Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Intervention
2.2. Clinical Assessment, Samples and Biochemical Measurements
2.3. Predicted 10-Year Risk for ASCVD
2.4. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Baseline Characteristics in the Participants
3.2. Changes in Biochemical Characteristics Post Vitamin D Supplementation
3.3. ASCVD Risk Scores as per Improvement in Circulating 25(OH) Vitamin D Levels Post Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Saquib, N.; Zaghloul, M.S.; Mazrou, A.; Saquib, J. Cardiovascular disease research in Saudi Arabia: A bibliometric analysis. Scientometrics 2017, 112, 111–140. [Google Scholar] [CrossRef]
- Aljefree, N.; Ahmed, F. Prevalence of cardiovascular disease and associated risk factors among adult population in the Gulf region: A systematic review. Adv. Public Health 2015, 2015, 235101. [Google Scholar] [CrossRef]
- Kannel, W.B. The Framingham Study: Historical insight on the impact of cardiovascular risk factors in men versus women. J. Gend.-Specif. Med. JGSM Off. J. Partnersh. Women’s Health Columbia 2002, 5, 27–37. [Google Scholar]
- Ferns, G.A. New and emerging risk factors for CVD: Symposium on ‘Diet and CVD’. Proc. Nutr. Soc. 2008, 67, 223–231. [Google Scholar] [CrossRef]
- Al-Hazzaa, H.M. Physical inactivity in Saudi Arabia revisited: A systematic review of inactivity prevalence and perceived barriers to active living. Int. J. Health Sci. 2018, 12, 50–64. [Google Scholar]
- Fareed, M.; Salam, N.; Khoja, A.T.; Mahmoud, A.M.; Ahamed, M. Life style related risk factors of type 2 diabetes mellitus and its increased prevalence in Saudi Arabia: A brief review. Int. J. Med. Res. Health Sci. 2017, 6, 125–132. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Kannel, W.B.; Dawber, T.R.; Kagan, A.; Revotskie, N.; Stokes, J., III. Factors of risk in the development of coronary heart disease—Six-year follow-up experience: The Framingham Study. Ann. Intern. Med. 1961, 55, 33–50. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection; Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III); The Program. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Shah, S.H.; Kraus, W.E.; Newgard, C.B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: Form and function. Circulation 2012, 126, 1110–1120. [Google Scholar] [CrossRef]
- Brown, H.A. Lipidomics: When apocrypha becomes canonical. Curr. Opin. Chem. Biol. 2012, 16, 221–226. [Google Scholar] [CrossRef]
