Microbiome and Asthma: Microbial Dysbiosis and the Origins, Phenotypes, Persistence, and Severity of Asthma
Abstract
1. Introduction
2. Gut and Airway Microbiome: Gut-Lung Axis
2.1. Gut Microbiome
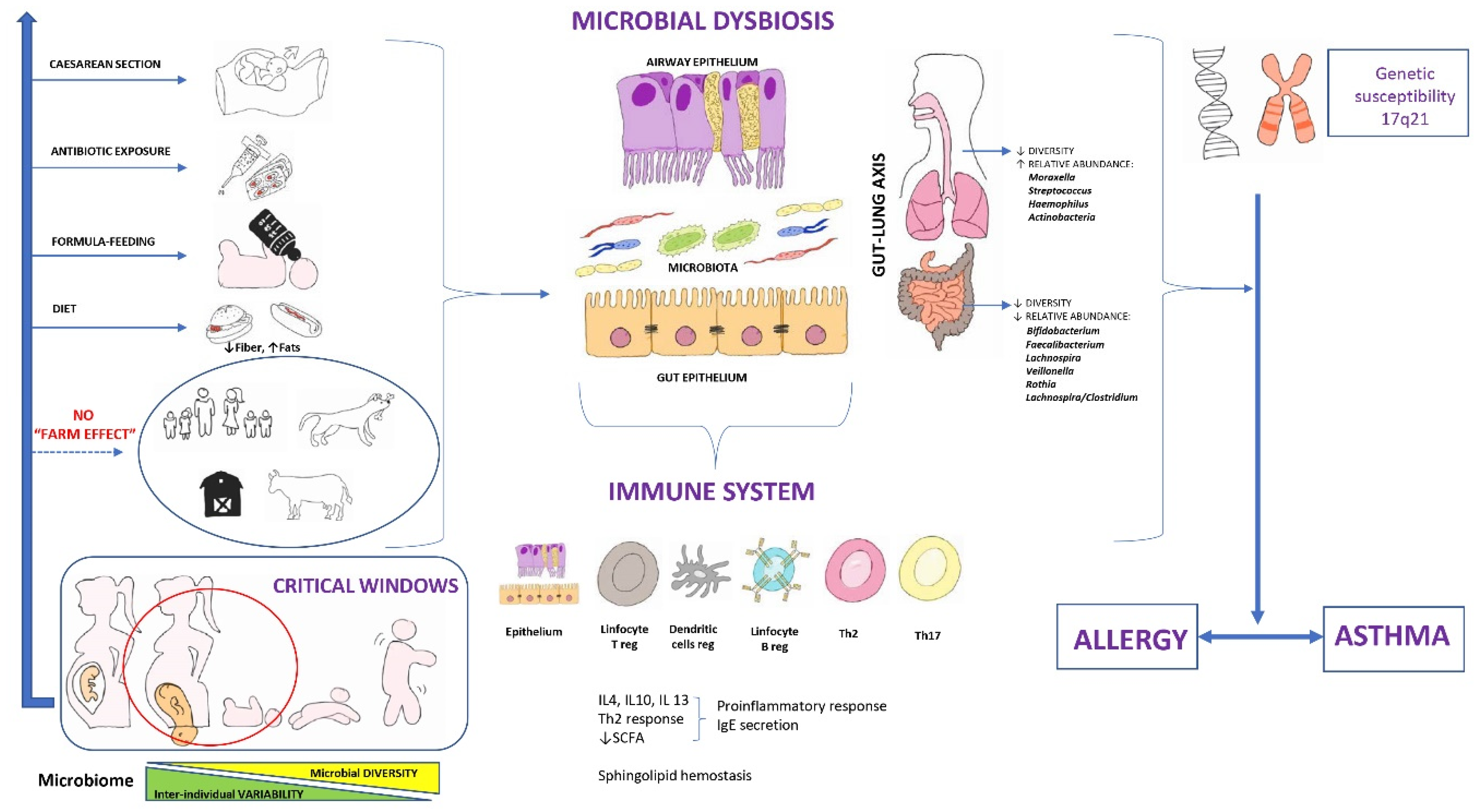
2.1.1. Factors That Affect the Microbiome
Maternal Factors during Pregnancy
Mode of Delivery
Prematurity
Type of Lactation
Early Contact with a Greater Number of Persons
Antibiotic Use
Introduction of Solid Foods and Diet
Environmental and Geographical Factors
2.2. Microbiome of the Airways
2.3. The Gut-Lung Axis
3. Microbiome and Immune System
4. Microbiome and Asthma in an Animal Model
5. Evidence of the Association between Microbial Dysbiosis and Asthma
5.1. Microbial Dysbiosis and Asthma Origin
5.1.1. Gut Microbiome and Asthma Origin
5.1.2. Airway Microbiome and Asthma Origin
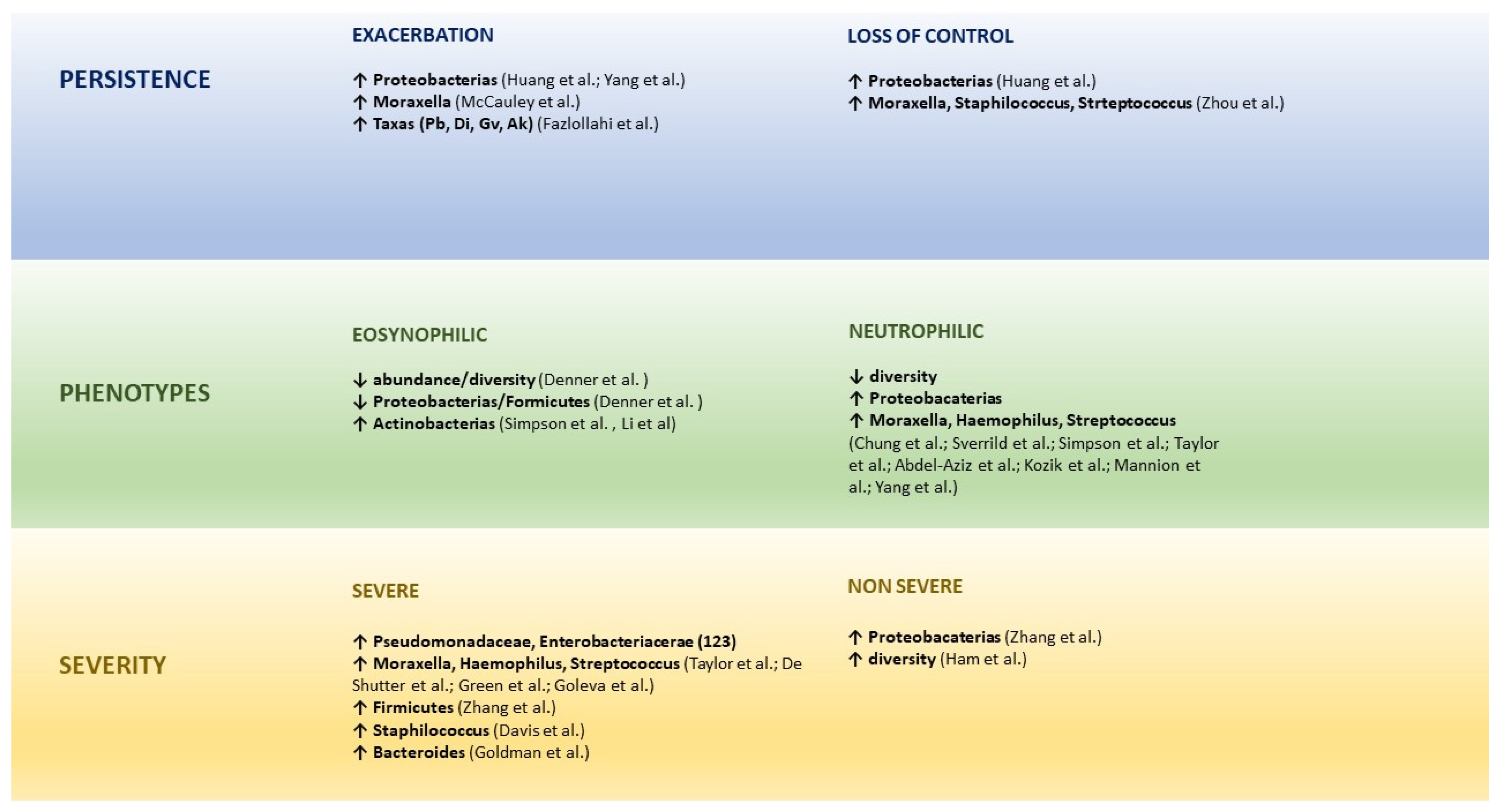
6. Microbial Dysbiosis and the Persistence, Phenotypes, and Severity of Asthma
6.1. Microbiome and Persistence of Asthma
6.2. Microbiome and Asthma Phenotypes
6.3. Microbiome and Asthma severity
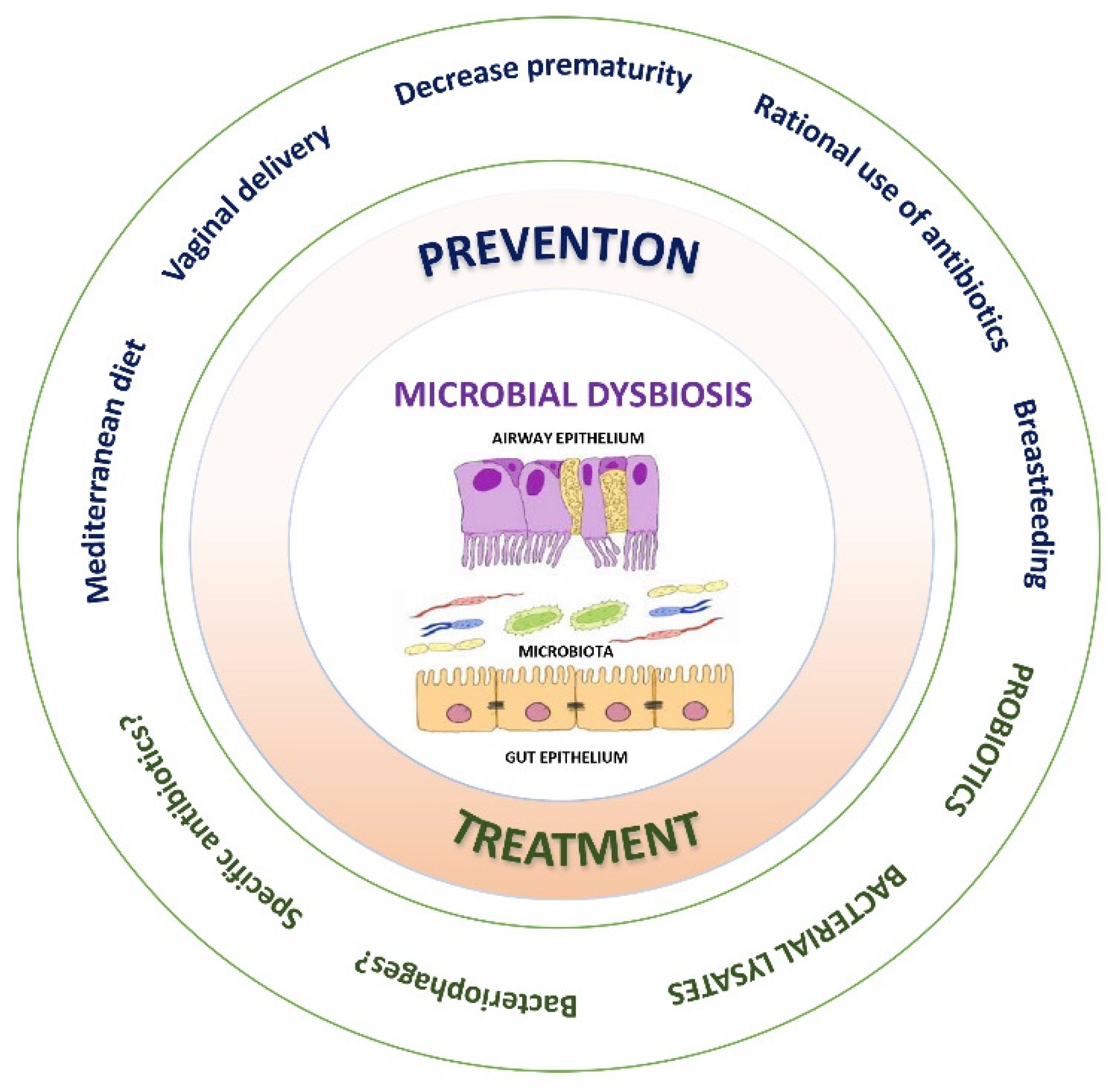
7. Prevention and Treatment of Microbial Dysbiosis
7.1. Probiotics
7.2. Bacterial Lysates
8. Clinical Implications and Future Perspectives
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scherzer, R.; Grayson, M.H. Heterogeneity and the origins of asthma. Ann. Allergy Asthma Immunol. 2018, 121, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Jatzlauk, G.; Bartel, S.; Heine, H.; Schloter, M.; Krauss-Etschmann, S. Influences of environmental bacteria and their metabolites on allergies, asthma, and host microbiota. Allergy 2017, 72, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Lynch, S.V. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe 2015, 17, 592–602. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Martinez, F.D.; Guerra, S. Early Origins of Asthma. Role of Microbial Dysbiosis and Metabolic Dysfunction. Am. J. Respir. Crit. Care Med. 2018, 197, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Boushey, H.A.; Lynch, S.V. Airway Microbiota and the Implications of Dysbiosis in Asthma. Curr. Allergy Asthma Rep. 2016, 16, 52. [Google Scholar] [CrossRef]
- Rivas, M.N.; Crother, T.R.; Arditi, M. The microbiome in asthma. Curr. Opin. Pediatr. 2016, 28, 764–771. [Google Scholar] [CrossRef]
- Loss, G.J.; Depner, M.; Hose, A.J.; Genuneit, J.; Karvonen, A.M.; Hyvärinen, A.; Roduit, C.; Kabesch, M.; Lauener, R.; Pfefferle, P.I.; et al. The Early Development of Wheeze. Environmental Determinants and Genetic Susceptibility at 17q21. Am. J. Respir. Crit. Care Med. 2016, 193, 889–897. [Google Scholar] [CrossRef]
- Tang, H.H.F.; Teo, S.M.; Sly, P.D.; Holt, P.G.; Inouye, M. The intersect of genetics, environment, and microbiota in asthma-perspectives and challenges. J. Allergy Clin. Immunol. 2021, 147, 781–793. [Google Scholar] [CrossRef]
- Hu, T.; Dong, Y.; Yang, C.; Zhao, M.; He, Q. Pathogenesis of Children’s Allergic Diseases: Refocusing the Role of the Gut Microbiota. Front. Physiol. 2021, 12, 749544. [Google Scholar] [CrossRef]
- Sokolowska, M.; Frei, R.; Lunjani, N.; Akdis, C.A.; O’Mahony, L. Microbiome and asthma. Asthma Res. Pract. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Ver Heul, A.; Planer, J.; Kau, A.L. The Human Microbiota and Asthma. Clin. Rev. Allergy Immunol. 2019, 57, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Bunyavanich, S. The airway microbiome and pediatric asthma. Curr. Opin. Pediatr. 2021, 33, 639–647. [Google Scholar] [CrossRef]
- Alcazar, C.G.M.; Paes, V.M.; Shao, Y.; Oesser, C.; Miltz, A.; Lawley, T.D.; Brocklehurst, P.; Rodger, A.; Field, N. The association between early-life gut microbiota and childhood respiratory diseases: A systematic review. Lancet Microbe 2022, 3, E867–E880. [Google Scholar] [CrossRef]
- Renz, H.; Brandtzaeg, P.; Hornef, M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 2011, 12, 9–23. [Google Scholar] [CrossRef]
