Thwarting Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) with Common Bean: Dose- and Sex-Dependent Protection against Hepatic Steatosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Feeding Study
2.2. Diet Formulations
2.3. Histopathology
2.3.1. Fixation
2.3.2. Hematoxylin and Eosin (H&E) Staining
2.3.3. Trichrome Staining
2.3.4. Oil Red O (ORO) Staining
2.4. Quantification of Liver Lipid Content
2.5. RNA Isolation and RNA-Seq Analysis
2.6. Statistical Evaluation
3. Results
3.1. Livers Accumulate Less Lipid upon Increasing Consumption of Bean
3.2. Dietary Bean Visually Reduces Steatosis Severity in the Liver Tissue
3.3. Liver Transcriptomic Signature upon Consumption of Bean Indicates Protection from Hepatotoxicity
3.4. Bean Consumption Maps to Hepatic Fatty Acid Metabolic Pathways and Regulators
3.5. Consumption of Beans Reduces Uptake and De Novo Lipogenesis of Free Fatty Acids and Differentially Affects Transport, Oxidation, and Incorporation into Lipid Droplets
4. Discussion
4.1. Dietary Bean-Induced Liver Phenotype
4.2. Triacylglycerol as the Main Chemical Form of Hepatic Lipid
4.3. Contribution of Extracellular Free Fatty Acids Uptake
4.4. Hepatocellular Oxidation of Fatty Acids
4.5. Bean Effects Shared by Both Sex Cohorts
4.6. Differential Sex-Dependent Effects of Common Bean
4.7. Translational Considerations
4.8. Limitations and Strengths
5. Conclusions
- ❖
- females exhibited lower hepatic lipid content at the 35% dose, whereas males achieved a similar reduction with the 70% dose of bean;
- ❖
- common bean dose-responsively inhibited de novo lipogenesis in both sex cohorts;
- ❖
- additionally, females showed greater capacity for FA oxidation, whereas males significantly reduced uptake of extracellular free FAs;
- ❖
- bean consumption significantly affected genes involved in TAGs metabolism consistent with enhanced hydrolysis and diminished biosynthesis;
- ❖
- bean-induced DEGs patterns indicated that reduced PPARα signaling and activated by dietary carbohydrates MLXIPL signaling may be at the core of lower susceptibility of male mice to dietary bean protection from hepatic steatosis;
- ❖
- hepatic CYPs-regulated FA oxidation, oxidative stress, cholesterol metabolism, bile acid metabolism, and inflammation appear to also be significantly affected by increasing doses of beans in the diet and will be assessed in further communications.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic syndrome pathophysiology and predisposing factors. Int. J. Sport. Med. 2021, 42, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Fedacko, J.; Takahashi, T.; Singh, R.B.; Pella, D.; Chibisov, S.; Hristova, K.; Pella, D.; Elkilany, G.N.; Juneja, L.R.; Behl, S.; et al. Chapter 1—Western diets and risk of non-communicable diseases. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 3–21. [Google Scholar]
- Akter, S.; Akhter, H.; Chaudhury, H.S.; Rahman, M.H.; Gorski, A.; Hasan, M.N.; Shin, Y.; Rahman, M.A.; Nguyen, M.N.; Choi, T.G.; et al. Dietary carbohydrates: Pathogenesis and potential therapeutic targets to obesity-associated metabolic syndrome. BioFactors 2022, 48, 1036–1059. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A.; et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Xian, Y.X.; Weng, J.P.; Xu, F. MAFLD vs. NAFLD: Shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy. Chin. Med. J. 2020, 134, 8–19. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Lazarus, J.V.; Mark, H.E.; Villota-Rivas, M.; Palayew, A.; Carrieri, P.; Colombo, M.; Ekstedt, M.; Esmat, G.; George, J.; Marchesini, G.; et al. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J. Hepatol. 2022, 76, 771–780. [Google Scholar] [CrossRef]
