Combined Exercise Training and Nutritional Interventions or Pharmacological Treatments to Improve Exercise Capacity and Body Composition in Chronic Obstructive Pulmonary Disease: A Narrative Review
Abstract
1. Introduction
- -
- Summarizes the main skeletal muscle adaptations in response to exercise training in healthy individuals and patients with COPD;
- -
- Discusses the potential additional beneficial effects of nutrition when combined with exercise training to target exercise capacity;
- -
- Describes the potential additional effects of nutrition or pharmacological treatments when combined with exercise training to target muscle perseverance and/or growth;
- -
- Presents the potential additional effects of nutrition or pharmacological treatments when combined with exercise training to target weight/fat loss;
- -
- Explores novel compounds that have shown ergogenic potential in healthy populations with limited evidence in patients with COPD.
2. Methods
2.1. Different Types of Exercise Training
2.2. Resistance Training
2.3. mTOR Signaling
2.4. AMPK Signaling
2.5. Resistance Training in Patients with COPD
3. Endurance Training
Endurance Training in Patients with COPD
4. Neuromuscular Electrical Training
NMES in Patients with COPD
5. Nutrition to Support Exercise Training Effects on Physical Functioning
5.1. Nutritional Support
5.2. Nutritional Support in COPD
5.3. Multinutrient Supplements
5.4. Omega-3 Polyunsaturated Fatty Acids (PUFAs)
5.5. Caffeine
5.6. Creatine
5.7. Nitrate and Beetroot Juice (BRJ)
5.8. Beta-Alanine
5.9. Vitamin B12
5.10. Vitamin D
5.11. L-Carnitine
| Nutritional Intervention | Study | Supplement, Dose, Frequency | Exercise Intervention | Benefits from Combined Interventions |
|---|---|---|---|---|
| Multinutrient drinks and supplements | Steiner et al., 2003 [96] RCT N = 81 | CHO-rich supplement, 570 kcal, 3 times per day. Trial duration: 7 weeks | 14 PR sessions in 7 weeks, including endurance training and conditioning exercises | ↑ shuttle walking performance in well-nourished patients |
| Van de Bool et al., 2017 [35] RCT N = 81 | Nutritional supplement with CHO, protein, fat, and enriched with leucine, vitamin D and PUFAs, 187.5 kcal per portion, 2–3 portions per day. Trial duration: 4 months | 4-month outpatient PR program of 40 training sessions, including high-intensity endurance exercise, treadmill walking, and progressive resistance training | ↑ lower-limb muscle strength ↑ cycle endurance time | |
| Sugawara et al., 2010 [34] RCT N = 32 | Nutritional supplement with CHO, protein, and fat, enriched with PUFAs and vitamin A, 400 kcal, one time per day. Trial duration: 12 weeks | Home-based low-intensity exercise training, including upper and lower-limb exercises, level walking, and respiratory muscle training for 12 weeks in malnourished patients with COPD | ↑ quadricep muscle force ↑ walking distance | |
| Huhn et al., 2022 [99] RCT N = 9 | Acute supplementation of a meal rich in CHO and protein (white bun (60 g) with sour-milk cheese (100 g), one time before maximal strength tests. Trial duration: 2 times 2 days (crossover design) | A supervised strength training was performed by the patients according to usual protocol in physiotherapeutic setting. Maximal muscle strength was measured by knee extensor strength and chest press | ↑ maximal muscle strength | |
| Van Wetering et al. 2010 [102] RCT N = 39 | Liquid nutritional supplementation of 564 kcal per day. Trial duration: 4 months | Twice a week intensive supervised exercise training for 30 min | ↑ muscle strength ↑ exercise capacity | |
| PUFAs | Broek-huizen et al. 2005 [110] RCT N = 80 | PUFA supplementation, 9 capsules of 1 g PUFA blend per day. Trial duration: 2 months | 8-week PR program consisting of general physical training, including cycle ergometry, treadmill walking, swimming, sports, and games | ↑ cycling performance ↑ muscle strength |
| Van de Bool et al. 2017 [35] RCT N = 81 | Nutritional supplement with CHO, protein, fat, and enriched with leucine, vitamin D and PUFAs, 187.5 kcal per portion, 2–3 portions per day. Trial duration: 4 months | 4-month outpatient PR program of 40 training sessions, including high-intensity endurance exercise, treadmill walking, and progressive resistance training | ↑ lower-limb muscle strength ↑ cycle endurance time | |
| BRJ | Pavitt et al. 2020 [138] RCT N = 165 | BRJ supplementation, 140 mL containing 0.8 g nitrate, consumed once, 3 h prior to exercise training during PR. Trial duration: 8 weeks | Patients were enrolled in PR that involved 8 weeks exercise training, including aerobic and strength training | ↑ exercise capacity |
| L-carnitine | Borghi-Silva 2006 [174] RCT N = 16 | L-carnitine supplementation, 1 g/day, two times a day. Trial duration: 6 weeks | 6-week endurance training program of three one-hour training sessions per week. Each training session consisted of treadmill walking and inspiratory muscle training | ↑ walking tolerance |
6. Nutrition to Support Muscle Perseverance and Growth
6.1. Protein and EAA
6.2. Leucine
6.3. Anabolic-Androgenic Steroids (AAS)
6.4. Activin Type II Receptor Blockade
6.5. Ghrelin
| Nutritional or Pharmaco-Logical Agent | Study | Supplement, Dose, Frequency | Exercise Intervention | Benefits from Combined Interventions |
|---|---|---|---|---|
| EAA | Baldi et al. 2010 [183] RCT N = 28 | EAA supplementation, 4 g given twice a day. Trial duration: 12 weeks | Patients were enrolled in a PR program | ↑ body weight ↑ FFM (p = 0.05) |
| Leucine | Van de Bool et al. 2017 [35] RCT N = 81 | Nutritional supplement with CHO, protein, fat, and enriched with leucine, vitamin D and PUFAs, 187.5 kcal per portion, 2–3 portions per day. Trial duration: 4 months | 4-month outpatient PR program of 40 training sessions including, high-intensity endurance exercise, treadmill walking, and progressive resistance training | ↑ skeletal muscle mass |
| Anabolic-androgenic steroids (AAS) | Creutzberg et al. 2003 [222] RCT N = 63 | Nandrolone decanoate administration, intramuscular injection of 50 mg on days 1, 15, 29 and 43 of PR. Trial duration: 8 weeks | All patients participated in an 8-week standardized PR program consisting of general physical training with particular attention to exercise in relation to daily activities such as cycle ergometry, treadmill walking, swimming, sports, and games | ↑ FFM |
| Ferreira et al. 1998 [223] RCT N = 23 | Intramuscular injection of 250 mg testosterone at baseline, and 12 mg of oral stanozolol per day. Trial duration: 27 weeks | Patients enrolled in a PR program that included cycle ergometer exercises during weeks 18–27 of PR | ↑ BMI ↑ lean body mass ↑ arm and thigh anthropometrics |
7. Nutritional and Pharmacological Treatments to Support Weight Loss
7.1. Hypocaloric Diets
7.2. Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs)
8. Novel Compounds
8.1. Sodium Bicarbonate
8.2. Vitamins C and E
8.3. Resveratrol
8.4. Magnesium
8.5. (-)-Epicatechin
8.6. Nicotinamide Riboside (NR)
8.7. β-Hydroxy β-Methylbutyrate (HMB)
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yousuf, A.; McAuley, H.; Elneima, O.; Brightling, C.E. The different phenotypes of COPD. Br. Med. Bull. 2021, 137, 82–97. [Google Scholar] [CrossRef]
- Raherison, C.; Girodet, P.O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213. [Google Scholar] [CrossRef]
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Keogh, E.; Mark Williams, E. Managing malnutrition in COPD: A review. Respir. Med. 2021, 176, 106248. [Google Scholar] [CrossRef]
- Alvar, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef]
- van Iersel, L.E.J.; Beijers, R.; Gosker, H.R.; Schols, A. Nutrition as a modifiable factor in the onset and progression of pulmonary function impairment in COPD: A systematic review. Nutr. Rev. 2022, 80, 1434–1444. [Google Scholar] [CrossRef]
- Kim, V.; Criner, G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.J.; Spruit, M.A.; Van ‘t Hul, A.J.; Peters, J.B.; Van Herck, M.; Nakken, N.; Djamin, R.S.; Burtin, C.; Thong, M.S.Y.; Coors, A.; et al. Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation. Ther. Adv. Respir. Dis. 2019, 13, 1753466619878128. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Lippi, L.; Aprile, V.; Calafiore, D.; Folli, A.; D’Abrosca, F.; Moalli, S.; Lucchi, M.; Ammendolia, A.; Invernizzi, M. Pharmacological, Nutritional, and Rehabilitative Interventions to Improve the Complex Management of Osteoporosis in Patients with Chronic Obstructive Pulmonary Disease: A Narrative Review. J. Pers. Med. 2022, 12, 1626. [Google Scholar] [CrossRef] [PubMed]