- Walker, W.A. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr. Res. 2017, 82, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar] [CrossRef]
- Huang, Y.J.; Porsche, C.; Kozik, A.J.; Lynch, S.V. Microbiome–Immune Interactions in Allergy and Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 2244–2251. [Google Scholar] [CrossRef]
- Kozik, A.J.; Holguin, F.; Segal, L.N.; Chatila, T.A.; Dixon, A.E.; Gern, J.E.; Lozupone, C.; Lukacs, N.; Lumeng, C.; Molyneaux, P.L.; et al. Microbiome, Metabolism, and Immunoregulation of Asthma: An American Thoracic Society and National Institute of Allergy and Infectious Diseases Workshop Report. Am. J. Respir. Cell Mol. Biol. 2022, 67, 155–163. [Google Scholar] [CrossRef]
- Valverde-Molina, J.; Valverde-Fuentes, J. La disbiosis microbiana como origen precoz del asma. Rev. Asma 2018, 3, 36–45. [Google Scholar]
- Santo, C.E.; Caseiro, C.; Martins, M.J.; Monteiro, R.; Brandão, I. Gut Microbiota, in the Halfway between Nutrition and Lung Function. Nutrients 2021, 13, 1716. [Google Scholar] [CrossRef]
- Shi, C.Y.; Yu, C.H.; Yu, W.Y.; Ying, H.Z. Gut-Lung Microbiota in Chronic Pulmonary Diseases: Evolution, Pathogenesis, and Therapeutics. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 9278441. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ximenez, C.; Torres, J. Development of Microbiota in Infants and its Role in Maturation of Gut Mucosa and Immune System. Arch. Med. Res. 2017, 48, 666–680. [Google Scholar] [CrossRef]
- Mishra, A.; Lai, G.C.; Yao, L.J.; Aung, T.T.; Shental, N.; Rotter-Maskowitz, A.; Shepherdson, E.; Singh, G.S.N.; Pai, R.; Shanti, A.; et al. Microbial exposure during early human development primes fetal immune cells. Cell 2021, 184, 3394–3409.e20. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Johnson, C.C.; Ownby, D.R. Allergies and Asthma: Do Atopic Disorders Result from Inadequate Immune Homeostasis arising from Infant Gut Dysbiosis? Expert Rev. Clin. Immunol. 2016, 12, 379–388. [Google Scholar] [CrossRef]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Blaser, M.J. Asthma: Undoing millions of years of coevolution in early life? Sci. Transl. Med. 2015, 7, 307fs39. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 852. [Google Scholar] [CrossRef]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef]
- Martín, R.; Heilig, G.H.J.; Zoetendal, E.G.; Smidt, H.; Rodríguez, J.M. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J. Appl. Microbiol. 2007, 103, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics 2007, 119, e724–e732. [Google Scholar] [CrossRef] [PubMed]
- Gorlanova, O.; Illi, S.; Toncheva, A.A.; Usemann, J.; Latzin, P.; Kabesch, M.; Dalphin, J.C.; Lauener, R.; Pekkanen, J.R.; Von Mutius, E.; et al. Protective effects of breastfeeding on respiratory symptoms in infants with 17q21 asthma risk variants. Allergy 2018, 73, 2388–2392. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef]
- Harmsen, H.J.; Wildeboer-Veloo, A.C.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef]
- Tanaka, S.; Kobayashi, T.; Songjinda, P.; Tateyama, A.; Tsubouchi, M.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K.; Nakayama, J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 2009, 56, 80–87. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A. Diversity of Microbial Signatures in Asthmatic Airways. Int. J. Gen. Med. 2021, 14, 1367–1378. [Google Scholar] [CrossRef]
- Biesbroek, G.; Tsivtsivadze, E.; Sanders, E.A.M.; Montijn, R.; Veenhoven, R.H.; Keijser, B.J.F.; Bogaert, D. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am. J. Respir. Crit. Care Med. 2014, 190, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities—2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [CrossRef]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015, 6, e00037. [Google Scholar] [CrossRef]