- Cholankeril, G.; Wong, R.J.; Hu, M.; Perumpail, R.B.; Yoo, E.R.; Puri, P.; Younossi, Z.M.; Harrison, S.A.; Ahmed, A. Liver transplantation for nonalcoholic steatohepatitis in the US: Temporal trends and outcomes. Dig. Dis. Sci. 2017, 62, 2915–2922. [Google Scholar] [CrossRef]
- Goldberg, D.; Ditah, I.C.; Saeian, K.; Lalehzari, M.; Aronsohn, A.; Gorospe, E.C.; Charlton, M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients with Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology 2017, 152, 1090–1099.e1. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Alalwani, J.; Eljazzar, S.; Basil, M.; Tayyem, R. The impact of health status, diet and lifestyle on non-alcoholic fatty liver disease: Narrative review. Clin. Obes. 2022, 12, e12525. [Google Scholar] [CrossRef] [PubMed]
- Didinger, C.; Thompson, H.J. Defining nutritional and functional niches of legumes: A Call for clarity to distinguish a future role for pulses in the Dietary Guidelines for Americans. Nutrients 2021, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- Didinger, C.; Thompson, H. Motivating pulse-centric eating patterns to benefit human and environmental well-being. Nutrients 2020, 12, 3500. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Blanco Mejia, S.; Kendall, C.W.C.; Sievenpiper, J.L. Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Ann. N. Y. Acad. Sci. 2017, 1392, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 85–96. [Google Scholar] [CrossRef]
- Kazemi, M.; Buddemeyer, S.; Fassett, C.M.; Gans, W.M.; Johnston, K.M.; Lungu, E.; Savelle, R.L.; Tolani, P.N.; Dahl, W.J. Pulses and Prevention and Management of Chronic Disease. In Health Benefits of Pulses; Dahl, W.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 55–72. [Google Scholar]
- Lutsiv, T.; Weir, T.L.; McGinley, J.N.; Neil, E.S.; Wei, Y.; Thompson, H.J. Compositional Changes of the High-Fat Diet-Induced Gut Microbiota upon Consumption of Common Pulses. Nutrients 2021, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.N.; Fitzgerald, V.K.; Neil, E.S.; Omerigic, H.M.; Heuberger, A.L.; Weir, T.L.; McGee, R.; Vandemark, G.; Thompson, H.J. Pulse Crop Effects on Gut Microbial Populations, Intestinal Function, and Adiposity in a Mouse Model of Diet-Induced Obesity. Nutrients 2020, 12, 593. [Google Scholar] [CrossRef]
- Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Brick, M.A. Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism. Nutrients 2017, 9, 998. [Google Scholar] [CrossRef]
- Neil, E.S.; McGinley, J.N.; Fitzgerald, V.K.; Lauck, C.A.; Tabke, J.A.; Streeter-McDonald, M.R.; Yao, L.; Broeckling, C.D.; Weir, T.L.; Foster, M.T.; et al. White Kidney Bean (Phaseolus vulgaris L.) Consumption Reduces Fat Accumulation in a Polygenic Mouse Model of Obesity. Nutrients 2019, 11, 2780. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, Z.; McGinley, J.N.; El, B.K.; Manni, A.; Thompson, H.J. Identification of a molecular signature underlying inhibition of mammary carcinoma growth by dietary N-3 fatty acids. Cancer Res. 2012, 72, 3795–3806. [Google Scholar] [CrossRef] [PubMed]
- Mensack, M.M.; McGinley, J.N.; Thompson, H.J. Metabolomic analysis of the effects of edible dry beans (Phaseolus vulgaris L.) on tissue lipid metabolism and carcinogenesis in rats. Br. J. Nutr. 2012, 108, S155–S165. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agricultur; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025; U.S. Department of Agriculture: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/ (accessed on 15 April 2022).