- Vanfleteren, L.E.G.W.; Spruit, M.A.; Groenen, M.; Gaffron, S.; van Empel, V.P.M.; Bruijnzeel, P.L.B.; Rutten, E.P.A.; Op ’t Roodt, J.; Wouters, E.F.M.; Franssen, F.M.E. Clusters of Comorbidities Based on Validated Objective Measurements and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 728–735. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaré, R.; Dekhuijzen, P.N.R.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update on Limb Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62. [Google Scholar] [CrossRef] [PubMed]
- Annegarn, J.; Meijer, K.; Passos, V.L.; Stute, K.; Wiechert, J.; Savelberg, H.H.; Schols, A.M.; Wouters, E.F.; Spruit, M.A. Problematic activities of daily life are weakly associated with clinical characteristics in COPD. J. Am. Med. Dir. Assoc. 2012, 13, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.W.; Delbressine, J.M.L.; Mesquita, R.; Goertz, Y.M.J.; Janssen, D.J.A.; Nakken, N.; Franssen, F.M.E.; Vanfleteren, L.; Wouters, E.F.M.; Spruit, M.A. Impact of pulmonary rehabilitation on activities of daily living in patients with chronic obstructive pulmonary disease. J. Appl. Physiol. (1985) 2019, 126, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Ambrosino, N.; Calverley, P.; Cazzola, M.; Donner, C.F.; Make, B. Treatments for COPD. Respir. Med. 2005, 99, S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- British Thoracic Society Standards of Care Subcommittee on Pulmonary Rehabilitation. Pulmonary rehabilitation. Thorax 2001, 56, 827. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.S. Pulmonary Rehabilitation. Proc. Am. Thorac. Soc. 2006, 3, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Troosters, T.; Casaburi, R.; Gosselink, R.; Decramer, M. Pulmonary Rehabilitation in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2005, 172, 19–38. [Google Scholar] [CrossRef]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Pison, C.; Rutten-van Mölken, M.; Slinde, F.; Steiner, M.C.; et al. Nutritional assessment and therapy in COPD: A European Respiratory Society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef]
- Schols, A.M.; Slangen, J.; Volovics, L.; Wouters, E.F. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1791–1797. [Google Scholar] [CrossRef]
- Prescott, E.; Almdal, T.; Mikkelsen, K.L.; Tofteng, C.L.; Vestbo, J.; Lange, P. Prognostic value of weight change in chronic obstructive pulmonary disease: Results from the Copenhagen City Heart Study. Eur. Respir. J. 2002, 20, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Prescott, E.; Almdal, T.; Dahl, M.; Nordestgaard, B.G.; Andersen, T.; Sørensen, T.I.; Lange, P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: Findings from the Copenhagen City Heart Study. Am. J. Respir. Crit. Care Med. 2006, 173, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Broekhuizen, R.; Weling-Scheepers, C.A.; Wouters, E.F. Body composition and mortality in chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2005, 82, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Joppa, P.; Tkacova, R.; Franssen, F.M.; Hanson, C.; Rennard, S.I.; Silverman, E.K.; McDonald, M.L.; Calverley, P.M.; Tal-Singer, R.; Spruit, M.A.; et al. Sarcopenic Obesity, Functional Outcomes, and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. J. Am. Med. Dir. Assoc. 2016, 17, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Gosselink, R.; Troosters, T.; De Paepe, K.; Decramer, M. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur. Respir. J. 2002, 19, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Kongsgaard, M.; Backer, V.; Jørgensen, K.; Kjær, M.; Beyer, N. Heavy resistance training increases muscle size, strength and physical function in elderly male COPD-patients—A pilot study. Respir. Med. 2004, 98, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Panton, L.B.; Golden, J.; Broeder, C.E.; Browder, K.D.; Cestaro-Seifer, D.J.; Seifer, F.D. The effects of resistance training on functional outcomes in patients with chronic obstructive pulmonary disease. Eur. J. Appl. Physiol. 2004, 91, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bourjeily, G.; Rochester, C.L. Exercise Training in Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2000, 21, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, M.; Antons, J.; Vercoulen, J.H.; Goërtz, Y.M.J.; Ebadi, Z.; Burtin, C.; Janssen, D.J.A.; Thong, M.S.Y.; Otker, J.; Coors, A.; et al. Pulmonary Rehabilitation Reduces Subjective Fatigue in COPD: A Responder Analysis. J. Clin. Med. 2019, 8, 1264. [Google Scholar] [CrossRef] [PubMed]
- Beijers, R.; Steiner, M.C.; Schols, A. The role of diet and nutrition in the management of COPD. Eur. Respir. Rev. 2023, 32, 230003. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Brooks, D.; White, J.; Goldstein, R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, 12, Cd000998. [Google Scholar] [CrossRef] [PubMed]
- Rawal, G.; Yadav, S. Nutrition in chronic obstructive pulmonary disease: A review. J. Transl. Intern. Med. 2015, 3, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.R.; Gaynor-Sodeifi, K.; Lewthwaite, H.; Triandafilou, J.; Belo, L.F.; de Oliveira, M.F.; Jensen, D. Efficacy of interventions to alter measures of fat-free mass in people with COPD: A systematic review and meta-analysis. ERJ Open Res. 2023, 9, 00102–2023. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Takahashi, H.; Kasai, C.; Kiyokawa, N.; Watanabe, T.; Fujii, S.; Kashiwagura, T.; Honma, M.; Satake, M.; Shioya, T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir. Med. 2010, 104, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- van de Bool, C.; Rutten, E.P.A.; van Helvoort, A.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J. Cachexia Sarcopenia Muscle 2017, 8, 748–758. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.M.; Gibson, P.G.; Scott, H.A.; Baines, P.J.; Hensley, M.J.; Pretto, J.J.; Wood, L.G. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology 2016, 21, 875–882. [Google Scholar] [CrossRef]
- ACSM. Benefits and Risks Associated with Physical Activity, 10th ed.; ACSM: Indianapolis, IN, USA, 2019; p. 1. [Google Scholar]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Close, G.L.; Hamilton, D.L.; Philp, A.; Burke, L.M.; Morton, J.P. New strategies in sport nutrition to increase exercise performance. Free Radic. Biol. Med. 2016, 98, 144–158. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef]
- Lambert, M.I. General Adaptations to Exercise: Acute Versus Chronic and Strength Versus Endurance Training. In Exercise and Human Reproduction: Induced Fertility Disorders and Possible Therapies; Vaamonde, D., du Plessis, S.S., Agarwal, A., Eds.; Springer: New York, NY, USA, 2016; pp. 93–100. [Google Scholar]
- Booth, F.W.; Thomason, D.B. Molecular and cellular adaptation of muscle in response to exercise: Perspectives of various models. Physiol. Rev. 1991, 71, 541–585. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Phillips, S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: Strategies to enhance anabolism. Nutr. Metab. 2012, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S. Influence of nutrition on responses to resistance training. Med. Sci. Sports Exerc. 2004, 36, 689–696. [Google Scholar] [CrossRef]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; West, D.W.; Staples, A.W.; Atherton, P.J.; Baker, J.M.; Moore, D.R.; Holwerda, A.M.; Parise, G.; Rennie, M.J.; Baker, S.K.; et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS ONE 2010, 5, e12033. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Wolfe, R.R. Exercise, protein metabolism, and muscle growth. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 109–132. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Babraj, J.; Smith, K.; Wilkes, E.; Fedele, M.J.; Esser, K.; Rennie, M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E731–E738. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Phillips, S.M.; Atherton, P.J.; Patel, R.; Yarasheski, K.E.; Tarnopolsky, M.A.; Rennie, M.J. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 2008, 586, 3701–3717. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Esser, K.A. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 230–235. [Google Scholar] [CrossRef]
