. Perivascular Inflammation in Pulmonary Arterial Hypertension. Cells 2020, 9, 2338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Yuan, W.; Meng, L.K.; Zhong, J.C.; Liu, X.Y. The Role and Mechanism of Gut Microbiota in Pulmonary Arterial Hypertension. Nutrients 2022, 14, 4278. [Google Scholar] [CrossRef]
- Quatredeniers, M.; Mendes-Ferreira, P.; Santos-Ribeiro, D.; Nakhleh, M.K.; Ghigna, M.R.; Cohen-Kaminsky, S.; Perros, F. Iron Deficiency in Pulmonary Arterial Hypertension: A Deep Dive into the Mechanisms. Cells 2021, 10, 477. [Google Scholar] [CrossRef]
- Callejo, M.; Barberá, J.A.; Duarte, J.; Perez-Vizcaino, F. Impact of Nutrition on Pulmonary Arterial Hypertension. Nutrients 2020, 12, 169. [Google Scholar] [CrossRef]
- Viethen, T.; Gerhardt, F.; Dumitrescu, D.; Knoop-Busch, S.; ten Freyhaus, H.; Rudolph, T.K.; Baldus, S.; Rosenkranz, S. Ferric carboxymaltose improves exercise capacity and quality of life in patients with pulmonary arterial hypertension and iron deficiency: A pilot study. Int. J. Cardiol. 2014, 175, 233–239. [Google Scholar] [CrossRef]
- Ruiter, G.; Manders, E.; Happé, C.M.; Schalij, I.; Groepenhoff, H.; Howard, L.S.; Wilkins, M.R.; Bogaard, H.J.; Westerhof, N.; van der Laarse, W.J.; et al. Intravenous iron therapy in patients with idiopathic pulmonary arterial hypertension and iron deficiency. Pulm. Circ. 2015, 5, 466–472. [Google Scholar] [CrossRef]
- Wong, C.M.; Preston, I.R.; Hill, N.S.; Suzuki, Y.J. Iron chelation inhibits the development of pulmonary vascular remodeling. Free Radic. Biol. Med. 2012, 53, 1738–1747. [Google Scholar] [CrossRef]
- Lakhal-Littleton, S.; Crosby, A.; Frise, M.C.; Mohammad, G.; Carr, C.A.; Loick, P.A.M.; Robbins, P.A. Intracellular iron deficiency in pulmonary arterial smooth muscle cells induces pulmonary arterial hypertension in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 13122–13130. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Hong, J.; Che, Y.; Chen, R.; Hu, Z.; Hu, X.; Wu, Q.; Hu, J.; Zhang, M. Iron deficiency promotes aortic medial degeneration via destructing cytoskeleton of vascular smooth muscle cells. Clin. Transl. Med. 2021, 11, e276. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, Z.; Wu, Q.; Zhong, X.; Zhang, M.; Li, B.; Ren, W.; Yuan, S.; Chen, Y. Iron deficiency promotes aortic media degeneration by activating endoplasmic reticulum stress-mediated IRE1 signaling pathway. Pharmacol. Res. 2022, 183, 106366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, X.; Huo, B.; He, Y.; Guo, X.; Zhang, Z.; Zhong, X.; Feng, X.; Fang, Z.M.; Zhu, X.H.; et al. BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol. Res. 2022, 177, 106122. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Park, J.H.; Lee, G.S.; Lee, J.Y.; Shin, J.H.; Kim, M.W.; Kim, Y.S.; Kim, J.Y.; Oh, K.J.; Han, B.S.; et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.J.; Zhou, Y.J.; Xiong, X.M.; Li, N.S.; Zhang, J.J.; Luo, X.J.; Peng, J. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic. Biol. Med. 2021, 162, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, L.; Wu, W.; Wu, J.; Sun, Z.; Ren, J. USP22 Protects against Myocardial Ischemia-Reperfusion Injury via the SIRT1-p53/SLC7A11-Dependent Inhibition of Ferroptosis-Induced Cardiomyocyte Death. Front. Physiol. 2020, 11, 551318. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, Y.; Zhang, Y.; Leng, Y.; Tao, J.; Li, L.; Qiu, Z.; Xia, Z. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones 2021, 27, 149–164. [Google Scholar] [CrossRef]