- Chu, D.T.; Malinowska, E.; Jura, M.; Kozak, L.P. C57BL/6J mice as a polygenic developmental model of diet-induced obesity. Physiol. Rep. 2017, 5, e13093. [Google Scholar] [CrossRef] [PubMed]
- Morán-Costoya, A.; Proenza, A.M.; Gianotti, M.; Lladó, I.; Valle, A. Sex Differences in Nonalcoholic Fatty Liver Disease: Estrogen Influence on the Liver–Adipose Tissue Crosstalk. Antioxid. Redox Signal. 2021, 35, 753–774. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, L.; Forsberg, G.-B.; Ståhlman, M. The BUME method: A new rapid and simple chloroform-free method for total lipid extraction of animal tissue. Sci. Rep. 2016, 6, 27688. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2013, 30, 523–530. [Google Scholar] [CrossRef]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr. Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef]
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef]
- Nassir, F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Kim, M.; Lazar, M.A. Nuclear receptors and transcriptional regulation in non-alcoholic fatty liver disease. Mol. Metab. 2021, 50, 101119. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Jakobsson, T.; Fan, R.; Treuter, E. The Nuclear Receptor-Co-repressor Complex in Control of Liver Metabolism and Disease. Front. Endocrinol. 2019, 10, 411. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, D.; Chakraborty, P.; Jolly, M.K. Emergent Properties of the HNF4α-PPARγ Network May Drive Consequent Phenotypic Plasticity in NAFLD. J. Clin. Med. 2020, 9, 870. [Google Scholar] [CrossRef]
