The Role of Maternal Vitamin D Deficiency in Offspring Obesity: A Narrative Review
Abstract
1. Introduction
2. The Relationship of VD Deficiency during Pregnancy and Obesity-Related Diseases in Offspring
2.1. VD Metabolism
2.2. VD Biological Effects
2.3. VD Deficiency in Pregnant Women
2.4. Clinical Evidence of VD Deficiency in Obese Pregnant Women and Its Effect on Offspring
2.5. Clinical Evidence of VD Deficiency in GDM and Its Effects on Offspring
2.6. Clinical Evidence of VD Deficiency in Pregnant Women with Dyslipidemia, and Its Effect on Offspring
2.7. Clinical Evidence of VD Deficiency in Pregnant Women with Other Disorders and Its Effect on Offspring
2.7.1. Polycystic Ovary Syndrome (PCOS)
2.7.2. Metabolic Syndrome (MetS)
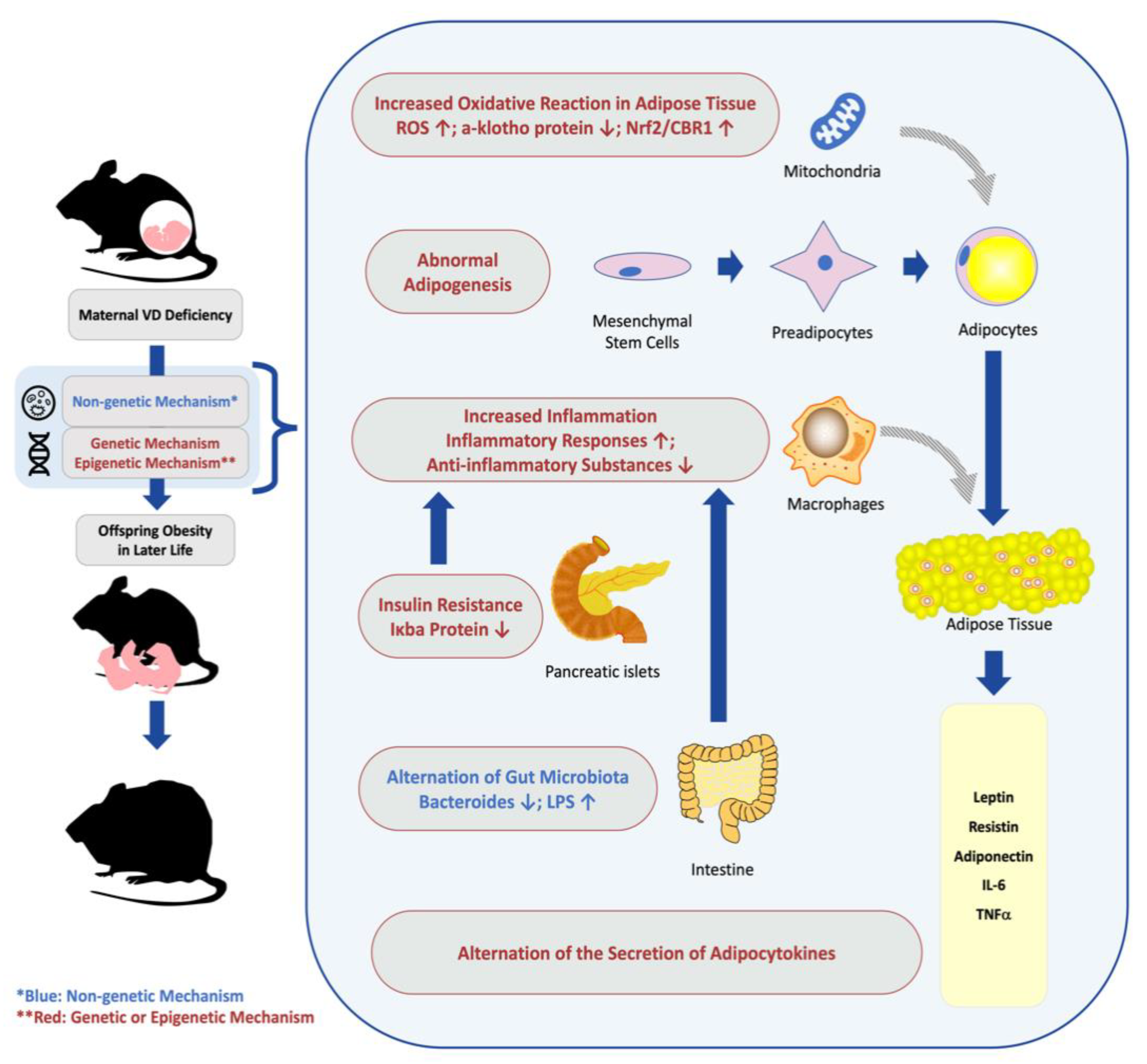
3. The Effects of Maternal VD Deficiency on Offspring Obesity, and Involved Mechanism
3.1. The Effects of Maternal VD Deficiency on the Adipogenesis Process in Offspring
3.2. The Effect of Maternal VD Deficiency on Adipocytokine Secretion in Offspring
3.2.1. Leptin
3.2.2. Resistin
3.2.3. Adiponectin
3.3. The Effect of Maternal VD Deficiency on Insulin Resistance in Offspring
3.4. The Effect of Maternal VD Deficiency on the Inflammatory Response in Offspring
3.5. The Effect of Maternal VD Deficiency on Alternations of Gut Microbiota in Offspring
3.6. The Effect of Maternal VD Status on Offspring Oxidative Stress
4. Conclusions and Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 20 August 2022).
- Bapat, S.P.; Whitty, C.; Mowery, C.T.; Liang, Y.; Yoo, A.; Jiang, Z.; Peters, M.C.; Zhang, L.J.; Vogel, I.; Zhou, C.; et al. Obesity alters pathology and treatment response in inflammatory disease. Nature 2022, 604, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kivimaki, M.; Strandberg, T.; Pentti, J.; Nyberg, S.T.; Frank, P.; Jokela, M.; Ervasti, J.; Suominen, S.B.; Vahtera, J.; Sipila, P.N.; et al. Body-mass index and risk of obesity-related complex multimorbidity: An observational multicohort study. Lancet Diabetes Endocrinol. 2022, 10, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Buklijas, T.; Hanson, M.A. The Developmental Origins of Health and Disease (DOHaD) Concept. In The Epigenome and Developmental Origins of Health and Disease; Rosenfeld, C.S., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 1–15. [Google Scholar]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef]
- Goyal, D.; Limesand, S.W.; Goyal, R. Epigenetic responses and the developmental origins of health and disease. J. Endocrinol. 2019, 242, T105–T119. [Google Scholar] [CrossRef]
- Antoun, E.; Kitaba, N.T.; Titcombe, P.; Dalrymple, K.V.; Garratt, E.S.; Barton, S.J.; Murray, R.; Seed, P.T.; Holbrook, J.D.; Kobor, M.S.; et al. Maternal dysglycaemia, changes in the infant’s epigenome modified with a diet and physical activity intervention in pregnancy: Secondary analysis of a randomised control trial. PLoS Med. 2020, 17, e1003229. [Google Scholar] [CrossRef] [PubMed]
- Laker, R.C.; Lillard, T.S.; Okutsu, M.; Zhang, M.; Hoehn, K.L.; Connelly, J.J.; Yan, Z. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1alpha gene and age-dependent metabolic dysfunction in the offspring. Diabetes 2014, 63, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xiao, X.H. Gut microbiota: Closely tied to the regulation of circadian clock in the development of type 2 diabetes mellitus. Chin. Med. J. 2020, 133, 817–825. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Varde, P.A.; Abdallah, S.L.; Najjar, S.M.; MohanKumar, P.S.; MohanKumar, S.M. Differential effects of prenatal stress on metabolic programming in diet-induced obese and dietary-resistant rats. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E582–E588. [Google Scholar] [CrossRef]
- Rogers, J.M. Smoking and pregnancy: Epigenetics and developmental origins of the metabolic syndrome. Birth Defects Res. 2019, 111, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Krela-Kazmierczak, I.; Szymczak, A.; Lykowska-Szuber, L.; Eder, P.; Stawczyk-Eder, K.; Klimczak, K.; Linke, K.; Horst-Sikorska, W. The importance of vitamin D in the pathology of bone metabolism in inflammatory bowel diseases. Arch. Med. Sci. 2015, 11, 1028–1032. [Google Scholar] [CrossRef]
- Ogeyingbo, O.D.; Ahmed, R.; Gyawali, M.; Venkatesan, N.; Bhandari, R.; Botleroo, R.A.; Kareem, R.; Elshaikh, A.O. The Relationship Between Vitamin D and Asthma Exacerbation. Cureus 2021, 13, e17279. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef]