- Magryś, A. Microbiota: A Missing Link in The Pathogenesis of Chronic Lung Inflammatory Diseases. Pol. J. Microbiol. 2021, 70, 25–32. [Google Scholar] [CrossRef]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.T.H.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Airway microbial dysbiosis in asthmatic patients: A target for prevention and treatment? J. Allergy Clin. Immunol. 2017, 139, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Cereta, A.D.; Oliveira, V.R.; Costa, I.P.; Guimarães, L.L.; Afonso, J.P.R.; Fonseca, A.L.; de Souza, A.R.T.; Silva, G.A.M.; Mello, D.A.C.P.G.; de Oliveira, L.V.F.; et al. Early Life Microbial Exposure and Immunity Training Effects on Asthma Development and Progression. Front. Med. 2021, 8, 662262. [Google Scholar] [CrossRef] [PubMed]
- Stiemsma, L.T.; Turvey, S.E. Asthma and the microbiome: Defining the critical window in early life. Allergy Asthma Clin. Immunol. 2017, 13, 3. [Google Scholar] [CrossRef]
- Samuelson, D.R.; Welsh, D.A.; Shellito, J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015, 6, 1085. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, H.K. The Role of CD4+ T Cells and Microbiota in the Pathogenesis of Asthma. Int. J. Mol. Sci. 2021, 22, 11822. [Google Scholar] [CrossRef]
- Torow, N.; Hornef, M.W. The Neonatal Window of Opportunity: Setting the Stage for Life-Long Host-Microbial Interaction and Immune Homeostasis. J. Immunol. 2017, 198, 557–563. [Google Scholar] [CrossRef]
- Smolinska, S.; Groeger, D.; O’Mahony, L. Biology of the Microbiome 1: Interactions with the Host Immune Response. Gastroenterol. Clin. N. Am. 2017, 46, 19–35. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bollrath, J.; Powrie, F. Immunology. Feed your Tregs more fiber. Science 2013, 341, 463–464. [Google Scholar] [CrossRef]
- Van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Herbst, T.; Sichelstiel, A.; Schär, C.; Yadava, K.; Bürki, K.; Cahenzli, J.; McCoy, K.; Marsland, B.J.; Harris, N.L. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 2011, 184, 198–205. [Google Scholar] [CrossRef]
- Gollwitzer, E.S.; Saglani, S.; Trompette, A.; Yadava, K.; Sherburn, R.; McCoy, K.D.; Nicod, L.P.; Lloyd, C.M.; Marsland, B.J. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014, 20, 642–647. [Google Scholar] [CrossRef]
- Stein, M.M.; Hrusch, C.L.; Gozdz, J.; Igartua, C.; Pivniouk, V.; Murray, S.E.; Ledford, J.G.; Marques Dos Santos, M.; Anderson, R.L.; Metwali, N.; et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N. Engl. J. Med. 2016, 375, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, X.; Zhang, Q.; He, X.; Zhang, Z.; Wang, X. Bifidobacterium infantis Relieves Allergic Asthma in Mice by Regulating Th1/Th2. Med. Sci. Monit. 2020, 26, e920583. [Google Scholar] [CrossRef]
- Wasserman, E.; Worgall, S. Perinatal origins of chronic lung disease: Mechanisms-prevention-therapy-sphingolipid metabolism and the genetic and perinatal origins of childhood asthma. Mol. Cell. Pediatr. 2021, 8, 22. [Google Scholar] [CrossRef]
- Van Nimwegen, F.A.; Penders, J.; Stobberingh, E.E.; Postma, D.S.; Koppelman, G.H.; Kerkhof, M.; Reijmerink, N.E.; Dompeling, E.; van den Brandt, P.A.; Ferreira, I.; et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol. 2011, 128, 948–955.e3. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Arrieta, M.C.; Dimitriu, P.A.; Cheng, J.; Thorson, L.; Lefebvre, D.L.; Azad, M.B.; Subbarao, P.; Mandhane, P.; Becker, A.; et al. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin. Sci. 2016, 130, 2199–2207. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Bisgaard, H.; Hermansen, M.N.; Buchvald, F.; Loland, L.; Halkjaer, L.B.; Bønnelykke, K.; Brasholt, M.; Heltberg, A.; Vissing, N.H.; Thorsen, S.V.; et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007, 357, 1487–1495. [Google Scholar] [CrossRef]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chan, Y.L.; Tsai, Y.S.; Chen, S.A.; Wang, C.J.; Chen, K.F.; Chung, I.F. Airway Microbial Diversity is Inversely Associated with Mite-Sensitized Rhinitis and Asthma in Early Childhood. Sci. Rep. 2017, 7, 1820. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.D.H.; Yap, G.C.; Tay, C.J.X.; Lim, A.S.M.; Huang, C.H.; Chu, C.W.; de Sessions, P.F.; Shek, L.P.; Goh, A.; Van Bever, H.P.S.; et al. Establishment of the nasal microbiota in the first 18 months of life: Correlation with early-onset rhinitis and wheezing. J. Allergy Clin. Immunol. 2018, 142, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Galazzo, G.; van Best, N.; Bervoets, L.; Dapaah, I.O.; Savelkoul, P.H.; Hornef, M.W.; GI-MDH Consortium; Lau, S.; Hamelmann, E.; Penders, J. Development of the Microbiota and Associations with Birth Mode, Diet, and Atopic Disorders in a Longitudinal Analysis of Stool Samples, Collected from Infancy Through Early Childhood. Gastroenterology 2020, 158, 1584–1596. [Google Scholar] [CrossRef]
- Teo, S.M.; Tang, H.H.F.; Mok, D.; Judd, L.M.; Watts, S.C.; Pham, K.; Holt, B.J.; Kusel, M.; Serralha, M.; Troy, N.; et al. Airway Microbiota Dynamics Uncover a Critical Window for Interplay of Pathogenic Bacteria and Allergy in Childhood Respiratory Disease. Cell Host Microbe 2018, 24, 341–352.e5. [Google Scholar] [CrossRef] [PubMed]
- Depner, M.; Ege, M.J.; Cox, M.J.; Dwyer, S.; Walker, A.W.; Birzele, L.T.; Genuneit, J.; Horak, E.; Braun-Fahrländer, C.; Danielewicz, H.; et al. Bacterial microbiota of the upper respiratory tract and childhood asthma. J. Allergy Clin. Immunol. 2017, 139, 826–834.e13. [Google Scholar] [CrossRef]
- Dzidic, M.; Abrahamsson, T.R.; Artacho, A.; Collado, M.C.; Mira, A.; Jenmalm, M.C. Oral microbiota maturation during the first 7 years of life in relation to allergy development. Allergy 2018, 73, 2000–2011. [Google Scholar] [CrossRef]
- Thorsen, J.; Rasmussen, M.A.; Waage, J.; Mortensen, M.; Brejnrod, A.; Bønnelykke, K.; Chawes, B.L.; Brix, S.; Sørensen, S.J.; Stokholm, J.; et al. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat. Commun. 2019, 10, 5001. [Google Scholar] [CrossRef]
- Powell, E.A.; Fontanella, S.; Boakes, E.; Belgrave, D.; Shaw, A.G.; Cornwell, E.; Fernandez-Crespo, R.; Fink, C.G.; Custovic, A.; Kroll, J.S. Temporal association of the development of oropharyngeal microbiota with early life wheeze in a population-based birth cohort. EBioMedicine 2019, 46, 486–498. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Zhang, N.; Wang, X.; Sun, L.; Chen, N.; Zhao, S.; He, Q. Airway microbiome, host immune response and recurrent wheezing in infants with severe respiratory syncytial virus bronchiolitis. Pediatr. Allergy Immunol. 2020, 31, 281–289. [Google Scholar] [CrossRef]
- Mansbach, J.M.; Luna, P.N.; Shaw, C.A.; Hasegawa, K.; Petrosino, J.F.; Piedra, P.A.; Sullivan, A.F.; Espinola, J.A.; Stewart, C.J.; Camrago, C.A. Increased Moraxella and Streptococcus species abundance after severe bronchiolitis is associated with recurrent wheezing. J. Allergy Clin. Immunol. 2020, 145, 518–527.e8. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Kelly, R.S.; Lasky-Su, J.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Laranjo, N.; Gold, D.R.; et al. Integrative analysis of the intestinal metabolome of childhood asthma. J. Allergy Clin. Immunol. 2019, 144, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.H.F.; Lang, A.; Teo, S.M.; Judd, L.M.; Gangnon, R.; Evans, M.D.; Lee, K.E.; Vrtis, R.; Holt, P.G.; Lemanske, R.F.; et al. Developmental patterns in the nasopharyngeal microbiome during infancy are associated with asthma risk. J. Allergy Clin. Immunol. 2021, 147, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, K.S.; Vidman, L.; Rydén, P.; West, C.E. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef]
- Thavagnanam, S.; Fleming, J.; Bromley, A.; Shields, M.D.; Cardwell, C.R. A meta-analysis of the association between Caesarean section and childhood asthma. Clin. Exp. Allergy 2008, 38, 629–633. [Google Scholar] [CrossRef]