- Dron, J.S.; Lazarte, J.; Hegele, R.A. Recent Highlights of ATVB: Lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e185–e197. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Johnston, H.E.; Singhania, A.; Alokail, M.S.; Alkharfy, K.M.; Abd-Alrahman, S.H.; Sabico, S.L.; Roumeliotis, T.I.; Manousopoulou-Garbis, A. Whole serum 3D LC-nESI-FTMS quantitative proteomics reveals sexual dimorphism in the milieu interieur of overweight and obese adults. J. Proteome Res. 2014, 13, 5094–5105. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Wani, K.; Sabico, S.; Garbis, S.D.; Chrousos, G.P.; Amer, O.E.; Ansari, M.G.A.; Al-Saleh, Y.; Aljohani, N.J.; Al-Attas, O.S. Sex-specific expression of apolipoprotein levels following replenishment of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 180, 129–136. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Mesa, M.D.; Gil, Á. Vitamin D: Role in chronic and acute diseases. Ref. Modul. Food Sci. 2022. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Mohammed, A.K.; Bukhari, I.; Rikli, M.; Abdi, S.; Ansari, M.G.A.; Sabico, S.; Hussain, S.D.; Alenad, A.; Al-Saleh, Y.; et al. Efficacy of vitamin D supplementation according to vitamin D-binding protein polymorphisms. Nutrition 2019, 63-64, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Harahap, I.A.; Landrier, J.-F.; Suliburska, J. Interrelationship between Vitamin D and Calcium in Obesity and Its Comorbid Conditions. Nutrients 2022, 14, 3187. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Muscogiuri, G.; Sorice, G.; Prioletta, A.; Salomone, E.; Pontecorvi, A.; Giaccari, A. Vitamin D deficiency: A new risk factor for type 2 diabetes. Ann. Nutr. Metab. 2012, 61, 337–348. [Google Scholar] [CrossRef]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Bassil, D.; Rahme, M.; Hoteit, M.; El-Hajj Fuleihan, G. Hypovitaminosis D in the Middle East and North Africa: Prevalence, risk factors and impact on outcomes. Dermatoendocrinology 2013, 5, 274–298. [Google Scholar] [CrossRef]
- Hayıroğlu, M.; Çınar, T.; Çinier, G.; Karakaya, A.; Yıldırım, M.; Güney, B.; Öz, A.; Gündoğmuş, P.D.; Ösken, A.; Özkan, A.; et al. The effect of 1-year mean step count on the change in the atherosclerotic cardiovascular disease risk calculation in patients with high cardiovascular risk: A sub-study of the LIGHT randomized clinical trial. Kardiol Pol. 2021, 79, 1140–1142. [Google Scholar] [CrossRef]