- Sacerdote, A.; Dave, P.; Lokshin, V.; Bahtiyar, G. Type 2 Diabetes Mellitus, Insulin Resistance, and Vitamin D. Curr. Diab. Rep. 2019, 19, 101. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J. Vitamin D: Emerging roles in infection and immunity. Expert Rev. Anti-Infect. Ther. 2010, 8, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Golzarand, M.; Hollis, B.W.; Mirmiran, P.; Wagner, C.L.; Shab-Bidar, S. Vitamin D supplementation and body fat mass: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 2015, 10, e0118108. [Google Scholar] [CrossRef]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC-Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef]
- Ruston, D. The National Diet and Nutrition Survey: Adults Aged 19 to 64 Years: Nutritional Status (Anthropometry and Blood Analytes), Blood Pressure and Physical Activity; Stationery Office: London, UK, 2004. [Google Scholar]
- Houghton, L.A.; Vieth, R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am. J. Clin. Nutr. 2006, 84, 694–697. [Google Scholar] [CrossRef]
- Tripkovic, L.; Wilson, L.R.; Hart, K.; Johnsen, S.; de Lusignan, S.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Elliott, R.; et al. Daily supplementation with 15 mug vitamin D2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: A 12-wk randomized, placebo-controlled food-fortification trial. Am. J. Clin. Nutr. 2017, 106, 481–490. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Recent insights into vitamin D, adipocyte, and adipose tissue biology. Obes. Rev. 2022, 23, e13453. [Google Scholar] [CrossRef] [PubMed]
- Luijten, I.H.N.; Feldmann, H.M.; von Essen, G.; Cannon, B.; Nedergaard, J. In the absence of UCP1-mediated diet-induced thermogenesis, obesity is augmented even in the obesity-resistant 129S mouse strain. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E729–E740. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef]
- Ribeiro, V.R.; Romao-Veiga, M.; Nunes, P.R.; de Oliveira, L.R.C.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Immunomodulatory effect of vitamin D on the STATs and transcription factors of CD4(+) T cell subsets in pregnant women with preeclampsia. Clin. Immunol. 2022, 234, 108917. [Google Scholar] [CrossRef]
- Huang, D.; Guo, Y.; Li, X.; Pan, M.; Liu, J.; Zhang, W.; Mai, K. Vitamin D3/VDR inhibits inflammation through NF-kappaB pathway accompanied by resisting apoptosis and inducing autophagy in abalone Haliotis discus hannai. Cell Biol. Toxicol. 2021. [Google Scholar] [CrossRef]
- Dimitrov, V.; Barbier, C.; Ismailova, A.; Wang, Y.; Dmowski, K.; Salehi-Tabar, R.; Memari, B.; Groulx-Boivin, E.; White, J.H. Vitamin D-regulated Gene Expression Profiles: Species-specificity and Cell-specific Effects on Metabolism and Immunity. Endocrinology 2021, 162, bqaa218. [Google Scholar] [CrossRef]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Vitamin D and Obesity/Adiposity-A Brief Overview of Recent Studies. Nutrients 2022, 14, 2049. [Google Scholar] [CrossRef]
- Wen, J.; Hong, Q.; Wang, X.; Zhu, L.; Wu, T.; Xu, P.; Fu, Z.; You, L.; Wang, X.; Ji, C.; et al. The effect of maternal vitamin D deficiency during pregnancy on body fat and adipogenesis in rat offspring. Sci. Rep. 2018, 8, 365. [Google Scholar] [CrossRef]
- Xue, J.; Gharaibeh, R.Z.; Pietryk, E.W.; Brouwer, C.; Tarantino, L.M.; Valdar, W.; Ideraabdullah, F.Y. Impact of vitamin D depletion during development on mouse sperm DNA methylation. Epigenetics 2018, 13, 959–974. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, L.; Wei, Z.; Liu, B.; Liu, X.; Yu, X. Vitamin D deficiency during pregnancy affects the function of Th1/Th2 cells and methylation of IFN-gamma gene in offspring rats. Immunol. Lett. 2019, 212, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.; Wu, J.; Zhong, Y.; Shen, X.; Petrov, B.; Cai, W. Maternal Vitamin D Deficiency Increases Intestinal Permeability and Programs Wnt/beta-Catenin Pathway in BALB/C Mice. JPEN J. Parenter. Enter. Nutr. 2021, 45, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.F. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D. Epigenetics 2018, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Molecular endocrinology of vitamin D on the epigenome level. Mol. Cell. Endocrinol. 2017, 453, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Fetahu, I.S.; Hobaus, J.; Kallay, E. Vitamin D and the epigenome. Front. Physiol. 2014, 5, 164. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef]
- Silvagno, F.; De Vivo, E.; Attanasio, A.; Gallo, V.; Mazzucco, G.; Pescarmona, G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS ONE 2010, 5, e8670. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Guillet, C.; Vaes, A.M.; Brouwer-Brolsma, E.M.; Rocher, C.; Giraudet, C.; Patrac, V.; Meugnier, E.; Montaurier, C.; et al. Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: Role in sarcopenia. Commun. Biol. 2022, 5, 1288. [Google Scholar] [CrossRef]