- Foliaki, S.; Pearce, N.; Björkstén, B.; Mallol, J.; Montefort, S.; von Mutius, E.; International Study of Asthma and Allergies in Childhood Phase III Study Group. Antibiotic use in infancy and symptoms of asthma, rhinoconjunctivitis, and eczema in children 6 and 7 years old: International Study of Asthma and Allergies in Childhood Phase III. J. Allergy Clin. Immunol. 2009, 124, 982–989. [Google Scholar] [CrossRef]
- Marra, F.; Marra, C.A.; Richardson, K.; Lynd, L.D.; Kozyrskyj, A.; Patrick, D.M.; Bowie, W.R.; Fitzgerald, J.M. Antibiotic use in children is associated with increased risk of asthma. Pediatrics 2009, 123, 1003–1010. [Google Scholar] [CrossRef]
- Klopp, A.; Vehling, L.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; CHILD Study Investigators; Azad, M.B. Modes of Infant Feeding and the Risk of Childhood Asthma: A Prospective Birth Cohort Study. J. Pediatr. 2017, 190, 192–199.e2. [Google Scholar] [CrossRef]
- Lodge, C.J.; Tan, D.J.; Lau, M.X.Z.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Kull, I.; Melen, E.; Alm, J.; Hallberg, J.; Svartengren, M.; van Hage, M.; Pershagen, G.; Wickman, M.; Bergström, A. Breast-feeding in relation to asthma, lung function, and sensitization in young schoolchildren. J. Allergy Clin. Immunol. 2010, 125, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.V.; Travers, C.; Aghai, Z.H.; Eipers, P.; Jilling, T.; Halloran, B.; Carlo, W.A.; Keeley, J.; Rezonzew, G.; Kumar, R.; et al. The Airway Microbiome at Birth. Sci. Rep. 2016, 6, 31023. [Google Scholar] [CrossRef]
- Davis, M.F.; Peng, R.D.; McCormack, M.C.; Matsui, E.C. Staphylococcus aureus colonization is associated with wheeze and asthma among US children and young adults. J. Allergy Clin. Immunol. 2015, 135, 811–813.e5. [Google Scholar] [CrossRef]
- Losol, P.; Choi, J.P.; Kim, S.H.; Chang, Y.S. The Role of Upper Airway Microbiome in the Development of Adult Asthma. Immune Netw. 2021, 21, e19. [Google Scholar] [CrossRef]
- Zhou, Y.; Jackson, D.; Bacharier, L.B.; Mauger, D.; Boushey, H.; Castro, M.; Durack, J.; Huang, Y.; Lemanske, R.F.; Storch, G.A.; et al. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat. Commun. 2019, 10, 5714. [Google Scholar] [CrossRef]
- Zhang, Q.; Cox, M.; Liang, Z.; Brinkmann, F.; Cardenas, P.A.; Duff, R.; Bhavsar, P.; Cookson, W.; Moffatt, M.; Chung, K.F. Airway Microbiota in Severe Asthma and Relationship to Asthma Severity and Phenotypes. PLoS ONE 2016, 11, e0152724. [Google Scholar] [CrossRef] [PubMed]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Nariya, S.; Harris, J.M.; Lynch, S.V.; Choy, D.F.; Arron, J.R.; Boushey, H. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J. Allergy Clin. Immunol. 2015, 136, 874–884. [Google Scholar] [CrossRef]
- Yang, H.J.; LoSavio, P.S.; Engen, P.A.; Naqib, A.; Mehta, A.; Kota, R.; Khan, R.J.; Tobin, M.C.; Green, S.J.; Schleimer, R.P.; et al. Association of nasal microbiome and asthma control in patients with chronic rhinosinusitis. Clin. Exp. Allergy 2018, 48, 1744–1747. [Google Scholar] [CrossRef]
- Fazlollahi, M.; Lee, T.D.; Andrade, J.; Oguntuyo, K.; Chun, Y.; Grishina, G.; Grishin, A.; Bunyavanich, S. The nasal microbiome in asthma. J. Allergy Clin. Immunol. 2018, 142, 834–843.e2. [Google Scholar] [CrossRef]
- McCauley, K.; Durack, J.; Valladares, R.; Fadrosh, D.W.; Lin, D.L.; Calatroni, A.; LeBeau, P.K.; Tran, H.T.; Fujimura, K.E.; LaMere, B.; et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J. Allergy Clin. Immunol. 2019, 144, 1187–1197. [Google Scholar] [CrossRef]