- Tekkeşin, A.İ.; Hayıroğlu, M.İ.; Çinier, G.; Özdemir, Y.S.; İnan, D.; Yüksel, G.; Pay, L.; Parsova, K.E.; Vatanoğlu, E.G.; Şeker, M.; et al. Lifestyle intervention using mobile technology and smart devices in patients with high cardiovascular risk: A pragmatic randomised clinical trial. Atherosclerosis 2021, 319, 21–27. [Google Scholar] [CrossRef]
- Aldawsari, G.M.; Sabico, S.; Alamro, A.A.; Alenad, A.; Wani, K.; Alnaami, A.M.; Khattak, M.N.K.; Masoud, M.S.; Al-Daghri, N.M.; Alokail, M.S. Angiogenin Levels and Their Association with Cardiometabolic Indices Following Vitamin D Status Correction in Saudi Adults. Biology 2022, 11, 286. [Google Scholar] [CrossRef]
- Al Saleh, Y.; Beshyah, S.A.; Hussein, W.; Almadani, A.; Hassoun, A.; Al Mamari, A.; Ba-Essa, E.; Al-Dhafiri, E.; Hassanein, M.; Fouda, M.A. Diagnosis and management of vitamin D deficiency in the Gulf Cooperative Council (GCC) countries: An expert consensus summary statement from the GCC vitamin D advisory board. Arch. Osteoporos. 2020, 15, 35. [Google Scholar] [CrossRef]
- ASCVD Risk Estimator Plus, American College of Cardiology. Available online: https://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/ (accessed on 20 February 2022).
- Li, Y.; Tong, C.H.; Rowland, C.M.; Radcliff, J.; Bare, L.A.; McPhaul, M.J.; Devlin, J.J. Association of changes in lipid levels with changes in vitamin D levels in a real-world setting. Sci. Rep. 2021, 11, 21536. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Rainsbury, J.; Kimball, S.M. Vitamin D supplementation, serum 25 (OH) D concentrations and cardiovascular disease risk factors: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2018, 5, 87. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Saleh, Y.; Aljohani, N.; Alokail, M.; Al-Attas, O.; Alnaami, A.M.; Sabico, S.; Alsulaimani, M.; Al-Harbi, M.; Alfawaz, H. Vitamin D deficiency and cardiometabolic risks: A juxtaposition of Arab adolescents and adults. PLoS ONE 2015, 10, e0131315. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alokail, M.S.; Manousopoulou, A.; Heinson, A.; Al-Attas, O.; Al-Saleh, Y.; Sabico, S.; Yakout, S.; Woelk, C.H.; Chrousos, G.P. Sex-specific vitamin D effects on blood coagulation among overweight adults. Eur. J. Clin. Investig. 2016, 46, 1031–1040. [Google Scholar] [CrossRef]