- Silvagno, F.; Pescarmona, G. Spotlight on vitamin D receptor, lipid metabolism and mitochondria: Some preliminary emerging issues. Mol. Cell. Endocrinol. 2017, 450, 24–31. [Google Scholar] [CrossRef]
- Ponsonby, A.L.; Lucas, R.M.; Lewis, S.; Halliday, J. Vitamin D status during pregnancy and aspects of offspring health. Nutrients 2010, 2, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of Maternal Vitamin D Deficiency with Pregnancy and Neonatal Complications in Developing Countries: A Systematic Review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef]
- Prentice, A. Vitamin D deficiency: A global perspective. Nutr. Rev. 2008, 66, S153–S164. [Google Scholar] [CrossRef]
- Yun, C.; Chen, J.; He, Y.; Mao, D.; Wang, R.; Zhang, Y.; Yang, C.; Piao, J.; Yang, X. Vitamin D deficiency prevalence and risk factors among pregnant Chinese women. Public Health Nutr. 2017, 20, 1746–1754. [Google Scholar] [CrossRef]
- Wong, R.S.; Tung, K.T.S.; Mak, R.T.W.; Leung, W.C.; Yam, J.C.; Chua, G.T.; Fung, G.P.G.; Ho, M.H.K.; Wong, I.C.K.; Ip, P. Vitamin D concentrations during pregnancy and in cord blood: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 2225–2236. [Google Scholar] [CrossRef]
- Lu, M.; Hollis, B.W.; Carey, V.J.; Laranjo, N.; Singh, R.J.; Weiss, S.T.; Litonjua, A.A. Determinants and Measurement of Neonatal Vitamin D: Overestimation of 25(OH)D in Cord Blood Using CLIA Assay Technology. J. Clin. Endocrinol. Metab. 2020, 105, e1085–e1092. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Antonopoulou, V.; Goulis, D.G.; Annweiler, C.; Pilz, S.; Bili, H.; Naughton, D.P.; Shah, I.; Harizopoulou, V.; et al. Characterizing neonatal vitamin D deficiency in the modern era: A maternal-neonatal birth cohort from Southern Europe. J. Steroid Biochem. Mol. Biol. 2020, 198, 105555. [Google Scholar] [CrossRef]
- Wang, C.; Gao, J.; Liu, N.; Yu, S.; Qiu, L.; Wang, D. Maternal factors associated with neonatal vitamin D deficiency. J. Pediatr. Endocrinol. Metab. 2019, 32, 167–172. [Google Scholar] [CrossRef]
- Weinert, L.S.; Silveiro, S.P. Maternal-fetal impact of vitamin D deficiency: A critical review. Matern. Child Health J. 2015, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Pereira, M.; Solé, D. Vitamin D deficiency in pregnancy and its impact on the fetus, the newborn and in childhood. Rev. Paul. Pediatr. 2015, 33, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Tous, M.; Villalobos, M.; Iglesias, L.; Fernández-Barrés, S.; Arija, V. Vitamin D status during pregnancy and offspring outcomes: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2020, 74, 36–53. [Google Scholar] [CrossRef]
- O’Loan, J.; Eyles, D.W.; Kesby, J.; Ko, P.; McGrath, J.J.; Burne, T.H. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology 2007, 32, 227–234. [Google Scholar] [CrossRef]
- Leffelaar, E.R.; Vrijkotte, T.G.; van Eijsden, M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: Results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br. J. Nutr. 2010, 104, 108–117. [Google Scholar] [CrossRef]
- Gale, C.R.; Robinson, S.M.; Harvey, N.C.; Javaid, M.K.; Jiang, B.; Martyn, C.N.; Godfrey, K.M.; Cooper, C. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 2008, 62, 68–77. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status during Pregnancy on Mother and her Developing Child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L.; Howard, C.R.; Ebeling, M.; Shary, J.R.; Smith, P.G.; Taylor, S.N.; Morella, K.; Lawrence, R.A.; Hulsey, T.C. Maternal Versus Infant Vitamin D Supplementation during Lactation: A Randomized Controlled Trial. Pediatrics 2015, 136, 625–634. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R.; Holick, M.F. Chapter 57B—The IOM—Endocrine Society Controversy on Recommended Vitamin D Targets: In Support of the Endocrine Society Position. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1091–1107. [Google Scholar]
- National Institute for Health and Care Excellence (Great Britain). Vitamin D: Supplement Use in Specific Population Groups; National Institute for Health and Care Excellence (NICE): London, UK, 2014. [Google Scholar]
- ACOG Committee Opinion. Vitamin D: Screening and supplementation during pregnancy. Obstet. Gynecol. 2011, 118, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Government of South Asutralia. Vitamin D Status in Pregnancy—SA Perinatal Practice Guidelines; Government of South Asutralia: Adelaide, Australia, 2021.