- Tipton, K.D.; Gurkin, B.E.; Matin, S.; Wolfe, R.R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J. Nutr. Biochem. 1999, 10, 89–95. [Google Scholar] [CrossRef]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Reynolds, N.; Downie, S.; Patel, A.; Rennie, M.J. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am. J. Physiol. 1998, 275, E73–E78. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; Fry, C.S.; Drummond, M.J.; Gundermann, D.M.; Walker, D.K.; Glynn, E.L.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J. Nutr. 2011, 141, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Weigl, L.G. Lost in translation: Regulation of skeletal muscle protein synthesis. Curr. Opin. Pharmacol. 2012, 12, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1011–E1018. [Google Scholar] [CrossRef]
- Drummond, M.J.; Fry, C.S.; Glynn, E.L.; Timmerman, K.L.; Dickinson, J.M.; Walker, D.K.; Gundermann, D.M.; Volpi, E.; Rasmussen, B.B. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J. Appl. Physiol. (1985) 2011, 111, 135–142. [Google Scholar] [CrossRef]
- Hundal, H.S.; Taylor, P.M. Amino acid transceptors: Gate keepers of nutrient exchange and regulators of nutrient signaling. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E603–E613. [Google Scholar] [CrossRef]
- Skumlien, S.; Aure Skogedal, E.; Skrede Ryg, M.; Bjørtuft, Ø. Endurance or resistance training in primary care after in-patient rehabilitation for COPD? Respir. Med. 2008, 102, 422–429. [Google Scholar] [CrossRef][Green Version]
- Vonbank, K.; Strasser, B.; Mondrzyk, J.; Marzluf, B.A.; Richter, B.; Losch, S.; Nell, H.; Petkov, V.; Haber, P. Strength training increases maximum working capacity in patients with COPD—Randomized clinical trial comparing three training modalities. Respir. Med. 2012, 106, 557–563. [Google Scholar] [CrossRef]
- Dourado, V.Z.; Tanni, S.E.; Antunes, L.C.; Paiva, S.A.; Campana, A.O.; Renno, A.C.; Godoy, I. Effect of three exercise programs on patients with chronic obstructive pulmonary disease. Braz. J. Med. Biol. Res. 2009, 42, 263–271. [Google Scholar] [CrossRef]
- Ortega, F.; Toral, J.; Cejudo, P.; Villagomez, R.; Sánchez, H.; Castillo, J.; Montemayor, T. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J.; Wagner, C.L. Effect of single-set resistance training on quality of life in COPD patients enrolled in pulmonary rehabilitation. Respir. Care 2013, 58, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Janaudis-Ferreira, T.; Hill, K.; Goldstein, R.S.; Robles-Ribeiro, P.; Beauchamp, M.K.; Dolmage, T.E.; Wadell, K.; Brooks, D. Resistance arm training in patients with COPD: A Randomized Controlled Trial. Chest 2011, 139, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, R.; Bhasin, S.; Cosentino, L.; Porszasz, J.; Somfay, A.; Lewis, M.I.; Fournier, M.; Storer, T.W. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 170, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Hoff, J.; Tjønna, A.E.; Steinshamn, S.; Høydal, M.; Richardson, R.S.; Helgerud, J. Maximal strength training of the legs in COPD: A therapy for mechanical inefficiency. Med. Sci. Sports Exerc. 2007, 39, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.; Killian, K.; McCartney, N.; Stubbing, D.G.; Jones, N.L. Randomised controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax 1992, 47, 70–75. [Google Scholar] [CrossRef]
- McKeough, Z.J.; Bye, P.T.; Alison, J.A. Arm exercise training in chronic obstructive pulmonary disease: A randomised controlled trial. Chron. Respir. Dis. 2012, 9, 153–162. [Google Scholar] [CrossRef]
- Hunter, G.R.; Singh, H.; Carter, S.J.; Bryan, D.R.; Fisher, G. Sarcopenia and its implications for metabolic health. J. Obes. 2019, 2019, 8031705. [Google Scholar] [CrossRef]
- Marillier, M.; Bernard, A.C.; Vergès, S.; Neder, J.A. Locomotor Muscles in COPD: The Rationale for Rehabilitative Exercise Training. Front. Physiol. 2019, 10, 1590. [Google Scholar] [CrossRef]
- Rivera-Brown, A.M.; Frontera, W.R. Principles of Exercise Physiology: Responses to Acute Exercise and Long-term Adaptations to Training. PM&R 2012, 4, 797–804. [Google Scholar] [CrossRef]
- Hawley, J.A. Adaptations Of Skeletal Muscle To Prolonged, Intense Endurance Training. Clin. Exp. Pharmacol. Physiol. 2002, 29, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Green, H.J.; Tarnopolsky, M.A.; Heigenhauser, G.J.; Grant, S.M. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 1996, 270, E265–E272. [Google Scholar] [CrossRef] [PubMed]
- Koulmann, N.; Bigard, A.X. Interaction between signalling pathways involved in skeletal muscle responses to endurance exercise. Pflug. Arch. 2006, 452, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Mador, M.J.; Bozkanat, E.; Aggarwal, A.; Shaffer, M.; Kufel, T.J. Endurance and Strength Training in Patients with COPD. Chest 2004, 125, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Normandin, E.A.; McCusker, C.; Connors, M.; Vale, F.; Gerardi, D.; ZuWallack, R.L. An evaluation of two approaches to exercise conditioning in pulmonary rehabilitation. Chest 2002, 121, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar]
- Reed, B. The Physiology of Neuromuscular Electrical Stimulation. Pediatr. Phys. Ther. 1997, 9, 96–102. [Google Scholar] [CrossRef]
- Langeard, A.; Bigot, L.; Chastan, N.; Gauthier, A. Does neuromuscular electrical stimulation training of the lower limb have functional effects on the elderly?: A systematic review. Exp. Gerontol. 2017, 91, 88–98. [Google Scholar] [CrossRef]
- Veldman, M.P.; Gondin, J.; Place, N.; Maffiuletti, N.A. Effects of Neuromuscular Electrical Stimulation Training on Endurance Performance. Front. Physiol. 2016, 7, 544. [Google Scholar] [CrossRef]
- Sillen, M.J.; Janssen, P.P.; Akkermans, M.A.; Wouters, E.F.; Spruit, M.A. The metabolic response during resistance training and neuromuscular electrical stimulation (NMES) in patients with COPD, a pilot study. Respir. Med. 2008, 102, 786–789. [Google Scholar] [CrossRef][Green Version]
- Sillen, M.J.; Franssen, F.M.; Delbressine, J.M.; Vaes, A.W.; Wouters, E.F.; Spruit, M.A. Efficacy of lower-limb muscle training modalities in severely dyspnoeic individuals with COPD and quadriceps muscle weakness: Results from the DICES trial. Thorax 2014, 69, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.J.H.; Speksnijder, C.M.; Eterman, R.-M.A.; Janssen, P.P.; Wagers, S.S.; Wouters, E.F.M.; Uszko-Lencer, N.H.M.K.; Spruit, M.A. Effects of Neuromuscular Electrical Stimulation of Muscles of Ambulation in Patients with Chronic Heart Failure or COPD: A Systematic Review of the English-Language Literature. Chest 2009, 136, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Man, W.D.; Gao, W.; Higginson, I.J.; Wilcock, A.; Maddocks, M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst. Rev. 2016, 10, Cd009419. [Google Scholar] [CrossRef]
- Hardie, D.G. Sensing of energy and nutrients by AMP-activated protein kinase. Am. J. Clin. Nutr. 2011, 93, 891s–896s. [Google Scholar] [CrossRef] [PubMed]
- Vandenbogaerde, T.J.; Hopkins, W.G. Effects of acute carbohydrate supplementation on endurance performance: A meta-analysis. Sports Med. 2011, 41, 773–792. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Clemente-Suárez, V.J.; Cupeiro, R.; Benítez-Muñoz, J.A.; Andreu Caravaca, L.; Rubio-Arias, J. The ergogenic effects of acute carbohydrate feeding on endurance performance: A systematic review, meta-analysis and meta-regression. Crit. Rev. Food Sci. Nutr. 2023, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A. A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Med. 2014, 44 (Suppl. S1), S25–S33. [Google Scholar] [CrossRef]
- Henselmans, M.; Bjørnsen, T.; Hedderman, R.; Vårvik, F.T. The Effect of Carbohydrate Intake on Strength and Resistance Training Performance: A Systematic Review. Nutrients 2022, 14, 856. [Google Scholar] [CrossRef]
- Tagawa, R.; Watanabe, D.; Ito, K.; Otsuyama, T.; Nakayama, K.; Sanbongi, C.; Miyachi, M. Synergistic Effect of Increased Total Protein Intake and Strength Training on Muscle Strength: A Dose-Response Meta-analysis of Randomized Controlled Trials. Sports Med.-Open 2022, 8, 110. [Google Scholar] [CrossRef]