- Loft, A.; Alfaro, A.J.; Schmidt, S.F.; Pedersen, F.B.; Terkelsen, M.K.; Puglia, M.; Chow, K.K.; Feuchtinger, A.; Troullinaki, M.; Maida, A.; et al. Liver-fibrosis-activated transcriptional networks govern hepatocyte reprogramming and intra-hepatic communication. Cell Metab. 2021, 33, 1685–1700.e9. [Google Scholar] [CrossRef]
- Shin, D.W. Lipophagy: Molecular Mechanisms and Implications in Metabolic Disorders. Mol. Cells 2020, 43, 686–693. [Google Scholar] [CrossRef]
- Grefhorst, A.; van de Peppel, I.P.; Larsen, L.E.; Jonker, J.W.; Holleboom, A.G. The Role of Lipophagy in the Development and Treatment of Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2020, 11, 601627. [Google Scholar] [CrossRef]
- Filali-Mouncef, Y.; Hunter, C.; Roccio, F.; Zagkou, S.; Dupont, N.; Primard, C.; Proikas-Cezanne, T.; Reggiori, F. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy 2022, 18, 50–72. [Google Scholar] [CrossRef]
- Ma, M.; Xie, W.; Li, X. Identification of Autophagy-Related Genes in the Progression from Non-Alcoholic Fatty Liver to Non-Alcoholic Steatohepatitis. Int. J. Gen. Med. 2021, 14, 3163–3176. [Google Scholar] [CrossRef]
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435–452. [Google Scholar] [PubMed]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, Y.; Hu, L.; Zhao, L.; Yang, P.; Moorhead, J.F.; Varghese, Z.; Chen, Y.; Ruan, X.Z. Rapamycin-mediated CD36 translational suppression contributes to alleviation of hepatic steatosis. Biochem. Biophys. Res. Commun. 2014, 447, 57–63. [Google Scholar] [CrossRef]