- McCauley, K.E.; Flynn, K.; Calatroni, A.; DiMassa, V.; LaMere, B.; Fadrosh, D.W.; Lynch, K.V.; Gill, M.A.; Pongracic, J.A.; Khurana Hershey, G.K.; et al. Seasonal airway microbiome and transcriptome interactions promote childhood asthma exacerbations. J. Allergy Clin. Immunol. 2022, 150, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, J.; Stokholm, J.; Rasmussen, M.A.; Mortensen, M.S.; Brejnrod, A.D.; Hjelmsø, M.; Shah, S.; Chawes, B.; Bønnelykke, K.; Sørensen, S.J.; et al. The Airway Microbiota Modulates Effect of Azithromycin Treatment for Episodes of Recurrent Asthma-like Symptoms in Preschool Children: A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 149–158. [Google Scholar] [CrossRef]
- Sverrild, A.; Kiilerich, P.; Brejnrod, A.; Pedersen, R.; Porsbjerg, C.; Bergqvist, A.; Erjefält, J.S.; Kristiansen, K.; Backer, V. Eosinophilic airway inflammation in asthmatic patients is associated with an altered airway microbiome. J. Allergy Clin. Immunol. 2017, 140, 407–417.e11. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Chen, Y.C.; Chen, Y.Y.; Kozyrskyj, A.L.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Bisgaard, H.; Stokholm, J.; Chawes, B.; et al. The maternal prenatal and offspring early-life gut microbiome of childhood asthma phenotypes. Allergy 2022. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Losada, M.; Authelet, K.J.; Hoptay, C.E.; Kwak, C.; Crandall, K.A.; Freishtat, R.J. Pediatric asthma comprises different phenotypic clusters with unique nasal microbiotas. Microbiome 2018, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Leong, L.E.X.; Choo, J.M.; Wesselingh, S.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J. Allergy Clin. Immunol. 2018, 141, 94–103.e15. [Google Scholar] [CrossRef]
- Denner, D.R.; Sangwan, N.; Becker, J.B.; Hogarth, D.K.; Oldham, J.; Castillo, J.; Sperling, A.I.; Solway, J.; Naureckas, E.T.; Gilbert, J.A.; et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J. Allergy Clin. Immunol. 2016, 137, 1398–1405.e3. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Teufelberger, A.; Van Nevel, S.; Krysko, D.V.; Bachert, C. Protease/antiprotease network in allergy: The role of Staphylococcus aureus protease-like proteins. Allergy 2019, 74, 2077–2086. [Google Scholar] [CrossRef]
- Bachert, C.; Humbert, M.; Hanania, N.A.; Zhang, N.; Holgate, S.; Buhl, R.; Bröker, B.A. Staphylococcus aureus its IgE-inducing enterotoxins in asthma: Current knowledge. Eur. Respir. J. 2020, 55, 1901592. [Google Scholar] [CrossRef]
- Stentzel, S.; Teufelberger, A.; Nordengrün, M.; Kolata, J.; Schmidt, F.; van Crombruggen, K.; Michalik, S.; Kumpfmüller, J.; Tischer, S.; Schweder, T.; et al. Staphylococcal serine protease-like proteins are pacemakers of allergic airway reactions to Staphylococcus aureus. J. Allergy Clin. Immunol. 2017, 139, 492–500.e8. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.I.; Brinkman, P.; Vijverberg, S.J.H.; Neerincx, A.H.; Riley, J.H.; Bates, S.; Hashimoto, S.; Kermani, N.Z.; Chung, K.F.; Djukanovic, R.; et al. Sputum microbiome profiles identify severe asthma phenotypes of relative stability at 12 to 18 months. J. Allergy Clin. Immunol. 2021, 147, 123–134. [Google Scholar] [CrossRef]
- Simpson, J.L.; Daly, J.; Baines, K.J.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Hugenholtz, P.; Willner, D.; et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur. Respir. J. 2016, 47, 792–800. [Google Scholar] [CrossRef]
- Kozik, A.J.; Huang, Y.J. The microbiome in asthma: Role in pathogenesis, phenotype, and response to treatment. Ann. Allergy Asthma Immunol. 2019, 122, 270–275. [Google Scholar] [CrossRef]
- Mannion, J.M.; McLoughlin, R.M.; Lalor, S.J. The Airway Microbiome-IL-17 Axis: A Critical Regulator of Chronic Inflammatory Disease. Clin. Rev. Allergy Immunol. 2022. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhao, S.; Wang, R.; Wang, C. Long-term exposure to low-dose Haemophilus influenzae during allergic airway disease drives a steroid-resistant neutrophilic inflammation and promotes airway remodeling. Oncotarget 2018, 9, 24898–24913. [Google Scholar] [CrossRef]