- Lin, Y.-N.; Tseng, T.-T.; Knuiman, P.; Chan, W.P.; Wu, S.-H.; Tsai, C.-L.; Hsu, C.-Y. Protein supplementation increases adaptations to endurance training: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 3123–3132. [Google Scholar] [CrossRef]
- McLellan, T.M.; Pasiakos, S.M.; Lieberman, H.R. Effects of Protein in Combination with Carbohydrate Supplements on Acute or Repeat Endurance Exercise Performance: A Systematic Review. Sports Med. 2014, 44, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Ramires, B.R.; de Oliveira, E.P.; Pimentel, G.D.; McLellan, K.C.P.; Nakato, D.M.; Faganello, M.M.; Galhardo, M.L.; de Souza Venâncio, L. Resting energy expenditure and carbohydrate oxidation are higher in elderly patients with COPD: A case control study. Nutr. J. 2012, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, S.; Eckert, I.C.; Burgel, C.F.; Teixeira, P.J.Z.; Silva, F.M. Increased energy and/or protein intake improves anthropometry and muscle strength in COPD patients: A systematic review with meta-analysis on randomized controlled clinical trials. Br. J. Nutr. 2022, 13, 1–55. [Google Scholar]
- Steiner, M.C.; Barton, R.L.; Singh, S.J.; Morgan, M.D. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: A randomised controlled trial. Thorax 2003, 58, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Iuliano, S.; Daly, R.M.; Duque, G. Effects of protein supplementation on muscle wasting disorders: A brief update of the evidence. Australas. J. Ageing 2020, 39, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Constantin, D.; Menon, M.K.; Houchen-Wolloff, L.; Morgan, M.D.; Singh, S.J.; Greenhaff, P.; Steiner, M.C. Skeletal muscle molecular responses to resistance training and dietary supplementation in COPD. Thorax 2013, 68, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Huhn, A.; Flenker, U.; Diel, P. Effects of Carbohydrate and Protein Administration by Food Items on Strength Response after Training in Stable COPD. Nutrients 2022, 14, 3565. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Punzi, L.; Soysal, P.; Incalzi, R.A.; Saller, A.; Maggi, S. Effect of nutritional supplementations on physical performance and muscle strength parameters in older people: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 51, 48–54. [Google Scholar] [CrossRef]
- Franssen, F.M.E.; Rutten, E.P.A.; Groenen, M.T.J.; Vanfleteren, L.E.; Wouters, E.F.M.; Spruit, M.A. New Reference Values for Body Composition by Bioelectrical Impedance Analysis in the General Population: Results From the UK Biobank. J. Am. Med. Dir. Assoc. 2014, 15, 448.e1–448.e6. [Google Scholar] [CrossRef]
- van Wetering, C.R.; Hoogendoorn, M.; Broekhuizen, R.; Geraerts-Keeris, G.J.; De Munck, D.R.; Rutten-van Mölken, M.P.; Schols, A.M. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: A prespecified subgroup analysis of the INTERCOM trial. J. Am. Med. Dir. Assoc. 2010, 11, 179–187. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Hunter, A.M.; Gray, S.R. Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism 2017, 66, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Khairallah, R.J.; Dabkowski, E.R. Update on lipids and mitochondrial function: Impact of dietary n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D. Omega-3 Polyunsaturated Fatty Acids in Physical Performance Optimization. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Shei, R.-J.; Lindley, M.R.; Mickleborough, T.D. Omega-3 Polyunsaturated Fatty Acids in the Optimization of Physical Performance. Mil. Med. 2014, 179, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G. Omega-3 polyunsaturated fatty acids and chronic obstructive pulmonary disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Broekhuizen, R.; Wouters, E.F.M.; Creutzberg, E.C.; Weling-Scheepers, C.A.P.M.; Schols, A.M.W.J. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005, 60, 376. [Google Scholar] [CrossRef]
- Southward, K.; Rutherfurd-Markwick, K.J.; Ali, A. The Effect of Acute Caffeine Ingestion on Endurance Performance: A Systematic Review and Meta–Analysis. Sports Med. 2018, 48, 1913–1928. [Google Scholar] [CrossRef]
- Raya-González, J.; Rendo-Urteaga, T.; Domínguez, R.; Castillo, D.; Rodríguez-Fernández, A.; Grgic, J. Acute Effects of Caffeine Supplementation on Movement Velocity in Resistance Exercise: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 717–729. [Google Scholar] [CrossRef]
- Grgic, J.; Mikulic, P.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. The Influence of Caffeine Supplementation on Resistance Exercise: A Review. Sports Med. 2019, 49, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.M.; Ferreira, G.; Okuno, N.M. Caffeine does not change incremental test performance and autonomic recovery response in COPD patients. Sport Sci. Health 2023, 19, 511–518. [Google Scholar] [CrossRef]
- Bemben, M.G.; Lamont, H.S. Creatine supplementation and exercise performance: Recent findings. Sports Med. 2005, 35, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Sarah, J.D.; Emma, E.V.; Sally, J.S.; Michael, C.S.; Paul, G.; Michael, D.M. Does creatine supplementation enhance the effects of physical training during pulmonary rehabilitation in COPD? Eur. Respir. Rev. 2006, 15, 187. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults—A meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sports Med. 2017, 8, 213–226. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Del Canale, S.; Vitali, P.; Coffrini, E.; Ronda, N.; Guariglia, A. Skeletal muscle energetics, acid-base equilibrium and lactate metabolism in patients with severe hypercapnia and hypoxemia. Chest 1987, 92, 883–887. [Google Scholar] [CrossRef]
- Al-Ghimlas, F.; Todd, D.C. Creatine supplementation for patients with COPD receiving pulmonary rehabilitation: A systematic review and meta-analysis. Respirology 2010, 15, 785–795. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44 (Suppl. S1), S35–S45. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Martin, M.P.; Mintz, J.A.; Rogers, R.R.; Ballmann, C.G. Effect of Acute Beetroot Juice Supplementation on Bench Press Power, Velocity, and Repetition Volume. J. Strength. Cond. Res. 2020, 34, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. (1985) 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef] [PubMed]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Gibala, M.J.; van Loon, L.J. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Eliot, K.; Heuertz, R.M.; Weiss, E. Whole beetroot consumption acutely improves running performance. J. Acad. Nutr. Diet. 2012, 112, 548–552. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.; Stinkens, R.; Lundberg, J.O.; Gibala, M.J.; van Loon, L.J. No improvement in endurance performance after a single dose of beetroot juice. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 470–478. [Google Scholar] [CrossRef]
- Peacock, O.; Tjønna, A.E.; James, P.; Wisløff, U.; Welde, B.; Böhlke, N.; Smith, A.; Stokes, K.; Cook, C.; Sandbakk, O. Dietary nitrate does not enhance running performance in elite cross-country skiers. Med. Sci. Sports Exerc. 2012, 44, 2213–2219. [Google Scholar] [CrossRef]
- Wilkerson, D.P.; Hayward, G.M.; Bailey, S.J.; Vanhatalo, A.; Blackwell, J.R.; Jones, A.M. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur. J. Appl. Physiol. 2012, 112, 4127–4134. [Google Scholar] [CrossRef]
- Bescós, R.; Ferrer-Roca, V.; Galilea, P.A.; Roig, A.; Drobnic, F.; Sureda, A.; Martorell, M.; Cordova, A.; Tur, J.A.; Pons, A. Sodium nitrate supplementation does not enhance performance of endurance athletes. Med. Sci. Sports Exerc. 2012, 44, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Ranchal-Sanchez, A.; Diaz-Bernier, V.M.; De La Florida-Villagran, C.A.; Llorente-Cantarero, F.J.; Campos-Perez, J.; Jurado-Castro, J.M. Acute effects of beetroot juice supplements on resistance training: A randomized double-blind crossover. Nutrients 2020, 12, 1912. [Google Scholar] [CrossRef] [PubMed]
- Poredoš, D.; Jenko Pražnikar, Z.; Kozinc, Ž. Acute Effects of Beetroot Juice Supplementation on Isometric Muscle Strength, Rate of Torque Development and Isometric Endurance in Young Adult Men and Women: A Randomized, Double-Blind, Controlled Cross-Over Pilot Study. Nutrients 2022, 14, 4759. [Google Scholar] [CrossRef] [PubMed]