- Mancuso, E.; Mannino, G.C.; Fuoco, A.; Leo, A.; Citraro, R.; Averta, C.; Spiga, R.; Russo, E.; De Sarro, G.; Andreozzi, F. Hdl (high-density lipoprotein) and apoa-1 (apolipoprotein a-1) potentially modulate pancreatic α-cell glucagon secretion. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2941–2952. [Google Scholar] [CrossRef]
- Radkhah, N.; Shabbidar, S.; Zarezadeh, M.; Safaeiyan, A.; Barzegar, A. Effects of vitamin D supplementation on apolipoprotein A1 and B100 levels in adults: Systematic review and meta-analysis of controlled clinical trials. J. Cardiovasc. Thorac. Res. 2021, 13, 190–197. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alkharfy, K.M.; Al-Othman, A.; El-Kholie, E.; Moharram, O.; Alokail, M.S.; Al-Saleh, Y.; Sabico, S.; Kumar, S.; Chrousos, G.P. Vitamin D supplementation as an adjuvant therapy for patients with T2DM: An 18-month prospective interventional study. Cardiovasc. Diabetol. 2012, 11, 85. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alkharfy, K.M.; Al-Saleh, Y.; Al-Attas, O.S.; Alokail, M.S.; Al-Othman, A.; Moharram, O.; El-Kholie, E.; Sabico, S.; Kumar, S. Modest reversal of metabolic syndrome manifestations with vitamin D status correction: A 12-month prospective study. Metabolism 2012, 61, 661–666. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A narrative review of the evidence for variations in serum 25-hydroxyvitamin D concentration thresholds for optimal health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the evidence from observational studies and randomized controlled trials for nonskeletal health effects of vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.; Al-Attas, O.; Alokail, M.; Alkharfy, K.; Yousef, M.; Nadhrah, H.; Al-Othman, A.; Al-Saleh, Y.; Sabico, S.; Chrousos, G. Hypovitaminosis D and cardiometabolic risk factors among non-obese youth. Open Medicine 2010, 5, 752–757. [Google Scholar] [CrossRef]
- Elmi, C.; Fan, M.M.; Le, M.; Cheng, G.; Khalighi, K. Association of serum 25-Hydroxy Vitamin D level with lipid, lipoprotein, and apolipoprotein level. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; März, W.; Pilz, S. Vitamin D and cardiovascular disease: An updated narrative review. Int. J. Mol. Sci. 2021, 22, 2896. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Vimaleswaran, K.S.; Zhou, A. Genetic Determinants of 25-Hydroxyvitamin D Concentrations and Their Relevance to Public Health. Nutrients 2022, 14, 4408. [Google Scholar] [CrossRef]
- Zhou, A.; Selvanayagam, J.B.; Hyppönen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2022, 43, 1731–1739. [Google Scholar] [CrossRef]