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Niño, J.F.; et al. Maternal 25(OH)D concentrations ≥ 40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef] [PubMed]
- Merewood, A.; Mehta, S.D.; Chen, T.C.; Bauchner, H.; Holick, M.F. Association between vitamin D deficiency and primary cesarean section. J. Clin. Endocrinol. Metab. 2009, 94, 940–945. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements and supplementation during pregnancy. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2011, 26, 2341–2357. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Gaillard, R.; Durmus, B.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Jaddoe, V.W. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 2013, 21, 1046–1055. [Google Scholar] [CrossRef]
- Alhomaid, R.M.; Mulhern, M.S.; Strain, J.; Laird, E.; Healy, M.; Parker, M.J.; McCann, M.T. Maternal obesity and baseline vitamin D insufficiency alter the response to vitamin D supplementation: A double-blind, randomized trial in pregnant women. Am. J. Clin. Nutr. 2021, 114, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Francis, E.C.; Hinkle, S.N.; Song, Y.; Rawal, S.; Donnelly, S.R.; Zhu, Y.; Chen, L.; Zhang, C. Longitudinal Maternal Vitamin D Status during Pregnancy Is Associated with Neonatal Anthropometric Measures. Nutrients 2018, 10, 1631. [Google Scholar] [CrossRef] [PubMed]
- Tint, M.T.; Chong, M.F.; Aris, I.M.; Godfrey, K.M.; Quah, P.L.; Kapur, J.; Saw, S.M.; Gluckman, P.D.; Rajadurai, V.S.; Yap, F.; et al. Association between maternal mid-gestation vitamin D status and neonatal abdominal adiposity. Int. J. Obes. 2018, 42, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Daraki, V.; Roumeliotaki, T.; Chalkiadaki, G.; Katrinaki, M.; Karachaliou, M.; Leventakou, V.; Vafeiadi, M.; Sarri, K.; Vassilaki, M.; Papavasiliou, S.; et al. Low maternal vitamin D status in pregnancy increases the risk of childhood obesity. Pediatr. Obes. 2018, 13, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Miliku, K.; Felix, J.F.; Voortman, T.; Tiemeier, H.; Eyles, D.W.; Burne, T.H.; McGrath, J.J.; Jaddoe, V.W.V. Associations of maternal and fetal vitamin D status with childhood body composition and cardiovascular risk factors. Matern. Child Nutr. 2019, 15, e12672. [Google Scholar] [CrossRef]
- Crozier, S.R.; Harvey, N.C.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Robinson, S.M. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: Findings from the Southampton Women’s Survey. Am. J. Clin. Nutr. 2012, 96, 57–63. [Google Scholar] [CrossRef]
- Morales, E.; Rodriguez, A.; Valvi, D.; Iñiguez, C.; Esplugues, A.; Vioque, J.; Marina, L.S.; Jiménez, A.; Espada, M.; Dehli, C.R.; et al. Deficit of vitamin D in pregnancy and growth and overweight in the offspring. Int. J. Obes. 2015, 39, 61–68. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, J.; Zhang, Y.; Teng, H.; Pei, J.; Zhang, C.; Guo, B.; Yin, J. Association between maternal vitamin D status with pregnancy outcomes and offspring growth in a population of Wuxi, China. Asia Pac. J. Clin. Nutr. 2021, 30, 464–476. [Google Scholar] [CrossRef]
- Zhang, H.; Chu, X.; Huang, Y.; Li, G.; Wang, Y.; Li, Y.; Sun, C. Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Iκbα methylation. Diabetologia 2014, 57, 2165–2172. [Google Scholar] [CrossRef]
- Shin, J.S.; Choi, M.Y.; Longtine, M.S.; Nelson, D.M. Vitamin D effects on pregnancy and the placenta. Placenta 2010, 31, 1027–1034. [Google Scholar] [CrossRef]
- Zhuang, W.; Lv, J.; Liang, Q.; Chen, W.; Zhang, S.; Sun, X. Adverse effects of gestational diabetes-related risk factors on pregnancy outcomes and intervention measures. Exp. Ther. Med. 2020, 20, 3361–3367. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; She, G.T.; Sun, L.Z.; Lu, H.; Wang, Y.P.; Miao, J.; Liu, K.Z.; Sun, C.F.; Ju, H.H. Correlation of serum vitamin D, adipose tissue vitamin D receptor, and peroxisome proliferator-activated receptor gamma in women with gestational diabetes mellitus. Chin. Med. J. 2019, 132, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Asadi, M.; Rahmani, S.; Akhavan Zanjani, M.; Sadeghi, O.; Hosseini, S.A.; Zare Javid, A. Circulating vitamin D and the risk of gestational diabetes: A systematic review and dose-response meta-analysis. Endocrine 2020, 70, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Abbasi, F.; Mousavi, S.M.; Esmaillzadeh, A. Maternal vitamin D status and risk of gestational diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Clin. Nutr. 2021, 40, 2576–2586. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, Y.; Xue, H.; Xiong, J.; Cheng, G. Vitamin D and gestational diabetes mellitus: A systematic review based on data free of Hawthorne effect. BJOG 2018, 125, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Aguero-Domenech, N.; Jover, S.; Sarrion, A.; Baranda, J.; Quesada-Rico, J.A.; Pereira-Exposito, A.; Gil-Guillen, V.; Cortes-Castell, E.; Garcia-Teruel, M.J. Vitamin D Deficiency and Gestational Diabetes Mellitus in Relation to Body Mass Index. Nutrients 2021, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Mo, M.; Xin, X.; Jiang, W.; Wu, J.; Huang, M.; Wang, S.; Muyiduli, X.; Si, S.; Shen, Y.; et al. The interaction between prepregnancy BMI and gestational vitamin D deficiency on the risk of gestational diabetes mellitus subtypes with elevated fasting blood glucose. Clin. Nutr. 2020, 39, 2265–2273. [Google Scholar] [CrossRef]