- Alshafie, S.; El-Helw, G.O.; Fayoud, A.M.; Elrashedy, A.A.; Gbreel, M.I.; Alfayoumi, S.S.; Mohamed, I.M.; Abdelwadoud, G.T.; Isa, A.S.; Ragab, K.M.; et al. Efficacy of dietary nitrate-rich beetroot juice supplementation in patients with chronic obstructive pulmonary disease (COPD): A systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 42, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pavitt, M.J.; Lewis, A.; Buttery, S.C.; Fernandez, B.O.; Mikus-Lelinska, M.; Banya, W.A.S.; Feelisch, M.; Polkey, M.I.; Hopkinson, N.S. Dietary nitrate supplementation to enhance exercise capacity in hypoxic COPD: EDEN-OX, a double-blind, placebo-controlled, randomised cross-over study. Thorax 2022, 77, 968–975. [Google Scholar] [CrossRef]
- Pavitt, M.J.; Tanner, R.J.; Lewis, A.; Buttery, S.; Mehta, B.; Jefford, H.; Curtis, K.J.; Banya, W.A.S.; Husain, S.; Satkunam, K.; et al. Oral nitrate supplementation to enhance pulmonary rehabilitation in COPD: ON-EPIC a multicentre, double-blind, placebo-controlled, randomised parallel group study. Thorax 2020, 75, 547–555. [Google Scholar] [CrossRef]
- Culbertson, J.Y.; Kreider, R.B.; Greenwood, M.; Cooke, M. Effects of Beta-Alanine on Muscle Carnosine and Exercise Performance: A Review of the Current Literature. Nutrients 2010, 2, 75–98. [Google Scholar] [CrossRef]
- Derave, W.; Everaert, I.; Beeckman, S.; Baguet, A. Muscle carnosine metabolism and beta-alanine supplementation in relation to exercise and training. Sports Med. 2010, 40, 247–263. [Google Scholar] [CrossRef]
- Hill, C.A.; Harris, R.C.; Kim, H.J.; Harris, B.D.; Sale, C.; Boobis, L.H.; Kim, C.K.; Wise, J.A. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 2007, 32, 225–233. [Google Scholar] [CrossRef]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef]
- Crozier, R.A.; Ajit, S.K.; Kaftan, E.J.; Pausch, M.H. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J. Neurosci. 2007, 27, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.R.; Cramer, J.T.; Zoeller, R.F.; Torok, D.; Costa, P.; Hoffman, J.R.; Harris, R.C.; O’Kroy, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 2007, 32, 381–386. [Google Scholar] [CrossRef] [PubMed]
- del Favero, S.; Roschel, H.; Solis, M.Y.; Hayashi, A.P.; Artioli, G.G.; Otaduy, M.C.; Benatti, F.B.; Harris, R.C.; Wise, J.A.; Leite, C.C.; et al. Beta-alanine (Carnosyn) supplementation in elderly subjects (60–80 years): Effects on muscle carnosine content and physical capacity. Amino Acids 2012, 43, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.R.; Graves, B.S.; Smith, A.E.; Hartman, M.J.; Cramer, J.T.; Beck, T.W.; Harris, R.C. The effect of beta-alanine supplementation on neuromuscular fatigue in elderly (55–92 Years): A double-blind randomized study. J. Int. Soc. Sports Nutr. 2008, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- McCormack, W.P.; Stout, J.R.; Emerson, N.S.; Scanlon, T.C.; Warren, A.M.; Wells, A.J.; Gonzalez, A.M.; Mangine, G.T.; Robinson, E.H.; Fragala, M.S.; et al. Oral nutritional supplement fortified with beta-alanine improves physical working capacity in older adults: A randomized, placebo-controlled study. Exp. Gerontol. 2013, 48, 933–939. [Google Scholar] [CrossRef]
- Van Thienen, R.; Van Proeyen, K.; Vanden Eynde, B.; Puype, J.; Lefere, T.; Hespel, P. Beta-alanine improves sprint performance in endurance cycling. Med. Sci. Sports Exerc. 2009, 41, 898–903. [Google Scholar] [CrossRef]
- Roveratti, M.C.; Jacinto, J.L.; Oliveira, D.B.; da Silva, R.A.; Andraus, R.A.C.; de Oliveira, E.P.; Ribeiro, A.S.; Aguiar, A.F. Effects of beta-alanine supplementation on muscle function during recovery from resistance exercise in young adults. Amino Acids 2019, 51, 589–597. [Google Scholar] [CrossRef]
- Kendrick, I.P.; Harris, R.C.; Kim, H.J.; Kim, C.K.; Dang, V.H.; Lam, T.Q.; Bui, T.T.; Smith, M.; Wise, J.A. The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids 2008, 34, 547–554. [Google Scholar] [CrossRef]
- De Brandt, J.; Derave, W.; Vandenabeele, F.; Pomiès, P.; Blancquaert, L.; Keytsman, C.; Barusso-Grüninger, M.S.; de Lima, F.F.; Hayot, M.; Spruit, M.A.; et al. Efficacy of 12 weeks oral beta-alanine supplementation in patients with chronic obstructive pulmonary disease: A double-blind, randomized, placebo-controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 2361–2372. [Google Scholar] [CrossRef]
- Jana De, B.; Wim, D.; Frank, V.; Joseph, A.; Laura, B.; Inge, E.; Martijn, S. Effect of oral beta-alanine supplementation on muscle carnosine in patients with COPD: A double blind, placebo-controlled, randomized trial. Eur. Respir. J. 2020, 56, 4748. [Google Scholar] [CrossRef]
- De Brandt, J.; Burtin, C.; Pomiès, P.; Vandenabeele, F.; Verboven, K.; Aumann, J.; Blancquaert, L.; Everaert, I.; Van Ryckeghem, L.; Cops, J.; et al. Carnosine, oxidative and carbonyl stress, antioxidants, and muscle fiber characteristics of quadriceps muscle of patients with COPD. J. Appl. Physiol. (1985) 2021, 131, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Tsiligianni, I.G.; van der Molen, T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir. Res. 2010, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef]
- Rosenbloom, C. Can Vitamins and Mineral Supplements Improve Sports Performance? Nutr. Today 2007, 42, 74–80. [Google Scholar] [CrossRef]
- Montoye, H.J.; Spata, P.J.; Pinckney, V.; Barron, L. Effects of vitamin B12 supplementation on physical fitness and growth of young boys. J. Appl. Physiol. 1955, 7, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Tin May, T.; Ma Win, M.; Khin Sann, A.; Mya-Tu, M. The effect of vitamin B12 on physical performance capacity. Br. J. Nutr. 1978, 40, 269–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paulin, F.V.; Zagatto, A.M.; Chiappa, G.R.; Müller, P.d.T. Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: A randomized and controlled study. Respir. Med. 2017, 122, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Sukumar, D.; Volpe, S.L. Magnesium and Vitamin D Supplementation on Exercise Performance. Transl. J. Am. Coll. Sports Med. 2021, 6, e000179. [Google Scholar] [CrossRef]
- Carswell, A.T.; Oliver, S.J.; Wentz, L.M.; Kashi, D.S.; Roberts, R.; Tang, J.C.Y.; Izard, R.M.; Jackson, S.; Allan, D.; Rhodes, L.E.; et al. Influence of Vitamin D Supplementation by Sunlight or Oral D3 on Exercise Performance. Med. Sci. Sports Exerc. 2018, 50, 2555–2564. [Google Scholar] [CrossRef]
- Alimoradi, K.; Nikooyeh, B.; Ravasi, A.A.; Zahedirad, M.; Shariatzadeh, N.; Kalayi, A.; Neyestani, T.R. Efficacy of Vitamin D Supplementation in Physical Performance of Iranian Elite Athletes. Int. J. Prev. Med. 2019, 10, 100. [Google Scholar] [CrossRef]
- Wyon, M.A.; Wolman, R.; Nevill, A.M.; Cloak, R.; Metsios, G.S.; Gould, D.; Ingham, A.; Koutedakis, Y. Acute Effects of Vitamin D3 Supplementation on Muscle Strength in Judoka Athletes: A Randomized Placebo-Controlled, Double-Blind Trial. Clin. J. Sport Med. 2016, 26, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Flueck, J.L.; Schlaepfer, M.W.; Perret, C. Effect of 12-Week Vitamin D Supplementation on 25[OH]D Status and Performance in Athletes with a Spinal Cord Injury. Nutrients 2016, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Calleja-González, J.; Urdampilleta, A.; León-Guereño, P.; Córdova, A.; Caballero-García, A.; Fernandez-Lázaro, D. Effects of Vitamin D Supplementation on Haematological Values and Muscle Recovery in Elite Male Traditional Rowers. Nutrients 2018, 10, 1968. [Google Scholar] [CrossRef] [PubMed]
- Wim, J.; Roger, B.; Bart, C.; Claudia, C.; An, L.; Ian, B.; Johan, C.; Chantal, M.; Marc, D.; Diether, L. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215. [Google Scholar] [CrossRef]
- Black, P.N.; Scragg, R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest 2005, 128, 3792–3798. [Google Scholar] [CrossRef]
- Hornikx, M.; Van Remoortel, H.; Lehouck, A.; Mathieu, C.; Maes, K.; Gayan-Ramirez, G.; Decramer, M.; Troosters, T.; Janssens, W. Vitamin D supplementation during rehabilitation in COPD: A secondary analysis of a randomized trial. Respir. Res. 2012, 13, 84. [Google Scholar] [CrossRef]
- Mølmen, K.S.; Hammarström, D.; Pedersen, K.; Lian Lie, A.C.; Steile, R.B.; Nygaard, H.; Khan, Y.; Hamarsland, H.; Koll, L.; Hanestadhaugen, M. Vitamin D3 supplementation does not enhance the effects of resistance training in older adults. J. Cachexia Sarcopenia Muscle 2021, 12, 599–628. [Google Scholar] [CrossRef]