- Tu, H.; Zhou, Y.J.; Tang, L.J.; Xiong, X.M.; Zhang, X.J.; Ali Sheikh, M.S.; Zhang, J.J.; Luo, X.J.; Yuan, C.; Peng, J. Combination of ponatinib with deferoxamine synergistically mitigates ischemic heart injury via simultaneous prevention of necroptosis and ferroptosis. Eur. J. Pharmacol. 2021, 898, 173999. [Google Scholar] [CrossRef]
- Ta, N.; Qu, C.; Wu, H.; Zhang, D.; Sun, T.; Li, Y.; Wang, J.; Wang, X.; Tang, T.; Chen, Q.; et al. Mitochondrial outer membrane protein FUNDC2 promotes ferroptosis and contributes to doxorubicin-induced cardiomyopathy. Proc. Natl. Acad. Sci. USA 2022, 119, e2117396119. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Liu, X.; Deng, F.; Wang, P.; Yang, L.; Hu, L.; Huang, K.; He, J. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ. 2022, 29, 1982–1995. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, J.; Zhang, X.; Wu, X.; Zhao, Y.; Ren, J. Iron homeostasis and disorders revisited in the sepsis. Free Radic. Biol. Med. 2021, 165, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Manceau, H.; Ausseil, J.; Masson, D.; Feugeas, J.P.; Sablonniere, B.; Guieu, R.; Puy, H.; Peoc’h, K. Neglected Comorbidity of Chronic Heart Failure: Iron Deficiency. Nutrients 2022, 14, 3214. [Google Scholar] [CrossRef]
- Zhabyeyev, P.; Oudit, G.Y. Unravelling the molecular basis for cardiac iron metabolism and deficiency in heart failure. Eur. Heart J. 2017, 38, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef]
- Feng, Y.; Madungwe, N.B.; Imam Aliagan, A.D.; Tombo, N.; Bopassa, J.C. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem. Biophys. Res. Commun. 2019, 520, 606–611. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Yuan, W.; Sun, L.; Xia, Z.; Zhang, Z.; Yao, W. Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J. Cell Mol. Med. 2020, 24, 6670–6679. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, Z.; Du, K.; Lu, X. Protective Effects of Dexazoxane on Rat Ferroptosis in Doxorubicin-Induced Cardiomyopathy through Regulating HMGB1. Front. Cardiovasc. Med. 2021, 8, 685434. [Google Scholar] [CrossRef]
- Zhang, M.W.; Li, X.T.; Zhang, Z.Z.; Liu, Y.; Song, J.W.; Liu, X.M.; Chen, Y.H.; Wang, N.; Guo, Y.; Liang, L.R.; et al. Elabela blunts doxorubicin-induced oxidative stress and ferroptosis in rat aortic adventitial fibroblasts by activating the KLF15/GPX4 signaling. Cell Stress Chaperones 2022, 28, 1–13. [Google Scholar] [CrossRef]
| Experimental Model | Interventions | Effects and Mechanisms | Ref. |
|---|---|---|---|
| Mice with AS | Fer-1 | Atherosclerotic lesion area↓, iron levels↓, GPX4↑, SLC7A11↑, MDA↓ | [9] |
| ApoE−/− mice | GPX4-Tg | Atherosclerotic lesions↓, lipid peroxidation↓ | [41] |
| Ox-LDL-treated HCAECs | Overexpression of PDSS2 | Cell death↓, iron content↓, GSH↑, Nrf2↑, ROS↓ | [36] |
| Hypertensive mice | ELA-32 | Cardiac hypertrophy and remodeling↓, myocardial fibrosis and dysfunction↓, SBP↓, iron levels↓, GPX4↑, Nrf2↑, MDA↓ | [12] |
| Hypertensive mice | SIRT7 | Kidney injury and dysfunction↓, renal fibrosis↓, GPX4↑, GSH/GSSG↑, Nrf2↑, NOX4↓, MDA↓ | [44] |
| Rats with PH | Fer-1 | Vascular remodeling↓, right ventricular function↑, iron content↓, GPX4↑, HMGB1↓, TLR4↓, NLRP3 inflammasome↓ | [13] |
| Hypoxic PASMCs | SLC7A11 siRNA | GPX4↓, GSH/GSSG↓, MDA↑ | [45] |
| Mice with AAD | - | Aortic diameter↑, HMOX1↑, TfR↑, lipid peroxidation↑ | [46] |
| Mice with AAD | Lip-1 | AAD incidence↓, medial degeneration↓, HMOX1↓, 4-HNE↓ | [46] |
| Experimental Model | Interventions | Effects and Mechanisms | Ref. |
|---|---|---|---|
| Rats | |||