- Yue, C.Y.; Ying, C.M. Sufficience serum vitamin D before 20 weeks of pregnancy reduces the risk of gestational diabetes mellitus. Nutr. Metab. 2020, 17, 89. [Google Scholar] [CrossRef]
- Gao, M.; Cao, S.; Li, N.; Liu, J.; Lyu, Y.; Li, J.; Yang, X. Risks of overweight in the offspring of women with gestational diabetes at different developmental stages: A meta-analysis with more than half a million offspring. Obes. Rev. 2022, 23, e13395. [Google Scholar] [CrossRef]
- Sellers, E.A.; Dean, H.J.; Shafer, L.A.; Martens, P.J.; Phillips-Beck, W.; Heaman, M.; Prior, H.J.; Dart, A.B.; McGavock, J.; Morris, M.; et al. Exposure to Gestational Diabetes Mellitus: Impact on the Development of Early-Onset Type 2 Diabetes in Canadian First Nations and Non-First Nations Offspring. Diabetes Care 2016, 39, 2240–2246. [Google Scholar] [CrossRef]
- Yu, Y.; Arah, O.A.; Liew, Z.; Cnattingius, S.; Olsen, J.; Sorensen, H.T.; Qin, G.; Li, J. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: Population based cohort study with 40 years of follow-up. BMJ 2019, 367, l6398. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wang, Y.; Yu, X.; Liu, Y.; Zhang, Z.J. Maternal diabetes and risk of childhood malignancies in the offspring: A systematic review and meta-analysis of observational studies. Acta Diabetol. 2021, 58, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Hrudey, E.J.; Reynolds, R.M.; Oostvogels, A.J.; Brouwer, I.A.; Vrijkotte, T.G. The Association between Maternal 25-Hydroxyvitamin D Concentration during Gestation and Early Childhood Cardio-metabolic Outcomes: Is There Interaction with Pre-Pregnancy BMI? PLoS ONE 2015, 10, e0133313. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Veena, S.R.; Winder, N.R.; Hill, J.C.; Noonan, K.; Boucher, B.J.; Karat, S.C.; Fall, C.H. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: The Mysore Parthenon Study. Am. J. Clin. Nutr. 2011, 93, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Sucksdorff, M.; Brown, A.S.; Chudal, R.; Surcel, H.M.; Hinkka-Yli-Salomaki, S.; Cheslack-Postava, K.; Gyllenberg, D.; Sourander, A. Maternal Vitamin D Levels and the Risk of Offspring Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 142–151.e2. [Google Scholar] [CrossRef] [PubMed]
- Albinana, C.; Boelt, S.G.; Cohen, A.S.; Zhu, Z.; Musliner, K.L.; Vilhjalmsson, B.J.; McGrath, J.J. Developmental exposure to vitamin D deficiency and subsequent risk of schizophrenia. Schizophr. Res. 2021, 247, 26–32. [Google Scholar] [CrossRef]
- Arrhenius, B.; Upadhyaya, S.; Hinkka-Yli-Salomaki, S.; Brown, A.S.; Cheslack-Postava, K.; Ohman, H.; Sourander, A. Prenatal Vitamin D Levels in Maternal Sera and Offspring Specific Learning Disorders. Nutrients 2021, 13, 3321. [Google Scholar] [CrossRef]
- Shrestha, D.; Workalemahu, T.; Tekola-Ayele, F. Maternal dyslipidemia during early pregnancy and epigenetic ageing of the placenta. Epigenetics 2019, 14, 1030–1039. [Google Scholar] [CrossRef]
- Al-Ajlan, A.; Krishnaswamy, S.; Alokail, M.S.; Aljohani, N.J.; Al-Serehi, A.; Sheshah, E.; Alshingetti, N.M.; Fouda, M.; Turkistani, I.Z.; Al-Daghri, N.M. Vitamin D deficiency and dyslipidemia in early pregnancy. BMC Pregnancy Childbirth 2015, 15, 314. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr. Pharm. Biotechnol. 2014, 15, 24–31. [Google Scholar] [CrossRef]
- Herrera, E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 2002, 19, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Disturbances in lipid metabolism in diabetic pregnancy—Are these the cause of the problem? Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Mahindra, M.P.; Sampurna, M.T.A.; Mapindra, M.P.; Sutowo Putri, A.M. Maternal lipid levels in pregnant women without complications in developing risk of large for gestational age newborns: A study of meta-analysis. F1000Research 2020, 9, 1213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Moore, D.; Subramanian, A.; Cheng, K.K.; Toulis, K.A.; Qiu, X.; Saravanan, P.; Price, M.J.; Nirantharakumar, K. Gestational dyslipidaemia and adverse birthweight outcomes: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1256–1268. [Google Scholar] [CrossRef]
- Contreras-Duarte, S.; Carvajal, L.; Fuenzalida, B.; Cantin, C.; Sobrevia, L.; Leiva, A. Maternal Dyslipidaemia in Pregnancy with Gestational Diabetes Mellitus: Possible Impact on Foetoplacental Vascular Function and Lipoproteins in the Neonatal Circulation. Curr. Vasc. Pharmacol. 2019, 17, 52–71. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhang, H.P.; Yang, J.; Huang, Z.Q.; Xu, H.X.; Jin, J.; Xu, K.; Tong, Y.; Dong, Q.Q.; Zheng, J.Q. The relationship between maternal vitamin D deficiency and glycolipid metabolism and adverse pregnancy outcome. Clin. Endocrinol. 2020, 93, 713–720. [Google Scholar] [CrossRef]
- Kollmann, M.; Obermayer-Pietsch, B.; Lerchbaum, E.; Feigl, S.; Hochstatter, R.; Pregartner, G.; Trummer, C.; Klaritsch, P. Vitamin D Concentrations at Term Do Not Differ in Newborns and Their Mothers with and without Polycystic Ovary Syndrome. J. Clin. Med. 2021, 10, 537. [Google Scholar] [CrossRef]
- Lin, M.W.; Wu, M.H. The role of vitamin D in polycystic ovary syndrome. Indian J. Med. Res. 2015, 142, 238–240. [Google Scholar] [CrossRef]