- Oliveira, C.; Sousa, M. The effects of L-carnitine supplementation in athletic performance. Sci. Sports 2019, 34, 63–72. [Google Scholar] [CrossRef]
- Askarpour, M.; Hadi, A.; Miraghajani, M.; Symonds, M.E.; Sheikhi, A.; Ghaedi, E. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: An updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 151, 104554. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, J.C.; Minton, J.E.; Tokach, M.D.; Nelssen, J.L.; Goodband, R.D.; Dritz, S.S.; Koo, S.I.; Owen, K.Q. Dietary L-carnitine increases plasma leptin concentrations of gestating sows fed one meal per day. Domest. Anim. Endocrinol. 2004, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, W.; Chen, G.; Zhu, W.; Ding, W.; Ge, Z.; Tan, Y.; Ma, T.; Cui, G. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2017, 26, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Borghi-Silva, A.; Baldissera, V.; Sampaio, L.M.; Pires-DiLorenzo, V.A.; Jamami, M.; Demonte, A.; Marchini, J.S.; Costa, D. L-carnitine as an ergogenic aid for patients with chronic obstructive pulmonary disease submitted to whole-body and respiratory muscle training programs. Braz. J. Med. Biol. Res. 2006, 39, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Lach-Trifilieff, E.; Minetti, G.C.; Sheppard, K.; Ibebunjo, C.; Feige, J.N.; Hartmann, S.; Brachat, S.; Rivet, H.; Koelbing, C.; Morvan, F.; et al. An Antibody Blocking Activin Type II Receptors Induces Strong Skeletal Muscle Hypertrophy and Protects from Atrophy. Mol. Cell. Biol. 2014, 34, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.F.; Yang, I.A.; Chang, Y.C.; Vaughan, A. Nutritional support in chronic obstructive pulmonary disease (COPD): An evidence update. J. Thorac. Dis. 2019, 11, S2230–S2237. [Google Scholar] [CrossRef]
- Fitschen, P.J.; Wilson, G.J.; Wilson, J.M.; Wilund, K.R. Efficacy of β-hydroxy-β-methylbutyrate supplementation in elderly and clinical populations. Nutrition 2013, 29, 29–36. [Google Scholar] [CrossRef]
- van Bakel, S.I.J.; Gosker, H.R.; Langen, R.C.; Schols, A.M.W.J. Towards Personalized Management of Sarcopenia in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 25–40. [Google Scholar] [CrossRef]
- Marco, E.; Sánchez-Rodríguez, D.; Dávalos-Yerovi, V.N.; Duran, X.; Pascual, E.M.; Muniesa, J.M.; Rodríguez, D.A.; Aguilera-Zubizarreta, A.; Escalada, F.; Duarte, E. Malnutrition according to ESPEN consensus predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. Clin. Nutr. 2019, 38, 2180–2186. [Google Scholar] [CrossRef]
- Yoneda, T.; Yoshikawa, M.; Fu, A.; Tsukaguchi, K.; Okamoto, Y.; Takenaka, H. Plasma levels of amino acids and hypermetabolism in patients with chronic obstructive pulmonary disease. Nutrition 2001, 17, 95–99. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef]
- Baldi, S.; Aquilani, R.; Pinna, G.D.; Poggi, P.; De Martini, A.; Bruschi, C. Fat-free mass change after nutritional rehabilitation in weight losing COPD: Role of insulin, C-reactive protein and tissue hypoxia. Int. J. Chronic Obstr. Pulm. Dis. 2010, 5, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Ferrando, A.A.; Phillips, S.M.; Doyle, D., Jr.; Wolfe, R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. 1999, 276, E628–E634. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- Fabre, M.; Hausswirth, C.; Tiollier, E.; Molle, O.; Louis, J.; Durguerian, A.; Neveux, N.; Bigard, X. Effects of Postexercise Protein Intake on Muscle Mass and Strength During Resistance Training: Is There an Optimal Ratio Between Fast and Slow Proteins? Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 448–457. [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Guillet, C.; Prod’homme, M.; Balage, M.; Gachon, P.; Giraudet, C.; Morin, L.; Grizard, J.; Boirie, Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004, 18, 1586–1587. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Dobson, J.P.; Greene, N.P.; Wiggs, M.P.; Shimkus, K.L.; Wudeck, E.V.; Davis, A.R.; Laureano, M.L.; Fluckey, J.D. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J. 2013, 27, 3905–3916. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Holwerda, A.M.; Phillips, S.M.; van Loon, L.J. What is the optimal amount of protein to support post-exercise skeletal muscle reconditioning in the older adult? Sports Med. 2016, 46, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Apró, W.; Moberg, M.; Hamilton, D.L.; Ekblom, B.; Rooyackers, O.; Holmberg, H.C.; Blomstrand, E. Leucine does not affect mechanistic target of rapamycin complex 1 assembly but is required for maximal ribosomal protein s6 kinase 1 activity in human skeletal muscle following resistance exercise. FASEB J. 2015, 29, 4358–4373. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.K.; Nilsson, P.A.; Nilsson, J.; Chibalin, A.V.; Zierath, J.R.; Blomstrand, E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1–E7. [Google Scholar] [CrossRef]
- Koopman, R.; Wagenmakers, A.J.; Manders, R.J.; Zorenc, A.H.; Senden, J.M.; Gorselink, M.; Keizer, H.A.; van Loon, L.J. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E645–E653. [Google Scholar] [CrossRef]
- Moore, D.R.; Atherton, P.J.; Rennie, M.J.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol. 2011, 201, 365–372. [Google Scholar] [CrossRef]
- Dreyer, H.C.; Drummond, M.J.; Pennings, B.; Fujita, S.; Glynn, E.L.; Chinkes, D.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E392–E400. [Google Scholar] [CrossRef]
- Symons, T.B.; Sheffield-Moore, M.; Mamerow, M.M.; Wolfe, R.R.; Paddon-Jones, D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J. Nutr. Health Aging 2011, 15, 376–381. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef]
- Tipton, K.D.; Rasmussen, B.B.; Miller, S.L.; Wolf, S.E.; Owens-Stovall, S.K.; Petrini, B.E.; Wolfe, R.R. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E197–E206. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Macdonald, M.J.; Macdonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. (1985) 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Hartman, J.W.; Wilkinson, S.B. Dietary Protein to Support Anabolism with Resistance Exercise in Young Men. J. Am. Coll. Nutr. 2005, 24, 134S–139S. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Payette, H.; Morais, J.A.; Shatenstein, B.; Gaudreau, P.; Chevalier, S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: The Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). Am. J. Clin. Nutr. 2017, 106, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Morais, J.A.; Payette, H.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K.; Chevalier, S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am. J. Clin. Nutr. 2016, 104, 694–703. [Google Scholar] [CrossRef]
- Wirunsawanya, K.; Upala, S.; Jaruvongvanich, V.; Sanguankeo, A. Whey Protein Supplementation Improves Body Composition and Cardiovascular Risk Factors in Overweight and Obese Patients: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2018, 37, 60–70. [Google Scholar] [CrossRef]
- Baum, J.I.; Kim, I.Y.; Wolfe, R.R. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients 2016, 8, 359. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Engelen, M.P.K.J.; Rutten, E.P.A.; De Castro, C.L.N.; Wouters, E.F.M.; Schols, A.M.W.J.; Deutz, N.E.P. Casein protein results in higher prandial and exercise induced whole body protein anabolism than whey protein in Chronic Obstructive Pulmonary Disease. Metabolism 2012, 61, 1289–1300. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Hamer, H.M.; de Lange, A.; Kiskini, A.; Groen, B.B.; Senden, J.M.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Elliott, T.A.; Ferrando, A.A.; Aarsland, A.A.; Wolfe, R.R. Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl. Physiol. Nutr. Metab. 2009, 34, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Jonker, R.; Deutz, N.E.P.; Erbland, M.L.; Anderson, P.J.; Engelen, M.P.K.J. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin. Nutr. 2014, 33, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Jonker, R.; Deutz, N.E.P.; Schols, A.M.W.J.; Veley, E.A.; Harrykissoon, R.; Zachria, A.J.; Engelen, M.P.K.J. Whole body protein anabolism in COPD patients and healthy older adults is not enhanced by adding either carbohydrates or leucine to a serving of protein. Clin. Nutr. 2019, 38, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Choong, K.; Lakshman, K.M.; Bhasin, S. The physiological and pharmacological basis for the ergogenic effects of androgens in elite sports. Asian J. Androl. 2008, 10, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Thein, L.A.; Thein, J.M.; Landry, G.L. Ergogenic aids. Phys. Ther. 1995, 75, 426–439. [Google Scholar] [CrossRef]