- Lehner, R.; Quiroga, A.D. Chapter 5—Fatty Acid Handling in Mammalian Cells. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Elsevier: Boston, MA, USA, 2016; pp. 149–184. [Google Scholar]
- Anderson, C.M.; Stahl, A. SLC27 fatty acid transport proteins. Mol. Asp. Med. 2013, 34, 516–528. [Google Scholar] [CrossRef]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef]
- Mashek, D.G. Hepatic Fatty Acid Trafficking: Multiple Forks in the Road. Adv. Nutr. 2013, 4, 697–710. [Google Scholar] [CrossRef]
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, Fatty Acid Oxidation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Schreurs, M.; Kuipers, F.; Van Der Leij, F.R. Regulatory enzymes of mitochondrial β-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388. [Google Scholar] [CrossRef]
- Diebold, I.; Schön, U.; Horvath, R.; Schwartz, O.; Holinski-Feder, E.; Kölbel, H.; Abicht, A. HADHA and HADHB gene associated phenotypes—Identification of rare variants in a patient cohort by Next Generation Sequencing. Mol. Cell. Probes 2019, 44, 14–20. [Google Scholar] [CrossRef]
- Watkins, P.A.; Chen, L.; Braverman, N. Peroxisomal Disorders in Children. In Liver Disease in Children, 5th ed.; Suchy, F.J., Sokol, R.J., Balistreri, W.F., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 671–697. [Google Scholar]
- Wanders, R.J.A.; Ferdinandusse, S.; Brites, P.; Kemp, S. Peroxisomes, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2010, 1801, 272–280. [Google Scholar] [CrossRef]