- Acharya, P.; Dalia, T.; Ranka, S.; Sethi, P.; Oni, O.A.; Safarova, M.S.; Parashara, D.; Gupta, K.; Barua, R.S. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J. Endocr. Soc. 2021, 5, bvab124. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Substantial vitamin D supplementation is required during the prenatal period to improve birth outcomes. Nutrients 2022, 14, 899. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Hu, D.; Chen, J.; Li, Y.; Huang, J.; Liu, X.; Liu, F.; Cao, J.; Shen, C. Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: The China-PAR Project (Prediction for ASCVD Risk in China). Circulation 2016, 134, 1430–1440. [Google Scholar] [CrossRef]
- Moss, H.E.; An, R.; Nelson, T.; Li, K. Risk of Atherosclerotic Cardiovascular Disease Among US Adults: Use of 1999–2014 NHANES Data. J. Prim. Prev. 2019, 40, 569–573. [Google Scholar] [CrossRef]
- Alhabib, K.F.; Batais, M.A.; Almigbal, T.H.; Alshamiri, M.Q.; Altaradi, H.; Rangarajan, S.; Yusuf, S. Demographic, behavioral, and cardiovascular disease risk factors in the Saudi population: Results from the Prospective Urban Rural Epidemiology study (PURE-Saudi). BMC Public Health 2020, 20, 1213. [Google Scholar] [CrossRef]
- Barter, P.; Genest, J. HDL cholesterol and ASCVD risk stratification: A debate. Atherosclerosis 2019, 283, 7–12. [Google Scholar] [CrossRef]
- Casula, M.; Colpani, O.; Xie, S.; Catapano, A.L.; Baragetti, A. HDL in Atherosclerotic Cardiovascular Disease: In Search of a Role. Cells 2021, 10, 1869. [Google Scholar] [CrossRef]
- Yi, S.W.; Park, H.B.; Jung, M.H.; Yi, J.J.; Ohrr, H. High-density lipoprotein cholesterol and cardiovascular mortality: A prospective cohort study among 15.8 million adults. Eur. J. Prev. Cardiol. 2022, 29, 844–854. [Google Scholar] [CrossRef]
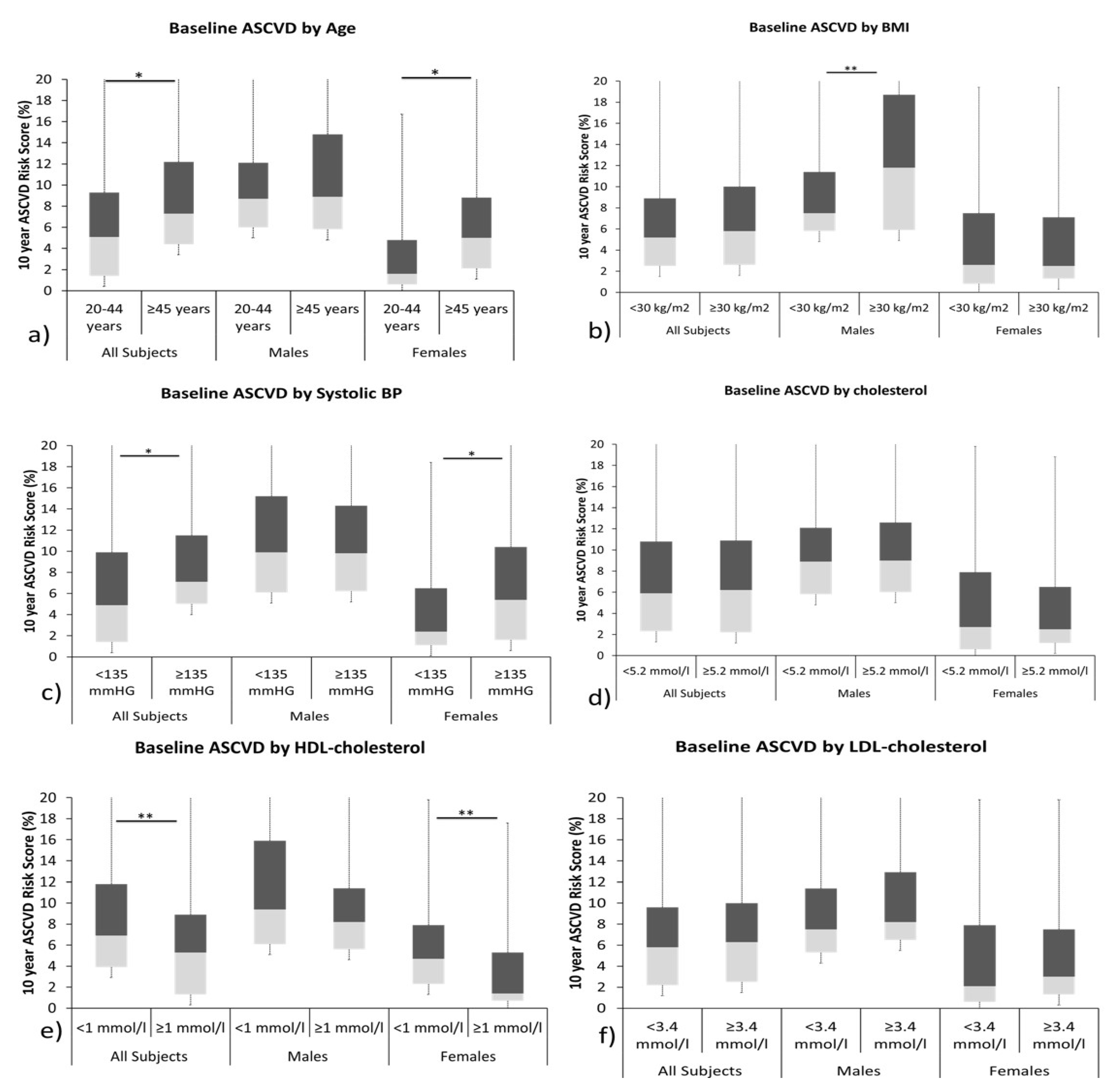
| Parameters | Total (N = 120) | Males (N = 58) | Females (N = 62) | p |
|---|---|---|---|---|
| BMI [N (%)] | ||||
| Lean Overweight Obese NA | 15 (12) 59 (49) 43 (36) 3 (2) | 7 (12) 34 (59) 16 (28) 1 (2) | 8 (13) 25 (40) 27 (44) 2 (3) | 0.12 |
| 25 (OH) D Status [N (%)] | ||||
| Severely deficient (<25 nmol/L) Deficient (25–50 nmol/L) | 32 (27) 88 (73) | 13 (22) 45 (78) | 19 (31) 43 (69) | 0.31 |
| Diabetes [N (%)] | ||||
| Yes No | 27 (22) 93 (78) | 12 (20.7) 46 (79) | 15 (24) 47 (76) | 0.65 |
| Family History of Diabetes [N (%)] | ||||