- Andrews, M.A.; Magee, C.D.; Combest, T.M.; Allard, R.J.; Douglas, K.M. Physical Effects of Anabolic-androgenic Steroids in Healthy Exercising Adults: A Systematic Review and Meta-analysis. Curr. Sports Med. Rep. 2018, 17, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, M.; Spruit, M.A.; Verleden, G.; Kasran, A.; Van Herck, E.; Pitta, F.; Bouillon, R.; Decramer, M. Hypogonadism, quadriceps weakness, and exercise intolerance in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 172, 1105–1111. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, C.; Du, J.; Lan, G.; Du, X.; Sun, Y.; Shi, G. Anabolic-androgenic steroids for patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Front. Med. 2022, 9, 915159. [Google Scholar] [CrossRef]
- Creutzberg, E.C.; Wouters, E.F.M.; Mostert, R.; Pluymers, R.J.; Schols, A.M.W.J. A Role for Anabolic Steroids in the Rehabilitation of Patients with COPD?*: A Double-Blind, Placebo-Controlled, Randomized Trial. Chest 2003, 124, 1733–1742. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Verreschi, I.T.; Nery, L.E.; Goldstein, R.S.; Zamel, N.; Brooks, D.; Jardim, J.R. The Influence of 6 Months of Oral Anabolic Steroids on Body Mass and Respiratory Muscles in Undernourished COPD Patients. Chest 1998, 114, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Passey, S.L.; Hansen, M.J.; Bozinovski, S.; McDonald, C.F.; Holland, A.E.; Vlahos, R. Emerging therapies for the treatment of skeletal muscle wasting in chronic obstructive pulmonary disease. Pharmacol. Ther. 2016, 166, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Onder, G.; Morley, J.E.; Gillette-Guyonet, S.; Abellan van Kan, G.; Vellas, B. Current and future pharmacologic treatment of sarcopenia. Clin. Geriatr. Med. 2011, 27, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Coleman, L.A.; Miller, R.; Rooks, D.S.; Laurent, D.; Petricoul, O.; Praestgaard, J.; Swan, T.; Wade, T.; Perry, R.G.; et al. Effect of Bimagrumab vs Placebo on Body Fat Mass among Adults with Type 2 Diabetes and Obesity: A Phase 2 Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2033457. [Google Scholar] [CrossRef]
- Kneppers, A.E.M.; Langen, R.C.J.; Gosker, H.R.; Verdijk, L.B.; Cebron Lipovec, N.; Leermakers, P.A.; Kelders, M.; de Theije, C.C.; Omersa, D.; Lainscak, M.; et al. Increased Myogenic and Protein Turnover Signaling in Skeletal Muscle of Chronic Obstructive Pulmonary Disease Patients with Sarcopenia. J. Am. Med. Dir. Assoc. 2017, 18, 637.e1–637.e11. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.R.; Chen, R.C. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir. Med. 2012, 106, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Man, W.D.; Natanek, S.A.; Riddoch-Contreras, J.; Lewis, A.; Marsh, G.S.; Kemp, P.R.; Polkey, M.I. Quadriceps myostatin expression in COPD. Eur. Respir. J. 2010, 36, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Polkey, M.I.; Praestgaard, J.; Berwick, A.; Franssen, F.M.E.; Singh, D.; Steiner, M.C.; Casaburi, R.; Tillmann, H.-C.; Lach-Trifilieff, E.; Roubenoff, R.; et al. Activin Type II Receptor Blockade for Treatment of Muscle Depletion in Chronic Obstructive Pulmonary Disease. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 313–320. [Google Scholar] [CrossRef]
- Kung, T.; Springer, J.; Doehner, W.; Anker, S.D.; von Haehling, S. Novel treatment approaches to cachexia and sarcopenia: Highlights from the 5th Cachexia Conference. Expert Opin. Investig. Drugs 2010, 19, 579–585. [Google Scholar] [CrossRef]
- Nagaya, N.; Itoh, T.; Murakami, S.; Oya, H.; Uematsu, M.; Miyatake, K.; Kangawa, K. Treatment of cachexia with ghrelin in patients with COPD. Chest 2005, 128, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Levinson, B.; Gertner, J. Randomized study of the efficacy and safety of SUN11031 (synthetic human ghrelin) in cachexia associated with chronic obstructive pulmonary disease. e-SPEN J. 2012, 7, e171–e175. [Google Scholar] [CrossRef]
- Miki, K.; Maekura, R.; Nagaya, N.; Nakazato, M.; Kimura, H.; Murakami, S.; Ohnishi, S.; Hiraga, T.; Miki, M.; Kitada, S.; et al. Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: A multicenter, randomized, double-blind, placebo-controlled trial. PLoS ONE 2012, 7, e35708. [Google Scholar] [CrossRef]
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Adv. Ther. 2021, 38, 2821–2839. [Google Scholar] [CrossRef]
- Zapatero, A.; Barba, R.; Ruiz, J.; Losa, J.E.; Plaza, S.; Canora, J.; Marco, J. Malnutrition and obesity: Influence in mortality and readmissions in chronic obstructive pulmonary disease patients. J. Hum. Nutr. Diet. 2013, 26 (Suppl. S1), 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hallin, R.; Gudmundsson, G.; Suppli Ulrik, C.; Nieminen, M.M.; Gislason, T.; Lindberg, E.; Brøndum, E.; Aine, T.; Bakke, P.; Janson, C. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir. Med. 2007, 101, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; von Haehling, S.; Doehner, W.; Sarc, I.; Jeric, T.; Ziherl, K.; Kosnik, M.; Anker, S.D.; Suskovic, S. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J. Cachexia Sarcopenia Muscle 2011, 2, 81–86. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Hasegawa, W.; Yasunaga, H.; Sunohara, M.; Jo, T.; Takami, K.; Matsui, H.; Fushimi, K.; Nagase, T. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Franssen, F.M.; O’Donnell, D.E.; Goossens, G.H.; Blaak, E.E.; Schols, A.M. Obesity and the lung: 5. Obesity and COPD. Thorax 2008, 63, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, R.F.; McDonald, V.M.; Gibson, P.G.; Scott, H.A.; Hensley, M.J.; MacDonald-Wicks, L.; Wood, L.G. The Impact of a Weight Loss Intervention on Diet Quality and Eating Behaviours in People with Obesity and COPD. Nutrients 2017, 9, 1147. [Google Scholar] [CrossRef]
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018, 10, 605. [Google Scholar] [CrossRef]
- Goisser, S.; Kemmler, W.; Porzel, S.; Volkert, D.; Sieber, C.C.; Bollheimer, L.C.; Freiberger, E. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons—A narrative review. Clin. Interv. Aging 2015, 10, 1267–1282. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Gonzalez, M.C.; Shen, W.; Redman, L.; Thomas, D. Weight loss composition is one-fourth fat-free mass: A critical review and critique of this widely cited rule. Obes. Rev. 2014, 15, 310–321. [Google Scholar] [CrossRef]
- Darmon, P. Intentional weight loss in older adults: Useful or wasting disease generating strategy? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 284–289. [Google Scholar] [CrossRef]
- Waters, D.L.; Ward, A.L.; Villareal, D.T. Weight loss in obese adults 65years and older: A review of the controversy. Exp. Gerontol. 2013, 48, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Christensen, M.; Junker, A.E.; Knop, F.K.; Gluud, L.L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ 2012, 344, d7771. [Google Scholar] [CrossRef] [PubMed]
- Altintas Dogan, A.D.; Hilberg, O.; Hess, S.; Jensen, T.T.; Bladbjerg, E.-M.; Juhl, C.B. Respiratory Effects of Treatment with a Glucagon-Like Peptide-1 Receptor Agonist in Patients Suffering from Obesity and Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mei, A.; Qian, H.; Li, D.; Xu, H.; Chen, J.; Yang, H.; Min, X.; Li, C.; Cheng, L.; et al. The Role of Glucagon-Like Peptide-1 Receptor Agonists in Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Practical considerations for bicarbonate loading and sports performance. Nestle Nutr. Inst. Workshop Ser. 2013, 75, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Rodriguez, R.F.; Garofolini, A.; Saunders, B.; Bishop, D.J.; Schoenfeld, B.J.; Pedisic, Z. Effects of Sodium Bicarbonate Supplementation on Muscular Strength and Endurance: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1361–1375. [Google Scholar] [CrossRef]