| MIRI | - | Infarct area↑, CK activity↑, iron content↑, GPX4↓, ACSL4↑, ROS↑ | [69] |
| MIRI | DFO | Infarct size↓, iron levels↓, GPX4↑, lipid peroxidation↓ | [69] |
| SIC | - | Myocardial function↓, iron levels↑, GPX4↓, PTGS2↑ | [16] |
| HF | TLR4-siRNA/NOX4-siRNA | Heart function↑, myocyte death↓, intracellular iron↓, GPX4↑, 4-HNE↓ | [18] |
| Mice | |||
| MIRI | ELAVL1-siRNA | Infarct size↓, iron levels↓, GPX4↑, ROS↓ | [14] |
| MIRI | GPX4-Tg | Myocardial impairments↓, lipid peroxides↓, TUNEL+ cells↓ | [15] |
| DIC | GPX4-Tg | Heart impairments↓, LVEF↑, MDA↓ | [42] |
| DIC | FUNDC2-KO | Cardiac function↑, cardiac fibrosis↓, 4-HNE↓, PTGS2↓ | [70] |
| DIC | rAAV9-PRMT4 | Myocardial injury↑, cardiac function↓, GPX4↓, GSH↓, ROS↑ | [71] |
| Dis. | Interventions | Targets | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| AS | Fer-1 | Inhibit lipid peroxidation | Atherosclerotic lesion↓, iron accumulation↓, GSH↑, SCL7A11↑, lipid peroxidation↓ | [9] |
| HT | ELA-32 | Inhibit ferroptosis | Myocardial fibrosis and dysfunction↓, SBP↓, iron levels↓, ROS↓, GPX4↑, Nrf2↑ | [12] |
| HT | Fer-1 | Inhibit lipid peroxidation | Cardiac hypertrophy and remodeling↓, GPX4↑, MDA↓ | [12] |
| PH | Fer-1 | Inhibit lipid peroxidation | Right ventricular hypertrophy↓, iron levels↓, HMGB1↓, TLR4↓, NLRP3 inflammasome↓ | [13] |
| AAD | Lip-1 | Inhibit lipid peroxidation | AAD incidence↓, mortality↓, TfR↓, HMOX1↓, lipid peroxidation↓ | [46] |
| AAD | BRD4770 | Inhibit ferroptosis | AAD mortality↓, aorta dilation↓, medial degradation↓, HMOX1↓, SLC7A11↑, FSP1↑, lipid peroxidation↓, neutrophil infiltration↓ | [63] |
| MIRI | P22077 | Inhibit USP7 | Infarct size↓, cardiac fiber loss↓, iron content↓, TfR1↓, GPX activity↑, ACSL4↓, lipid peroxidation↓ | [66] |
| MIRI | DFO | Iron chelation | Infarct size↓, CK activity↓, iron content↓, GPX4↑, ACSL4↓, lipid peroxidation↓ | [69] |
| MIRI | Lip-1 | Inhibit lipid peroxidation | Myocardial infarct size↓, mitochondrial structural integrity↑, GPX4↑, ROS↓, VDAC1↓ | [77] |
| DIMI | Vas2870 | Inhibit NOX2 | Cardiac injury↓, GPX4↑, oxidative stress↓, | [78] |
| SIC | Fer-1 | Inhibit lipid peroxidation | Cardiac function↑, iron content↓, GPX4↑, PTGS2↓, inflammatory cell infiltration↓, TLR4↓, NF-κB↓ | [16] |
| SIC | DXZ/Fer-1 | Iron chelation/Inhibit lipid peroxidation | Survival rate↑, cardiac injury↓, ferric iron↓, PTGS2↓, MDA↓, inflammatory cells↓ | [17] |
| DIC | ZnPP | Inhibit HMOX1 | Cardiac injury↓, MDA↓, 4-HNE↓, PTGS2↓ | [37] |
| DIC | DXZ/Fer-1 | Iron chelation/Inhibit lipid peroxidation | Myocardial hypertrophy↓, cardiac function↑, lipid peroxidation↓, PTGS2↓ | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, X.; Wang, S.; Miao, R.; Zhong, J. Targeting Iron Metabolism and Ferroptosis as Novel Therapeutic Approaches in Cardiovascular Diseases. Nutrients 2023, 15, 591. https://doi.org/10.3390/nu15030591
Chen Y, Li X, Wang S, Miao R, Zhong J. Targeting Iron Metabolism and Ferroptosis as Novel Therapeutic Approaches in Cardiovascular Diseases. Nutrients. 2023; 15(3):591. https://doi.org/10.3390/nu15030591
Chicago/Turabian StyleChen, Yufei, Xueting Li, Siyuan Wang, Ran Miao, and Jiuchang Zhong. 2023. "Targeting Iron Metabolism and Ferroptosis as Novel Therapeutic Approaches in Cardiovascular Diseases" Nutrients 15, no. 3: 591. https://doi.org/10.3390/nu15030591
APA StyleChen, Y., Li, X., Wang, S., Miao, R., & Zhong, J. (2023). Targeting Iron Metabolism and Ferroptosis as Novel Therapeutic Approaches in Cardiovascular Diseases. Nutrients, 15(3), 591. https://doi.org/10.3390/nu15030591