| Yes No NA | 66 (55) 17 (14) 37 (31) | 23 (40) 4 (7) 31 (53) | 43 (69) 13 (21) 6 (10) | <0.001 |
| Family History of CVD [N (%)] | ||||
| Yes No NA | 15 (12) 62 (52) 43 (36) | 5 (9) 17 (29) 36 (62) | 10 (16) 45 (73) 7 (11) | <0.001 |
| Family History of Dyslipidemia [N (%)] | ||||
| Yes No NA | 28 (23) 65 (54) 27 (22) | 16 (28) 19 (33) 23 (40) | 12 (19) 46 (74) 4 (6) | <0.001 |
| Age (years) # | 40.6 ± 10.8 | 42.8 ± 9.6 | 38.5 ± 11.6 | 0.03 |
| Anthropometrics | ||||
| Weight (kg) # | 77 ± 14 | 83 ± 14 | 72 ± 13 | <0.01 |
| BMI (kg/m2) # | 29 ± 5 | 29 ± 4 | 30 ± 6 | 0.36 |
| Waist (cm) # | 96 ± 13 | 98 ± 12 | 95 ± 14 | 0.19 |
| Hips (cm) # | 102 ± 14 | 99 ± 13 | 105 ± 14 | 0.04 |
| Systolic BP (mmHg) # | 127 ± 14 | 132 ± 10 | 121 ± 15 | <0.01 |
| Diastolic BP (mmHg) # | 79 ± 9 | 83 ± 7 | 76 ± 10 | <0.01 |
| Biochemical Characteristics | ||||
| Total cholesterol (mmol/L) # | 5.1 ± 1.2 | 5.1 ± 1.2 | 5.1 ± 1.2 | 0.77 |
| Triglycerides (mmol/L) $ | 1.5 (1.1,2.3) | 1.5 (1.1,2.5) | 1.5 (1.1,2.1) | 0.24 |
| HDL cholesterol (mmol/L) # | 1.01 ± 0.4 | 0.98 ± 0.3 | 1.03 ± 0.4 | 0.39 |
| LDL cholesterol (mmol/L) # | 3.2 ± 1.1 | 3.1 ± 1.1 | 3.3 ± 1.1 | 0.26 |
| Glucose (mmol/L) $ | 5.7 (5.1,6.6) | 5.7 (5.3,6.6) | 5.6 (4.9,6.5) | 0.35 |
| Total 25 (OH)D (nmol/L) # | 32.5 ± 10.7 | 34.7 ± 9.5 | 30.4 ± 11.5 | 0.03 |
| Category by Cardiovascular Risk Score (ASCVD) [N (%)] | ||||
| Low Borderline Intermediate High | 49 (41) 22 (18) 43 (36) 6 (5) | 42 (68) 4 (6) 16 (26) 0 (0) | 7 (12) 18 (31) 27 (47) 6 (10) | <0.001 |
| ASCVD risk score (%) $ | 6 (2,10) | 9 (6,13) | 3 (1, 8) | <0.001 |
| Parameters | All (N = 120) | Males (N = 58) | Females (N = 62) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-Up | Difference | p-Value | Follow-Up | Difference | p-Value | Follow-Up | Difference | p-Value | |
| Total cholesterol (mmol/L) # | 5.1 ± 1.3 | −0.02 | 0.83 | 5.2 ± 1.4 | 0.1 | 0.46 | 5.0 ± 1.2 | −0.1 | 0.31 |
| Triglycerides (mmol/L) $ | 1.6 (1.1,2.1) | 0.1 | 0.82 | 1.7 (1.3,2.3) | 0.2 | 0.60 | 1.4 (1,2) | −0.1 | 0.40 |
| HDL cholesterol (mmol/L) # | 1.1 ± 0.4 | 0.1 | 0.001 | 1.0 ± 0.3 | 0.1 | 0.001 | 1.2 ± 0.4 | 0.2 | 0.04 |
| LDL cholesterol (mmol/L) # | 3.1 ± 1.1 | −0.1 | 0.28 | 3.2 ± 1.3 | 0.1 | 0.65 | 3.1 ± 1 | −0.2 | 0.04 |
| Glucose (mmol/L) $ | 5.8 (5.2,6.4) | 0.1 | 0.62 | 5.9 (5.5,6.4) | 0.2 | 0.24 | 5.5 (4.8,6.4) | −0.1 | 0.74 |
| Total 25 (OH)D (nmol/L) # | 63.3 ± 16.5 | 30.8 | <0.001 | 61 ± 14.3 | 26.2 | <0.001 | 65.5 ± 18.1 | 35.1 | <0.001 |