- Coppoolse, R.; Barstow, T.J.; Stringer, W.W.; Carithers, E.; Casaburi, R. Effect of acute bicarbonate administration on exercise responses of COPD patients. Med. Sci. Sports Exerc. 1997, 29, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.A.; Bishop, D.J. Effects of Dietary Supplements on Adaptations to Endurance Training. Sports Med. 2020, 50, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Yfanti, C.; Fischer, C.P.; Nielsen, S.; Åkerström, T.; Nielsen, A.R.; Veskoukis, A.S.; Kouretas, D.; Lykkesfeldt, J.; Pilegaard, H.; Pedersen, B.K. Role of vitamin C and E supplementation on IL-6 in response to training. J. Appl. Physiol. 2012, 112, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Jeffries, O.; Stevenson, E.J.; Davies, K.A.B. The effects of vitamin C and E on exercise-induced physiological adaptations: A systematic review and Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ferrán, M.; Berlanga, L.A.; Barcelo-Guido, O.; Matos-Duarte, M.; Vicente-Campos, D.; Sánchez-Jorge, S.; Romero-Morales, C.; Munguía-Izquierdo, D.; Pareja-Galeano, H. Antioxidant vitamin supplementation on muscle adaptations to resistance training: A double-blind, randomized controlled trial. Nutrition 2023, 105, 111848. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, A.; Holmes, M.; Papoutsi, E.; Panton, L.; Koutakis, P. Resistance Training, Antioxidant Status, and Antioxidant Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 539–547. [Google Scholar] [CrossRef]
- Bjørnsen, T.; Salvesen, S.; Berntsen, S.; Hetlelid, K.J.; Stea, T.H.; Lohne-Seiler, H.; Rohde, G.; Haraldstad, K.; Raastad, T.; Køpp, U.; et al. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand. J. Med. Sci. Sports 2016, 26, 755–763. [Google Scholar] [CrossRef]
- Cumming, K.T.; Raastad, T.; Holden, G.; Bastani, N.E.; Schneeberger, D.; Paronetto, M.P.; Mercatelli, N.; Østgaard, H.N.; Ugelstad, I.; Caporossi, D. Effects of vitamin C and E supplementation on endogenous antioxidant systems and heat shock proteins in response to endurance training. Physiol. Rep. 2014, 2, e12142. [Google Scholar] [CrossRef]
- Beijers, R.; Gosker, H.R.; Schols, A. Resveratrol for patients with chronic obstructive pulmonary disease: Hype or hope? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 138–144. [Google Scholar] [CrossRef]
- Wiciński, M.; Leis, K.; Szyperski, P.; Węclewicz, M.M.; Mazur, E.; Pawlak-Osińska, K. Impact of resveratrol on exercise performance: A review. Sci. Sports 2018, 33, 207–212. [Google Scholar] [CrossRef]
- Rahman, I. Antioxidant therapies in COPD. J. Chronic Obstr. Pulm. Dis. 2006, 1, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.P.; Liu, C.C.; Wang, H.F.; Bernard, J.R.; Huang, C.C.; Cheng, I.S. Oral Resveratrol supplementation attenuates exercise-induced Interleukin-6 but not Oxidative Stress after a high intensity cycling challenge in adults. Int. J. Med. Sci. 2021, 18, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Biensø, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Gliemann, L.; Biensø, R.; Schmidt, J.; Hellsten, Y.; Pilegaard, H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014, 592, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Scribbans, T.D.; Ma, J.K.; Edgett, B.A.; Vorobej, K.A.; Mitchell, A.S.; Zelt, J.G.; Simpson, C.A.; Quadrilatero, J.; Gurd, B.J. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl. Physiol. Nutr. Metab. 2014, 39, 1305–1313. [Google Scholar] [CrossRef]
- Beijers, R.J.; Gosker, H.R.; Sanders, K.J.; de Theije, C.; Kelders, M.; Clarke, G.; Cryan, J.F.; van den Borst, B.; Schols, A.M. Resveratrol and metabolic health in COPD: A proof-of-concept randomized controlled trial. Clin. Nutr. 2020, 39, 2989–2997. [Google Scholar] [CrossRef]
- Zanforlini, B.M.; Ceolin, C.; Trevisan, C.; Alessi, A.; Seccia, D.M.; Noale, M.; Maggi, S.; Guarnieri, G.; Vianello, A.; Sergi, G. Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin. Exp. Res. 2022, 34, 167–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, P.; Wang, R.; Mao, L.; He, K. Can Magnesium Enhance Exercise Performance? Nutrients 2017, 9, 946. [Google Scholar] [CrossRef]
- Schwarz, N.A.; Blahnik, Z.J.; Prahadeeswaran, S.; McKinley-Barnard, S.K.; Holden, S.L.; Waldhelm, A. (–)-Epicatechin Supplementation Inhibits Aerobic Adaptations to Cycling Exercise in Humans. Front. Nutr. 2018, 5, 132. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef]
- Gutiérrez-Salmeán, G.; Ortiz-Vilchis, P.; Vacaseydel, C.M.; Rubio-Gayosso, I.; Meaney, E.; Villarreal, F.; Ramírez-Sánchez, I.; Ceballos, G. Acute effects of an oral supplement of (-)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food Funct. 2014, 5, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Allgrove, J.; Farrell, E.; Gleeson, M.; Williamson, G.; Cooper, K. Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Daussin, F.N.; Heyman, E.; Burelle, Y. Effects of (−)-epicatechin on mitochondria. Nutr. Rev. 2020, 79, 25–41. [Google Scholar] [CrossRef]
- Schwarz, N.A.; Theodore, A.P.; Funderburg, B.R.; Waldhelm, A.; McKinley-Barnard, S.K.; Hudson, G.M. Acute (-)-Epicatechin Consumption: Effects on Local Vasodilation Following Resistance Exercise and High-Intensity Exercise Performance. Sports 2020, 8, 22. [Google Scholar] [CrossRef]
- Mafi, F.; Biglari, S.; Ghardashi Afousi, A.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef]
- Damgaard, M.V.; Treebak, J.T. What is really known about the effects of nicotinamide riboside supplementation in humans. Sci. Adv. 2023, 9, eadi4862. [Google Scholar] [CrossRef]
- Custodero, C.; Saini, S.K.; Shin, M.J.; Jeon, Y.K.; Christou, D.D.; McDermott, M.M.; Leeuwenburgh, C.; Anton, S.D.; Mankowski, R.T. Nicotinamide riboside—A missing piece in the puzzle of exercise therapy for older adults? Exp. Gerontol. 2020, 137, 110972. [Google Scholar] [CrossRef]
- Gray, E.L. The Effects of Acute Nicotinamide Riboside Supplementation on Substrate Utilisation and 5 km Time-Trial Performance. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2019. [Google Scholar]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Limb, M.C.; Phillips, B.E.; Lund, J.; Williams, J.P.; Brook, M.S.; Cegielski, J.; Philp, A.; Ashcroft, S.; et al. Impact of the calcium form of β-hydroxy-β-methylbutyrate upon human skeletal muscle protein metabolism. Clin. Nutr. 2018, 37, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.J.; Wyke, S.M.; Tisdale, M.J. Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate. Cancer Res. 2004, 64, 8731–8735. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Lau, K.J.; D’Souza, A.C.; Nunes, E.A. An umbrella review of systematic reviews of β-hydroxy-β-methyl butyrate supplementation in ageing and clinical practice. J. Cachexia Sarcopenia Muscle 2022, 13, 2265–2275. [Google Scholar] [CrossRef]
- Hsieh, L.; Chien, S.; Huang, M.; Tseng, H.; Chang, C. Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac. J. Clin. Nutr. 2006, 15, 544. [Google Scholar]
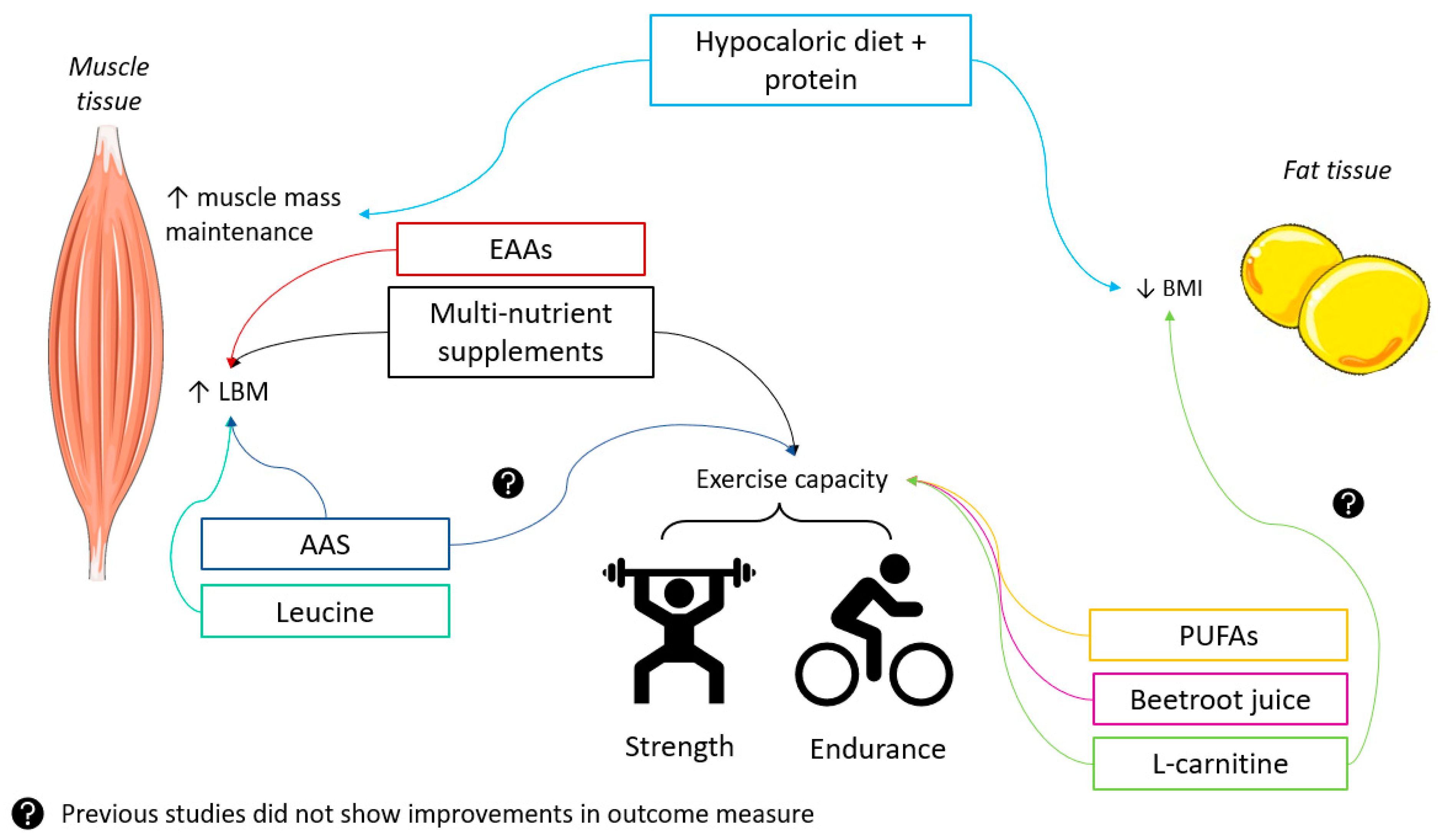
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauwers, B.; Machado, F.V.C.; Beijers, R.J.H.C.G.; Spruit, M.A.; Franssen, F.M.E. Combined Exercise Training and Nutritional Interventions or Pharmacological Treatments to Improve Exercise Capacity and Body Composition in Chronic Obstructive Pulmonary Disease: A Narrative Review. Nutrients 2023, 15, 5136. https://doi.org/10.3390/nu15245136
Brauwers B, Machado FVC, Beijers RJHCG, Spruit MA, Franssen FME. Combined Exercise Training and Nutritional Interventions or Pharmacological Treatments to Improve Exercise Capacity and Body Composition in Chronic Obstructive Pulmonary Disease: A Narrative Review. Nutrients. 2023; 15(24):5136. https://doi.org/10.3390/nu15245136
Chicago/Turabian StyleBrauwers, Bente, Felipe V. C. Machado, Rosanne J. H. C. G. Beijers, Martijn A. Spruit, and Frits M. E. Franssen. 2023. "Combined Exercise Training and Nutritional Interventions or Pharmacological Treatments to Improve Exercise Capacity and Body Composition in Chronic Obstructive Pulmonary Disease: A Narrative Review" Nutrients 15, no. 24: 5136. https://doi.org/10.3390/nu15245136
APA StyleBrauwers, B., Machado, F. V. C., Beijers, R. J. H. C. G., Spruit, M. A., & Franssen, F. M. E. (2023). Combined Exercise Training and Nutritional Interventions or Pharmacological Treatments to Improve Exercise Capacity and Body Composition in Chronic Obstructive Pulmonary Disease: A Narrative Review. Nutrients, 15(24), 5136. https://doi.org/10.3390/nu15245136