- Li, N.; Qiu, R.; Yang, Z.; Li, J.; Chung, K.F.; Zhong, N.; Zhang, Q. Sputum microbiota in severe asthma patients: Relationship to eosinophilic inflammation. Respir. Med. 2017, 131, 192–198. [Google Scholar] [CrossRef]
- De Schutter, I.; Dreesman, A.; Soetens, O.; De Waele, M.; Crokaert, F.; Verhaegen, J.; Piérard, D.; Malfroot, A. In young children, persistent wheezing is associated with bronchial bacterial infection: A retrospective analysis. BMC Pediatr. 2012, 12, 83. [Google Scholar] [CrossRef]
- Green, B.J.; Wiriyachaiporn, S.; Grainge, C.; Rogers, G.B.; Kehagia, V.; Lau, L.; Carroll, M.P.; Bruce, K.D.; Howarth, P.H. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE 2014, 9, e100645. [Google Scholar] [CrossRef]
- Zhang, Q.; Illing, R.; Hui, C.K.; Downey, K.; Carr, D.; Stearn, M.; Alshafi, K.; Menzies-Gow, A.; Zhong, N.; Chung, K.F. Bacteria in sputum of stable severe asthma and increased airway wall thickness. Respir. Res. 2012, 13, 35. [Google Scholar] [CrossRef]
- Goleva, E.; Jackson, L.P.; Harris, J.K.; Robertson, C.E.; Sutherland, E.R.; Hall, C.F.; Good, J.T.; Gelfand, E.W.; Martin, R.J.; Leung, D.Y.M. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Losol, P.; Park, H.S.; Song, W.J.; Hwang, Y.K.; Kim, S.H.; Holloway, J.W.; Chang, Y.S. Association of upper airway bacterial microbiota and asthma: Systematic review. Asia Pac. Allergy 2022, 12, e32. [Google Scholar] [CrossRef]
- Goldman, D.L.; Chen, Z.; Shankar, V.; Tyberg, M.; Vicencio, A.; Burk, R. Lower airway microbiota and mycobiota in children with severe asthma. J. Allergy Clin. Immunol. 2018, 141, 808–811.e7. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Do, A.; Grishina, G.; Grishin, A.; Fang, G.; Rose, S.; Spencer, C.; Vicencio, A.; Schadt, E.; Bunyavanich, S. Integrative study of the upper and lower airway microbiome and transcriptome in asthma. JCI Insight 2020, 5, 133707. [Google Scholar] [CrossRef] [PubMed]
- Millares, L.; Bermudo, G.; Pérez-Brocal, V.; Domingo, C.; Garcia-Nuñez, M.; Pomares, X.; Moya, A.; Monsó, E. The respiratory microbiome in bronchial mucosa and secretions from severe IgE-mediated asthma patients. BMC Microbiol. 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Kim, J.; Choi, S.; Park, J.; Baek, M.G.; Kim, Y.C.; Sohn, K.H.; Cho, S.H.; Yang, S.; Bae, Y.S.; et al. Interactions between NCR+ILC3s and the Microbiome in the Airways Shape Asthma Severity. Immune Netw. 2021, 21, e25. [Google Scholar] [CrossRef]
- Polk, B.I.; Bacharier, L.B. Potential Strategies and Targets for the Prevention of Pediatric Asthma. Immunol. Allergy Clin. N. Am. 2019, 39, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Jan, R.L.; Lin, Y.L.; Chen, H.H.; Wang, J.Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010, 45, 1111–1120. [Google Scholar] [CrossRef]
- Huang, C.F.; Chie, W.C.; Wang, I.J. Efficacy of Lactobacillus Administration in School-Age Children with Asthma: A Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 1678. [Google Scholar] [CrossRef]
- Cabana, M.D.; McKean, M.; Caughey, A.B.; Fong, L.; Lynch, S.; Wong, A.; Leong, R.; Boushey, H.A.; Hilton, J.F. Early Probiotic Supplementation for Eczema and Asthma Prevention: A Randomized Controlled Trial. Pediatrics 2017, 140, e20163000. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, L.; Wu, S.; Yuan, L.; Tang, S.; Xiang, Y.; Qu, X.; Liu, H.; Quin, X.; Liu, C. Efficacy of probiotic supplementary therapy for asthma, allergic rhinitis, and wheeze: A meta-analysis of randomized controlled trials. Allergy Asthma Proc. 2019, 40, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Emeryk, A.; Bartkowiak-Emeryk, M.; Raus, Z.; Braido, F.; Ferlazzo, G.; Melioli, G. Mechanical bacterial lysate administration prevents exacerbation in allergic asthmatic children—The EOLIA study. Pediatr. Allergy Immunol. 2018, 29, 394–401. [Google Scholar] [CrossRef]