- Scorletti, E.; Carr, R.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.; Benet, M.; Guzmán, C.; Tolosa, L.; García-Monzón, C.; Pareja, E.; Castell, J.V.; Jover, R. Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS ONE 2012, 7, e30014. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2022, 23, e13366. [Google Scholar] [CrossRef]
- Chia, A.; Ong, J.; Bundele, A.; Lim, Y.W. Social entrepreneurship in obesity prevention: A scoping review. Obes. Rev. 2022, 23, e13378. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Mark, H.E.; Colombo, M.; Demaio, S.; Dillon, J.F.; George, J.; Hagström, H.; Hocking, S.; Lee, N.; Nieuwenhuijsen, M.J.; et al. A sustainable development goal framework to guide multisectoral action on NAFLD through a societal approach. Aliment. Pharmacol. Ther. 2022, 55, 234–243. [Google Scholar] [CrossRef]
- Naeem, M.; Markus, M.R.P.; Mousa, M.; Schipf, S.; Dörr, M.; Steveling, A.; Aghdassi, A.; Kühn, J.-P.; Kromrey, M.-L.; Nauck, M.; et al. Associations of liver volume and other markers of hepatic steatosis with all-cause mortality in the general population. Liver Int. 2022, 42, 575–584. [Google Scholar] [CrossRef]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Zhang, Q.; Du, Z.; Gai, Z.; Wu, J.; et al. An Overview of Lipid Metabolism and Nonalcoholic Fatty Liver Disease. Biomed. Res. Int. 2020, 2020, 4020249. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Wilson, C.G.; Tran, J.L.; Erion, D.M.; Vera, N.B.; Febbraio, M.; Weiss, E.J. Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice. Endocrinology 2016, 157, 570–585. [Google Scholar] [CrossRef]