- Gunning, M.N.; Sir Petermann, T.; Crisosto, N.; van Rijn, B.B.; de Wilde, M.A.; Christ, J.P.; Uiterwaal, C.; de Jager, W.; Eijkemans, M.J.C.; Kunselman, A.R.; et al. Cardiometabolic health in offspring of women with PCOS compared to healthy controls: A systematic review and individual participant data meta-analysis. Hum. Reprod. Update 2020, 26, 103–117. [Google Scholar] [CrossRef]
- Grieger, J.A.; Bianco-Miotto, T.; Grzeskowiak, L.E.; Leemaqz, S.Y.; Poston, L.; McCowan, L.M.; Kenny, L.C.; Myers, J.E.; Walker, J.J.; Dekker, G.A.; et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med. 2018, 15, e1002710. [Google Scholar] [CrossRef]
- Park, J.E.; Pichiah, P.B.T.; Cha, Y.S. Vitamin D and Metabolic Diseases: Growing Roles of Vitamin D. J. Obes. Metab. Syndr. 2018, 27, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodriguez, L.; Costela-Ruiz, V.J.; Garcia-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, X.; Zheng, B.; Zheng, Z.; Zhang, H.; Zheng, J.; Sun, C.; Chen, H.; Yang, J.; Wang, Z.; et al. Maternal 25-Hydroxyvitamin D Deficiency Promoted Metabolic Syndrome and Downregulated Nrf2/CBR1 Pathway in Offspring. Front. Pharmacol. 2020, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Shih, H.H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.F.; Ke, L.Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef]
- Dix, C.F.; Barcley, J.L.; Wright, O.R.L. The role of vitamin D in adipogenesis. Nutr. Rev. 2018, 76, 47–59. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Bassatne, A.; Jafari, A.; Kassem, M.; Mantzoros, C.; Rahme, M.; El-Hajj Fuleihan, G. Delta-like 1 (DLK1) is a possible mediator of vitamin D effects on bone and energy metabolism. Bone 2020, 138, 115510. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.H.; Kang, J.; Yang, H.; Jeong, E.J.; Kim, H.P.; Kim, Y.C.; Sung, S.H. Salicortin-derivatives from Salix pseudo-lasiogyne twigs inhibit adipogenesis in 3T3-L1 cells via modulation of C/EBPalpha and SREBP1c dependent pathway. Molecules 2013, 18, 10484–10496. [Google Scholar] [CrossRef]
- Seong, S.; Kim, J.H.; Kim, K.; Kim, I.; Koh, J.T.; Kim, N. Alternative regulatory mechanism for the maintenance of bone homeostasis via STAT5-mediated regulation of the differentiation of BMSCs into adipocytes. Exp. Mol. Med. 2021, 53, 848–863. [Google Scholar] [CrossRef]
- Huang, X.Y.; Chen, J.X.; Ren, Y.; Fan, L.C.; Xiang, W.; He, X.J. Exosomal miR-122 promotes adipogenesis and aggravates obesity through the VDR/SREBF1 axis. Obesity 2022, 30, 666–679. [Google Scholar] [CrossRef]
- Nimitphong, H.; Holick, M.F.; Fried, S.K.; Lee, M.J. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 2012, 7, e52171. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.M.; Tzameli, I.; Astapova, I.; Lam, F.S.; Flier, J.S.; Hollenberg, A.N. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J. Biol. Chem. 2006, 281, 11205–11213. [Google Scholar] [CrossRef] [PubMed]
- Belenchia, A.M.; Jones, K.L.; Will, M.; Beversdorf, D.Q.; Vieira-Potter, V.; Rosenfeld, C.S.; Peterson, C.A. Maternal vitamin D deficiency during pregnancy affects expression of adipogenic-regulating genes peroxisome proliferator-activated receptor gamma (PPARgamma) and vitamin D receptor (VDR) in lean male mice offspring. Eur. J. Nutr. 2018, 57, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Belenchia, A.M.; Johnson, S.A.; Ellersieck, M.R.; Rosenfeld, C.S.; Peterson, C.A. In utero vitamin D deficiency predisposes offspring to long-term adverse adipose tissue effects. J. Endocrinol. 2017, 234, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G., Jr.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. 2010, 21, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Patriota, P.; Rezzi, S.; Guessous, I.; Marques-Vidal, P. Association between anthropometric markers of adiposity, adipokines and vitamin D levels. Sci. Rep. 2022, 12, 15435. [Google Scholar] [CrossRef]
- Khwanchuea, R.; Punsawad, C. Associations Between Body Composition, Leptin, and Vitamin D Varied by the Body Fat Percentage in Adolescents. Front. Endocrinol. 2022, 13, 876231. [Google Scholar] [CrossRef]
- Guo, L.; Miao, Z.; Ma, H.; Melnychuk, S. Effects of maternal vitamin D3 during pregnancy on FASN and LIPE mRNA expression in offspring pigs. J. Agric. Sci. 2020, 158, 128–135. [Google Scholar] [CrossRef]
- Harreiter, J.; Mendoza, L.C.; Simmons, D.; Desoye, G.; Devlieger, R.; Galjaard, S.; Damm, P.; Mathiesen, E.R.; Jensen, D.M.; Andersen, L.L.T.; et al. Vitamin D3 Supplementation in Overweight/Obese Pregnant Women: No Effects on the Maternal or Fetal Lipid Profile and Body Fat Distribution-A Secondary Analysis of the Multicentric, Randomized, Controlled Vitamin D and Lifestyle for Gestational Diabetes Prevention Trial (DALI). Nutrients 2022, 14, 3781. [Google Scholar] [CrossRef]
- Mousa, A.; Naderpoor, N.; Wilson, K.; Plebanski, M.; de Courten, M.P.J.; Scragg, R.; de Courten, B. Vitamin D supplementation increases adipokine concentrations in overweight or obese adults. Eur. J. Nutr. 2020, 59, 195–204. [Google Scholar] [CrossRef]