| ASCVD risk score (%) $ | 5.0 (1.5,8.3) | −1.1 | <0.001 | 7.4 (5.4,12) | −1.4 | 0.02 | 1.5 (0.8,3.5) | −1.1 | <0.001 |
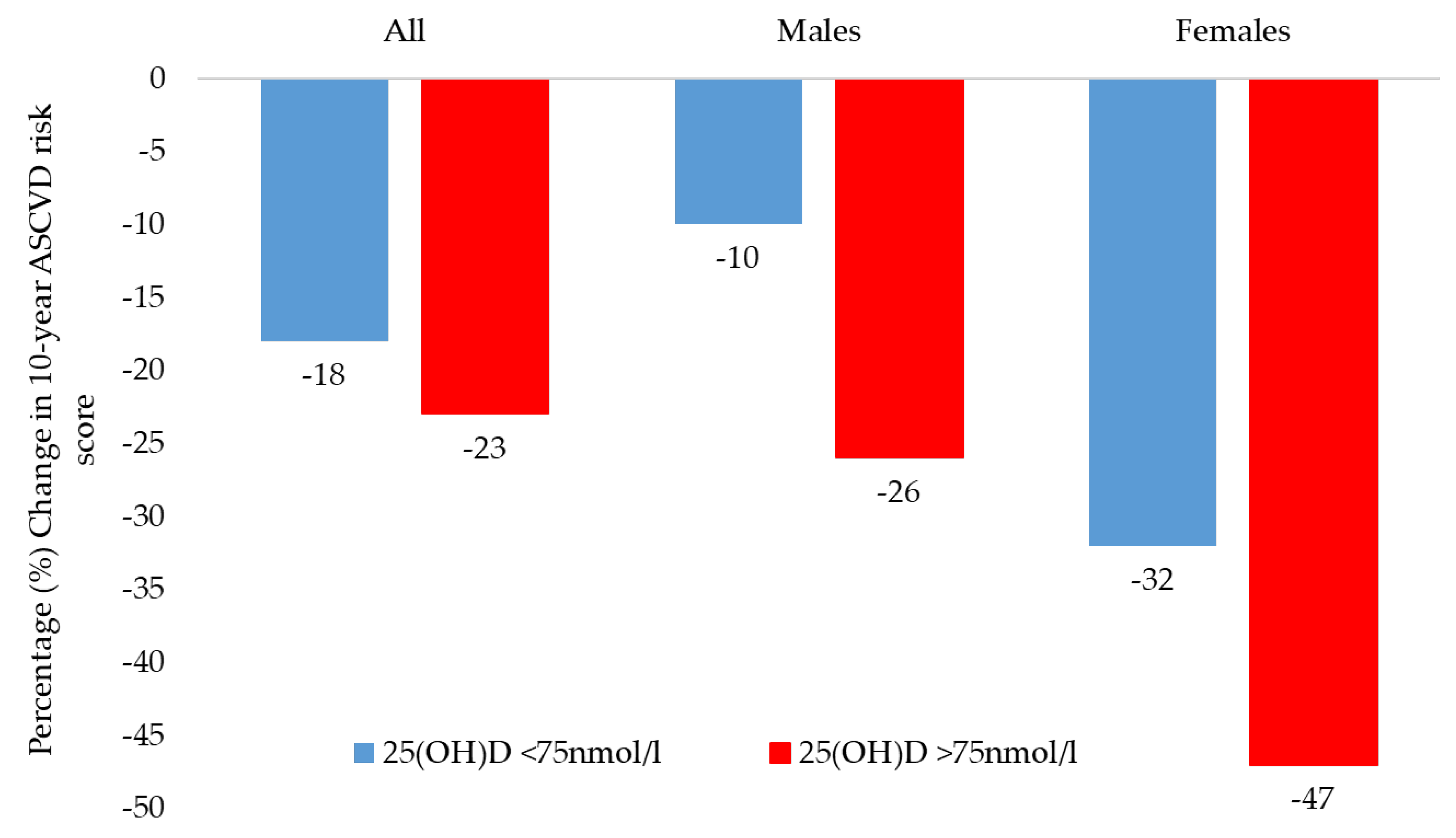
| Post-Intervention Status | 25(OH)D < 75 nmol/L | 25(OH)D ≥ 75 nmol/L | p-Value * | ||
|---|---|---|---|---|---|
| % Change | p-Value | % Change | p-Value | ||
| All Participants | 105 | 25 | |||
| 25(OH)D (nmol/L) | 76 | <0.001 | 159 | <0.001 | <0.001 |
| Total cholesterol (mmol/L) | −0.2 | 0.95 | −2 | 0.71 | 0.54 |
| Triglycerides (mmol/L) | 12 | 0.98 | −2 | 0.58 | 0.06 |
| HDL cholesterol (mmol/L) | 11 | 0.01 | 12 | 0.009 | 0.16 |
| LDL cholesterol (mmol/L) | −3 | 0.35 | −3 | 0.62 | 0.86 |
| Glucose (mmol/L) | 3 | 0.56 | −2 | 0.83 | 0.79 |
| 10-year ASCVD risk (%) | −18 | 0.01 | −23 | 0.03 | 0.41 |
| Males | 49 | 9 | |||
| 25(OH)D (nmol/L) | 62 | <0.001 | 149 | <0.001 | 0.002 |
| Total cholesterol (mmol/L) | 3 | 0.39 | −0.4 | 0.95 | 0.97 |
| Triglycerides (mmol/L) | 7 | 0.83 | 3 | 0.44 | 0.36 |
| HDL cholesterol (mmol/L) | 6 | 0.02 | 8 | 0.02 | 0.46 |
| LDL cholesterol (mmol/L) | 3 | 0.51 | −3 | 0.85 | 0.56 |
| Glucose (mmol/L) | 4 | 0.57 | 8.4 | 0.13 | 0.35 |
| 10-year ASCVD risk (%) | −10 | 0.02 | −26 | <0.001 | 0.71 |
| Females | 46 | 16 | |||
| 25(OH)D (nmol/L) | 95 | <0.001 | 166 | <0.001 | <0.001 |
| Total cholesterol (mmol/L) | −3 | 0.38 | −2 | 0.59 | 0.49 |
| Triglycerides (mmol/L) | 2 | 0.73 | −7 | 0.31 | 0.07 |
| HDL cholesterol (mmol/L) | 16 | 0.03 | 15 | 0.03 | 0.38 |
| LDL cholesterol (mmol/L) | −8 | 0.05 | −3 | 0.51 | 0.79 |
| Glucose (mmol/L) | −2 | 0.83 | −9 | 0.22 | 0.32 |
| 10-year ASCVD risk (%) | −32 | 0.004 | −47 | 0.002 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabico, S.; Wani, K.; Grant, W.B.; Al-Daghri, N.M. Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults. Nutrients 2023, 15, 551. https://doi.org/10.3390/nu15030551
Sabico S, Wani K, Grant WB, Al-Daghri NM. Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults. Nutrients. 2023; 15(3):551. https://doi.org/10.3390/nu15030551
Chicago/Turabian StyleSabico, Shaun, Kaiser Wani, William B. Grant, and Nasser M. Al-Daghri. 2023. "Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults" Nutrients 15, no. 3: 551. https://doi.org/10.3390/nu15030551
APA StyleSabico, S., Wani, K., Grant, W. B., & Al-Daghri, N. M. (2023). Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults. Nutrients, 15(3), 551. https://doi.org/10.3390/nu15030551