- Enjoji, M.; Kohjima, M.; Nakamuta, M. Lipid metabolism and the liver. In The Liver in Systemic Diseases; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–122. [Google Scholar]
- Chella Krishnan, K.; Kurt, Z.; Barrere-Cain, R.; Sabir, S.; Das, A.; Floyd, R.; Vergnes, L.; Zhao, Y.; Che, N.; Charugundla, S.; et al. Integration of Multi-omics Data from Mouse Diversity Panel Highlights Mitochondrial Dysfunction in Non-alcoholic Fatty Liver Disease. Cell Syst. 2018, 6, 103–115.e7. [Google Scholar] [CrossRef] [PubMed]
- Kurt, Z.; Barrere-Cain, R.; LaGuardia, J.; Mehrabian, M.; Pan, C.; Hui, S.T.; Norheim, F.; Zhou, Z.; Hasin, Y.; Lusis, A.J.; et al. Tissue-specific pathways and networks underlying sexual dimorphism in non-alcoholic fatty liver disease. Biol. Sex Differ. 2018, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Sexual dimorphism in cardiometabolic health: The role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 2021, 17, 47–66. [Google Scholar] [CrossRef]
- Link, J.C.; Reue, K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu. Rev. Nutr. 2017, 37, 225–245. [Google Scholar] [CrossRef]
- Daniel, P.V.; Mondal, P. Causative and Sanative dynamicity of ChREBP in Hepato-Metabolic disorders. Eur. J. Cell Biol. 2020, 99, 151128. [Google Scholar] [CrossRef] [PubMed]
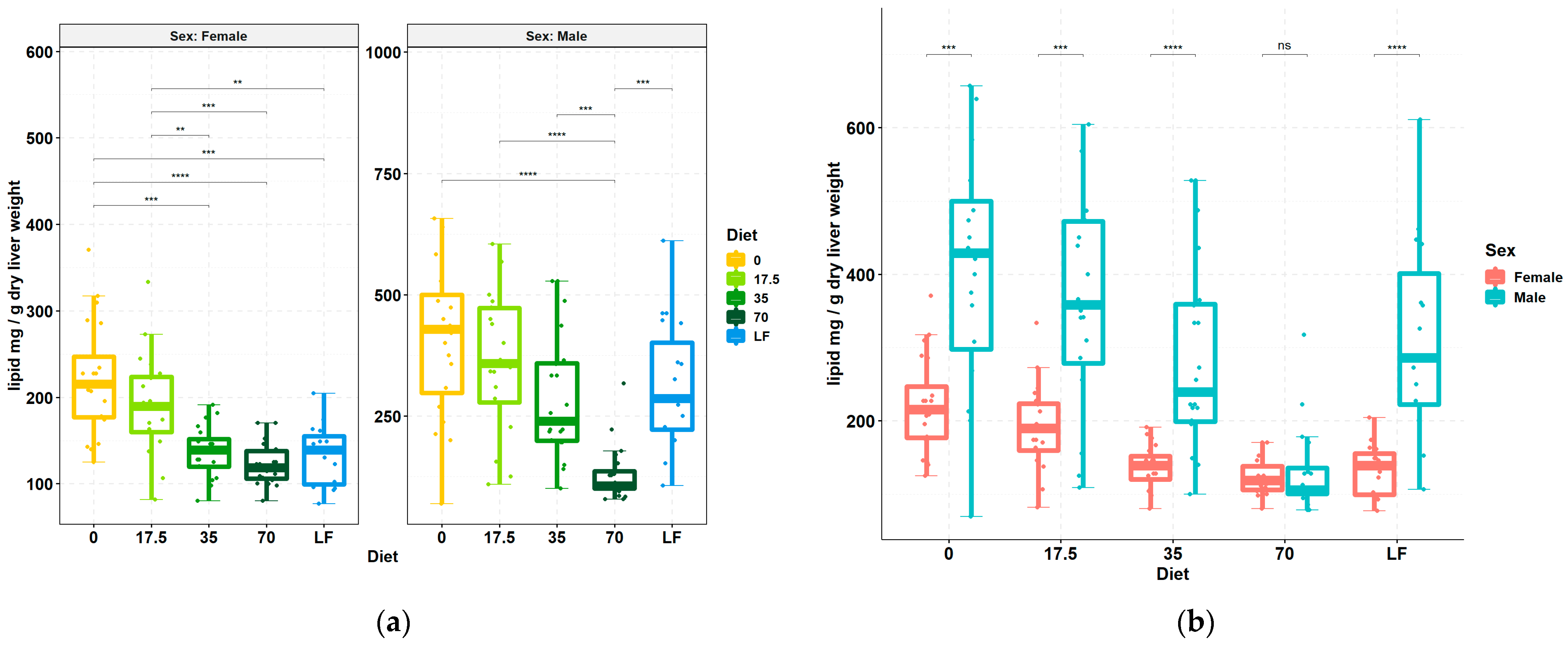
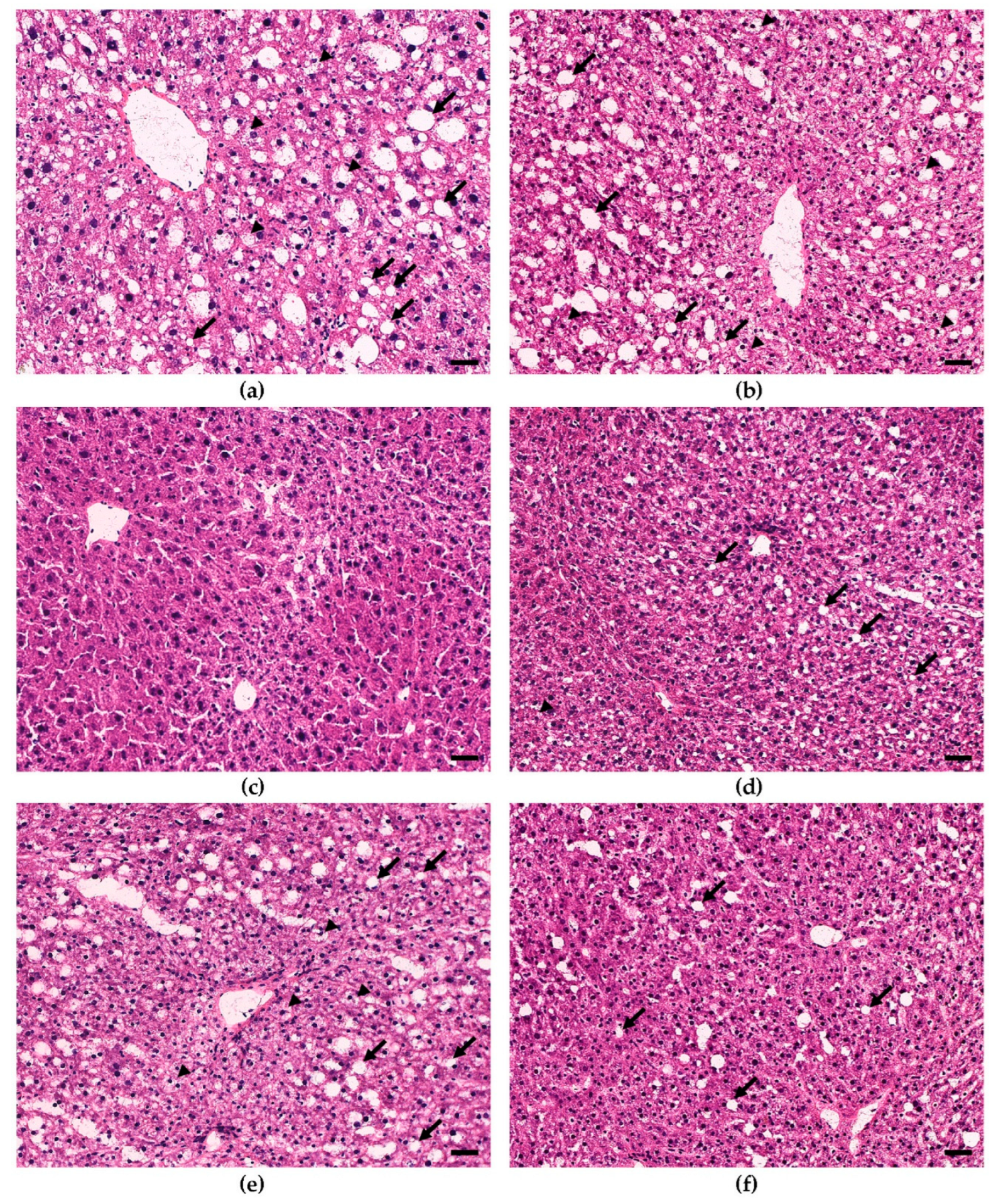

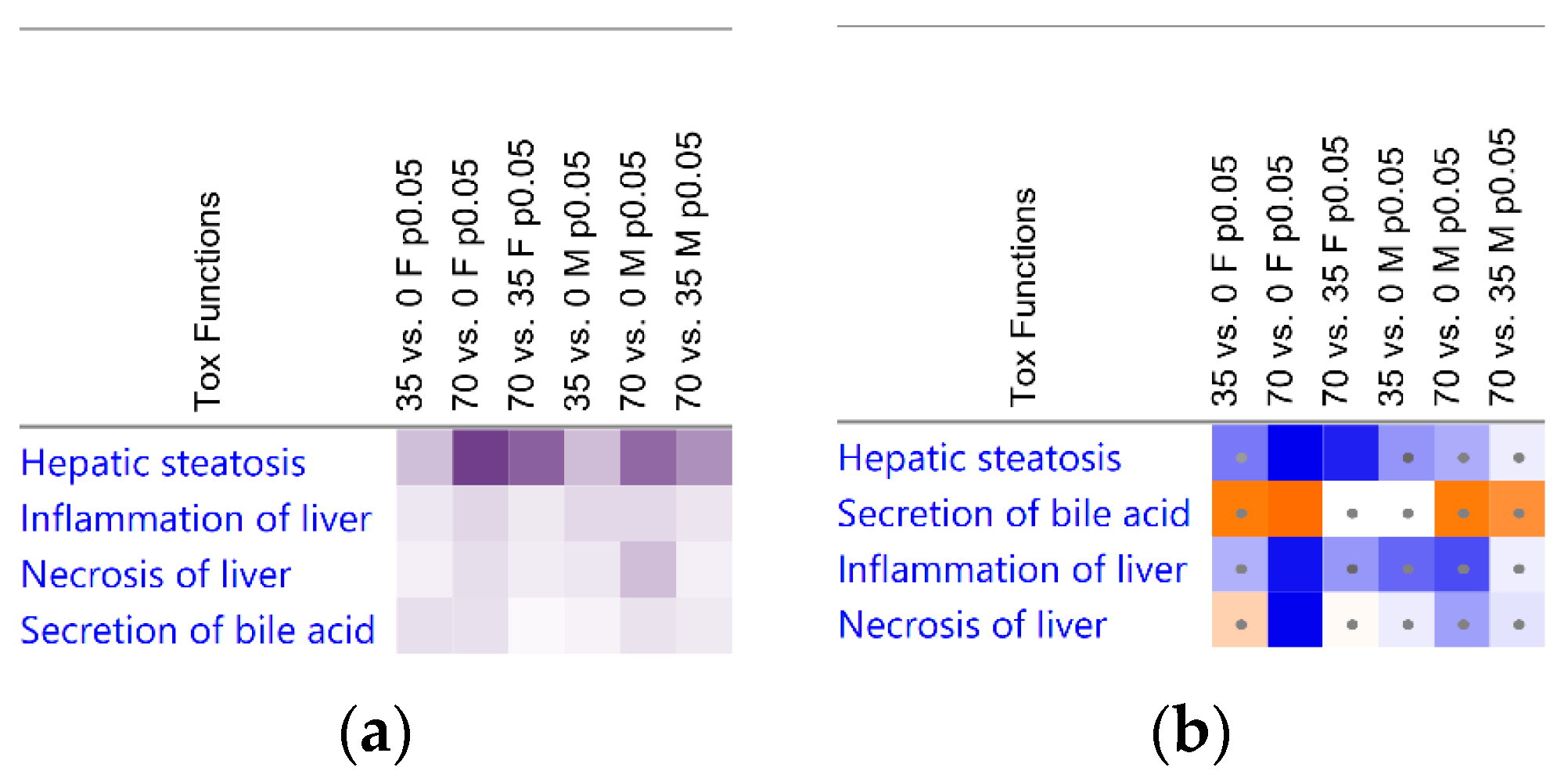
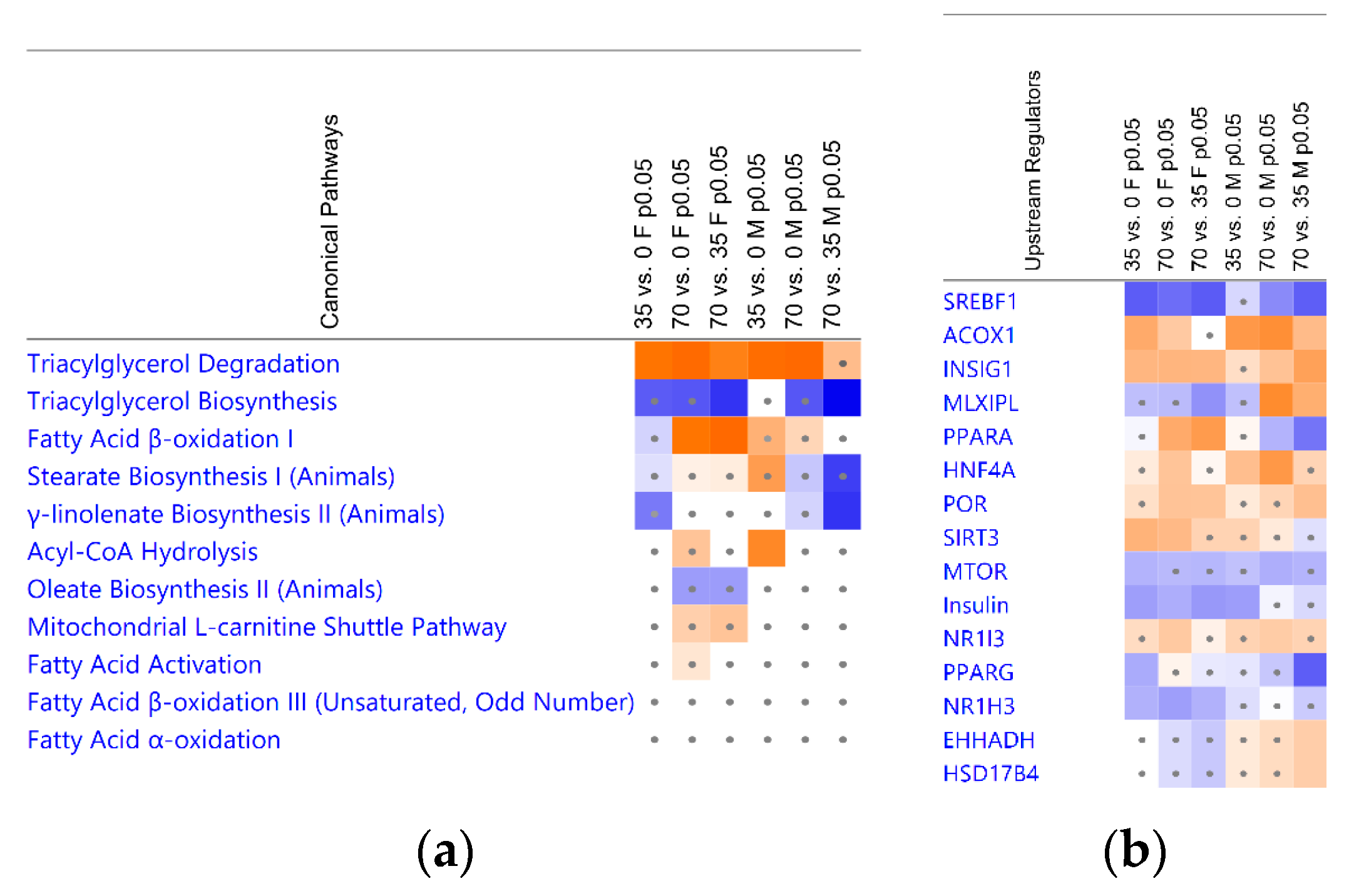