- Eglit, T.; Ringmets, I.; Lember, M. Obesity, high-molecular-weight (HMW) adiponectin, and metabolic risk factors: Prevalence and gender-specific associations in Estonia. PLoS ONE 2013, 8, e73273. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Abell, S.K.; Shorakae, S.; Harrison, C.L.; Naderpoor, N.; Hiam, D.; Moreno-Asso, A.; Stepto, N.K.; Teede, H.J.; de Courten, B. Relationship between vitamin D and gestational diabetes in overweight or obese pregnant women may be mediated by adiponectin. Mol. Nutr. Food Res. 2017, 61, 488. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.A.; Ceciliano, T.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Transgenerational effects on the liver and pancreas resulting from maternal vitamin D restriction in mice. J. Nutr. Sci. Vitaminol. 2013, 59, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Jangale, N.M.; Harsulkar, A.M.; Gokhale, M.K.; Joshi, B.N. Chronic maternal calcium and 25-hydroxyvitamin D deficiency in Wistar rats programs abnormal hepatic gene expression leading to hepatic steatosis in female offspring. J. Nutr. Biochem. 2017, 43, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Li, P.; Li, P.; Liu, Y.; Liu, W.; Zha, L.; Chen, X.; Zheng, R.; Qi, K.; Zhang, Y. Maternal vitamin D deficiency increases the risk of obesity in male offspring mice by affecting the immune response. Nutrition 2021, 87–88, 111191. [Google Scholar] [CrossRef]
- Mandell, E.; Ryan, S.; Seedorf, G.J.; Gonzalez, T.; Abman, S.H.; Fleet, J.C. Maternal vitamin D deficiency induces transcriptomic changes in newborn rat lungs. J. Steroid Biochem. Mol. Biol. 2020, 199, 105613. [Google Scholar] [CrossRef]
- Milliken, S.; Allen, R.M.; Lamont, R.F. The role of antimicrobial treatment during pregnancy on the neonatal gut microbiome and the development of atopy, asthma, allergy and obesity in childhood. Expert Opin. Drug Saf. 2019, 18, 173–185. [Google Scholar] [CrossRef]
- Niu, J.; Xu, L.; Qian, Y.; Sun, Z.; Yu, D.; Huang, J.; Zhou, X.; Wang, Y.; Zhang, T.; Ren, R.; et al. Evolution of the Gut Microbiome in Early Childhood: A Cross-Sectional Study of Chinese Children. Front. Microbiol. 2020, 11, 439. [Google Scholar] [CrossRef]
- Villa, C.R.; Taibi, A.; Chen, J.; Ward, W.E.; Comelli, E.M. Colonic Bacteroides are positively associated with trabecular bone structure and programmed by maternal vitamin D in male but not female offspring in an obesogenic environment. Int. J. Obes. 2018, 42, 696–703. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, Q.; Zhao, J.; Yao, D.; Wang, T.; Shen, Q.; Li, W.; Li, B.; Ding, X.; Liu, Z. Prenatal inflammation causes obesity and abnormal lipid metabolism via impaired energy expenditure in male offspring. Nutr. Metab. 2022, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.R.; Chen, J.; Wen, B.; Sacco, S.M.; Taibi, A.; Ward, W.E.; Comelli, E.M. Maternal vitamin D beneficially programs metabolic, gut and bone health of mouse male offspring in an obesogenic environment. Int. J. Obes. 2016, 40, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.R.; Chen, J.; Wen, B.; Sacco, S.M.; Taibi, A.; Ward, W.E.; Comelli, E.M. Maternal Dietary Vitamin D Does Not Program Systemic Inflammation and Bone Health in Adult Female Mice Fed an Obesogenic Diet. Nutrients 2016, 8, 675. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torres, I.; Castrejon-Tellez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Hu, C.; Yan, Y.; Ji, F.; Zhou, H. Maternal Obesity Increases Oxidative Stress in Placenta and It Is Associated with Intestinal Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 671347. [Google Scholar] [CrossRef]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Qasemi, R.; Ghavamzadeh, S.; Faghfouri, A.H.; Valizadeh, N.; Mohammadi, A.; Sayyadi, H. The effect of vitamin D supplementation on flow-mediated dilatation, oxidized LDL and intracellular adhesion molecule 1 on type 2 diabetic patients with hypertension: A randomized, placebo-controlled, double-blind trial. Diabetes Metab. Syndr. 2021, 15, 102200. [Google Scholar] [CrossRef]
- Gallo, L.A.; Barrett, H.L.; Dekker Nitert, M. Review: Placental transport and metabolism of energy substrates in maternal obesity and diabetes. Placenta 2017, 54, 59–67. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Blanco, I.; Agodi, A. Effects of Vitamin D Supplementation during Pregnancy on Birth Size: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 442. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, H.; Xie, H.; Zheng, J.; Lin, M.; Chen, J.; Tong, Y.; Jin, J.; Xu, K.; Yang, J.; et al. Maternal, umbilical arterial metabolic levels and placental Nrf2/CBR1 expression in pregnancies with and without 25-hydroxyvitamin D deficiency. Gynecol. Endocrinol. 2021, 37, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, B.A.; Sellers, E.A. Maternal health issues and cardio-metabolic outcomes in the offspring: A focus on Indigenous populations. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 43–53. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.R.; Reynolds, R.M. The risk of maternal obesity to the long-term health of the offspring. Clin. Endocrinol. 2013, 78, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Ehrenberg, H.M. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef]
- Williams, C.B.; Mackenzie, K.C.; Gahagan, S. The effect of maternal obesity on the offspring. Clin. Obstet. Gynecol. 2014, 57, 508–515. [Google Scholar] [CrossRef]
- Shankar, K.; Harrell, A.; Liu, X.; Gilchrist, J.M.; Ronis, M.J.; Badger, T.M. Maternal obesity at conception programs obesity in the offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R528–R538. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Trandafir, L.M.; Temneanu, O.R. Pre and post-natal risk and determination of factors for child obesity. J. Med. Life 2016, 9, 386–391. [Google Scholar]