- Bartkowiak-Emeryk, M.; Emeryk, A.; Roliński, J.; Wawryk-Gawda, E.; Markut-Miotła, E. Impact of Polyvalent Mechanical Bacterial Lysate on lymphocyte number and activity in asthmatic children: A randomized controlled trial. Allergy Asthma Clin. Immunol. 2021, 17, 10. [Google Scholar] [CrossRef]
- Nieto, A.; Mazón, A.; Nieto, M.; Calderón, R.; Calaforra, S.; Selva, B.; Uixera, S.; Palao, M.J.; Brandi, P.; Conejero, L.; et al. Bacterial Mucosal Immunotherapy with MV130 Prevents Recurrent Wheezing in Children: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 462–472. [Google Scholar] [CrossRef]
- Gibson, M.K.; Crofts, T.S.; Dantas, G. Antibiotics and the developing infant gut microbiota and resistome. Curr. Opin. Microbiol. 2015, 27, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, S.; Deschildre, A.; Le Rouzic, O.; Engelmann, I.; Dessein, R.; Pichavant, M.; Gosset, P. Childhood asthma heterogeneity at the era of precision medicine: Modulating the immune response or the microbiota for the management of asthma attack. Biochem. Pharmacol. 2020, 179, 114046. [Google Scholar] [CrossRef] [PubMed]
- Kloepfer, K.M.; Lee, W.M.; Pappas, T.E.; Kang, T.J.; Vrtis, R.F.; Evans, M.D.; Gangnon, R.E.; Bochkov, Y.A.; Jackson, D.J.; Lemanske, R.F.; et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J. Allergy Clin. Immunol. 2014, 133, 1301–1307.E3. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Sohn, K.H.; Jung, J.W.; Kang, M.G.; Yang, M.S.; Kim, S.; Choi, J.H.; Cho, S.H.; Kang, H.R.; Yi, H. Lung virome: New potential biomarkers for asthma severity and exacerbation. J. Allergy Clin. Immunol. 2021, 148, 1007–1015.e9. [Google Scholar] [CrossRef]
- de Dios Caballero, J.; Cantón, R.; Ponce-Alonso, M.; García-Clemente, M.M.; Gómez, G.; de la Pedrosa, E.; López-Campos, J.L.; Máiz, L.; del Campo, R.; Martínez-García, M.A. The Human Mycobiome in Chronic Respiratory Diseases: Current Situation and Future Perspectives. Microorganisms 2022, 10, 810. [Google Scholar] [CrossRef]
- Gutkowski, P.; Madalinski, K.; Grek, M.; Dmenska, H.; Syczewska, M.; Michalkiewicz, J. Clinical Immunology. Effect of orally administered probiotic strains Lactobacillus and Bifidobacterium in children with atopic asthma. Cent. Eur. J. Immunol. 2010, 35, 233–238. [Google Scholar]
- Wei, X.; Jiang, P.; Liu, J.; Sun, R.; Zhu, L. Association between probiotic supplementation and asthma incidence in infants: A meta-analysis of randomized controlled trials. J. Asthma 2020, 57, 167–178. [Google Scholar] [CrossRef]
- Martens, K.; Pugin, B.; De Boeck, I.; Spacova, I.; Steelant, B.; Seys, S.F.; Lebeer, S.; Hellings, P.W. Probiotics for the airways: Potential to improve epithelial and immune homeostasis. Allergy 2018, 73, 1954–1963. [Google Scholar] [CrossRef]
- Tzani-Tzanopoulou, P.; Skliros, D.; Megremis, S.; Xepapadaki, P.; Andreakos, E.; Chanishvili, N.; Flemetakis, E.; Kaltsas, G.; Taka, S.; Lebessi, E.; et al. Interactions of Bacteriophages and Bacteria at the Airway Mucosa: New Insights Into the Pathophysiology of Asthma. Front. Allergy 2020, 1, 617240. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valverde-Molina, J.; García-Marcos, L. Microbiome and Asthma: Microbial Dysbiosis and the Origins, Phenotypes, Persistence, and Severity of Asthma. Nutrients 2023, 15, 486. https://doi.org/10.3390/nu15030486
Valverde-Molina J, García-Marcos L. Microbiome and Asthma: Microbial Dysbiosis and the Origins, Phenotypes, Persistence, and Severity of Asthma. Nutrients. 2023; 15(3):486. https://doi.org/10.3390/nu15030486
Chicago/Turabian StyleValverde-Molina, José, and Luis García-Marcos. 2023. "Microbiome and Asthma: Microbial Dysbiosis and the Origins, Phenotypes, Persistence, and Severity of Asthma" Nutrients 15, no. 3: 486. https://doi.org/10.3390/nu15030486
APA StyleValverde-Molina, J., & García-Marcos, L. (2023). Microbiome and Asthma: Microbial Dysbiosis and the Origins, Phenotypes, Persistence, and Severity of Asthma. Nutrients, 15(3), 486. https://doi.org/10.3390/nu15030486