| Group Cohort | 35% vs. 0% Bean | 70% vs. 0% Bean | 70% vs. 35% Bean |
|---|---|---|---|
| DEGs1 | DEGs1 | DEGs1 | |
| Pre-filtered Mapped IDs2 | |||
| Females | 3547 | 3579 | 3541 |
| Males | 3539 | 3539 | 3497 |
| Core Analysis-ready IDs3 | |||
| Females | 461 | 966 | 389 |
| Males | 394 | 1130 | 535 |
| TOTAL DEGs 4 | 66,624 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutsiv, T.; McGinley, J.N.; Neil, E.S.; Foster, M.T.; Thompson, H.J. Thwarting Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) with Common Bean: Dose- and Sex-Dependent Protection against Hepatic Steatosis. Nutrients 2023, 15, 526. https://doi.org/10.3390/nu15030526
Lutsiv T, McGinley JN, Neil ES, Foster MT, Thompson HJ. Thwarting Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) with Common Bean: Dose- and Sex-Dependent Protection against Hepatic Steatosis. Nutrients. 2023; 15(3):526. https://doi.org/10.3390/nu15030526
Chicago/Turabian StyleLutsiv, Tymofiy, John N. McGinley, Elizabeth S. Neil, Michelle T. Foster, and Henry J. Thompson. 2023. "Thwarting Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) with Common Bean: Dose- and Sex-Dependent Protection against Hepatic Steatosis" Nutrients 15, no. 3: 526. https://doi.org/10.3390/nu15030526
APA StyleLutsiv, T., McGinley, J. N., Neil, E. S., Foster, M. T., & Thompson, H. J. (2023). Thwarting Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) with Common Bean: Dose- and Sex-Dependent Protection against Hepatic Steatosis. Nutrients, 15(3), 526. https://doi.org/10.3390/nu15030526