| Author [References] | Country/ Region | Subjects | Pre-Pregnancy BMI (kg/m2) | Outcomes |
|---|---|---|---|---|
| Tint et al. [89] | Singapore | 292 | 23.8 ± 5.1 (VD inadequacy group) 22.4 ± 4.6 (VD sufficiency group) | 2 weeks old: birth weight N *; abdominal subcutaneous adipose tissue volume ↑ |
| Daraki et al. [90] | Greek | 532 | 26.1 ± 5.8 (low VD group) 24.5 ± 4.5 (high VD group) | 4 years old: BMI ↑; waist circumference ↑; TL N; TG N; HDL-C N. 6 years old: BMI ↑; waist circumference ↑; fat percentage ↑; TL N; TG N; HDL-C N |
| Miliku et al. [91] | Netherlands | 4903 | 22.7 (18.1 ~ 34.8) | 6 years old: BMI N; fat percentage ↑; lean mass percentage ↓; TL N; TG N |
| Crozier et al. [92] | United Kingdom | 977 | 24.3 (22.2 ~ 27.6) | At birth: fat mass ↓; 4 and 6 years old: fat mass ↑ |
| Morales et al. [93] | Spain | 2223 | 18.5 ~ 25.0 | 1 years old: BMI ↑; 4 years old: BMI N |
| Jiang et al. [94] | China | 329 | 21.2 ± 2.9 (VD sufficiency group) 21.1 ± 3.5 (VD insufficiency group) 21.0 ± 3.2 (VD deficiency group) | At birth: risks of preterm birth, small for gestation age, and low birth weight N; 0 ~ 3 years old: weight N; length N; BMI N |
| Hrudey et al. [109] | Netherlands | 1882 | 24.2 ± 4.9 (VD sufficiency group) 23.6 ± 4.3 (VD insufficiency group) 22.2 ± 2.9 (VD deficiency group) | 5~6 years old: insulin resistance ↑; fat percentage ↑ |
| Krishnaveni et al. [110] | India | 568 | NA | 9.5 years old: insulin resistance ↑; muscle-grip strength N; arm-muscle area N |
| Chen et al. [122] | China | 425 | 22.6 ± 3.2 (VD deficiency group) 22.4 ± 2.8 (control group) | newborn: TG ↑; HDL-C ↓; HOMA-β ↓ |
| Author [References] | Animal Model | Intervention | Phenotypic Changes | Potential Mechanism |
|---|---|---|---|---|
| Wen et al. [42] | Sprague-Dawley rats | maternal VD * deficient diet | 10 weeks: weight ↑; 14 weeks: 24 h heat production ↑, peak blood glucose ↑, adipose tissue volume ↑, blood lipid ↑ | increased proliferation rate and number of lipid droplets for pre-adipocytes; hypermethylation and low expression of Vldlr gene; demethylation and high expression of Hif1α gene |
| Belenchia et al. [139] | C57BL/6J mouse | maternal VD deficient diet | body weight N, adipose pad weight N, adipocyte size N | male offspring: expression of PPAR-γ and VDR ↑ |
| Belenchia et al. [140] | C57BL/6J mouse | maternal VD deficient diet | at weaning: weight ↓; 4 weeks: weight ↑; 19 weeks: perigonadal adipose tissue ↑ | male offspring: adipocyte hypertrophy ↑; expression of PPAR-γ ↑ |
| Guo et al. [144] | pigs | maternal VD deficient diet | fat mass ↑; insulin ↑; leptin ↑ | FASN mRNA level ↑; altered LIPE gene expression in different tissues |
| Nascimento et al. [149] | Swiss Webster mouse | maternal VD deficient diet | body weight ↑; insulin ↑; AUC ↑; islet diameter ↑; liver steatosis | FASN expression ↑ |
| Sharma et al. [150] | Wistar rats | maternal VD deficient diet | VD ↓; TG ↑; liver steatosis | female offspring: PPAR-γ and UCP2 ↓, SREBP-1c, IL-6 and SOD-1 ↑; male offspring: UCP2 and SOD-1 ↓ |
| Zhang et al. [95] | Sprague-Dawley rats | maternal VD deficient diet | 16 weeks: insulin ↑; HOMA-IR ↑ | serum and liver IL-1β, IL-6, IL-8 and TNF-α ↑; hepatic Iκbα mRNA and IκBα protein ↓ |
| Li et al. [152] | C57BL/6J mouse | maternal VD deficient diet | weight ↑; adipose cells ↑; abnormal glucose and lipid metabolisms | serum IL-4, IL-10, interferon-γ and TNF-α ↑; adipose tissue dendritic cells, and CD4(+) and CD8(+) T cells ↑; percentages of M1 macrophages ↑, percentages of M2 macrophages ↓ |
| Villa et al. [156] | C57BL/6J mouse | maternal VD deficient diet | improved bone strength and structure | male offspring: colonic Bacteroides improved; systemic inflammation ↓; |
| Ni et al. [157] | C57BL/6J mouse | injection with 50 μg/kg LPS once | 20 weeks: weight ↑; fat percentage ↑; energy expenditure ↓ | mTOR/PPAR-γ ↑; serum bile acids level ↓, serum unsaturated fatty acids androgens and prostaglandins ↓ |
| Villa et al. [158,159] | C57BL/6J mouse | maternal VD supplementation | fasting glucose ↓; fat mass ↓ in male offspring but not female offspring | intestinal permeability↓; serum LPS ↓ in male offspring but not female offspring |
| Zhang et al. [129] | Sprague-Dawley rats | maternal VD deficient diet | TG ↑; fasting glucose ↑; insulin ↑; HDL-C ↓ | ROS level ↑; Nrf2/CBR1 pathway ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zeng, Y.; Zhang, Q.; Xiao, X. The Role of Maternal Vitamin D Deficiency in Offspring Obesity: A Narrative Review. Nutrients 2023, 15, 533. https://doi.org/10.3390/nu15030533
Wu Y, Zeng Y, Zhang Q, Xiao X. The Role of Maternal Vitamin D Deficiency in Offspring Obesity: A Narrative Review. Nutrients. 2023; 15(3):533. https://doi.org/10.3390/nu15030533
Chicago/Turabian StyleWu, Yifan, Yuan Zeng, Qian Zhang, and Xinhua Xiao. 2023. "The Role of Maternal Vitamin D Deficiency in Offspring Obesity: A Narrative Review" Nutrients 15, no. 3: 533. https://doi.org/10.3390/nu15030533
APA StyleWu, Y., Zeng, Y., Zhang, Q., & Xiao, X. (2023). The Role of Maternal Vitamin D Deficiency in Offspring Obesity: A Narrative Review. Nutrients, 15(3), 533. https://doi.org/10.3390/nu15030533







