Body Composition Evaluation and Clinical Markers of Cardiometabolic Risk in Patients with Phenylketonuria
Abstract
:1. Introduction
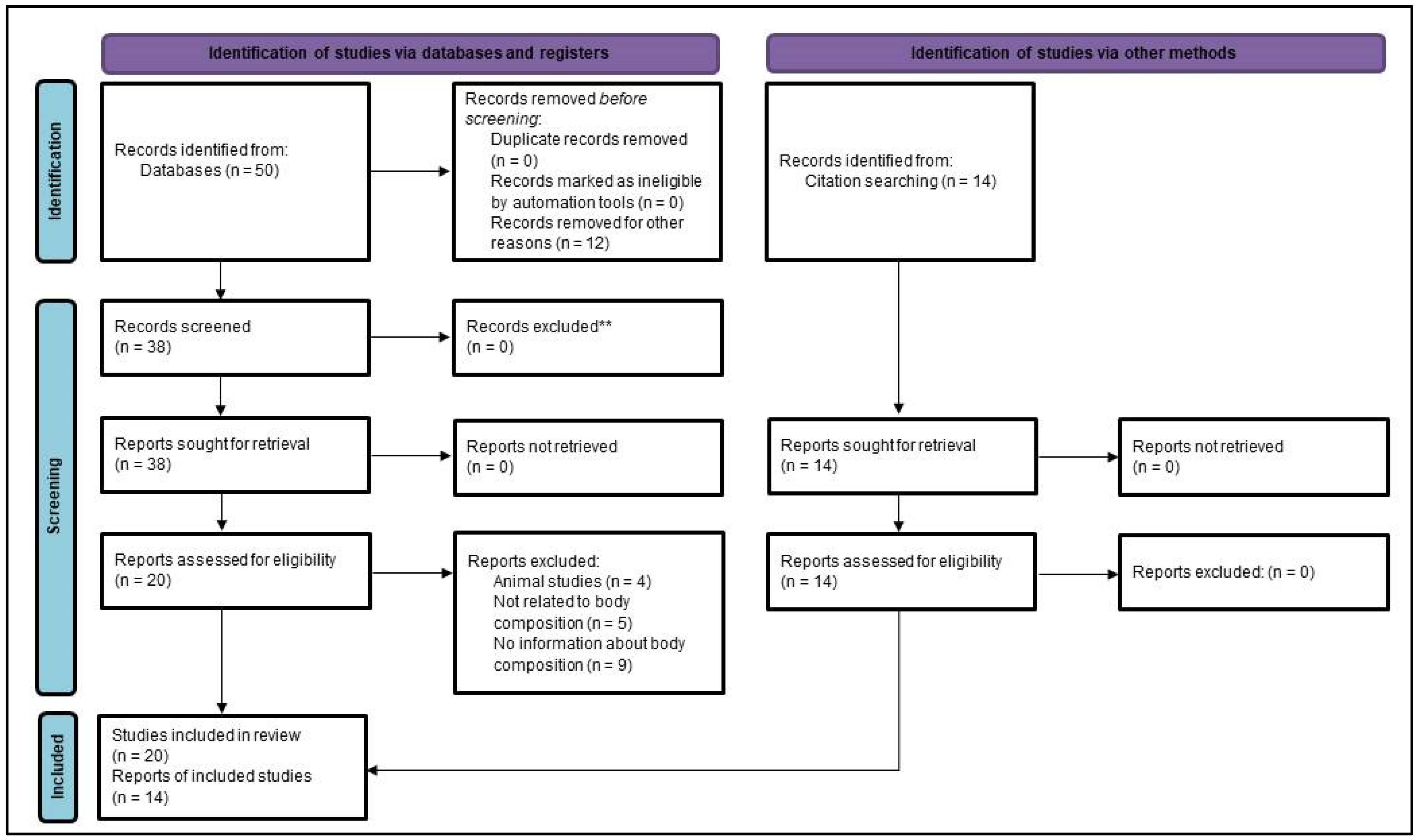
2. Materials and Methods
3. Results
3.1. Classical Clinical Markers of Cardiometabolic Risk
3.1.1. Anthropometric Markers
3.1.2. Metabolic Cardiovascular Risk Factors
3.2. Body Composition and Cardiometabolic Risk
3.2.1. Types of Adipose Tissue and Cardiometabolic Risk
3.2.2. Sarcopenia, Myosteatosis, and Cardiometabolic Risk
3.3. Techniques to Evaluate Quantity and Quality of Adipose and Muscle Tissues
3.3.1. Dual-Energy X-ray Absorptiometry
3.3.2. Bioelectrical Impedance Analysis
3.3.3. Computed Tomography
3.3.4. Magnetic Resonance Imaging
3.3.5. Air-Displacement Plethysmography
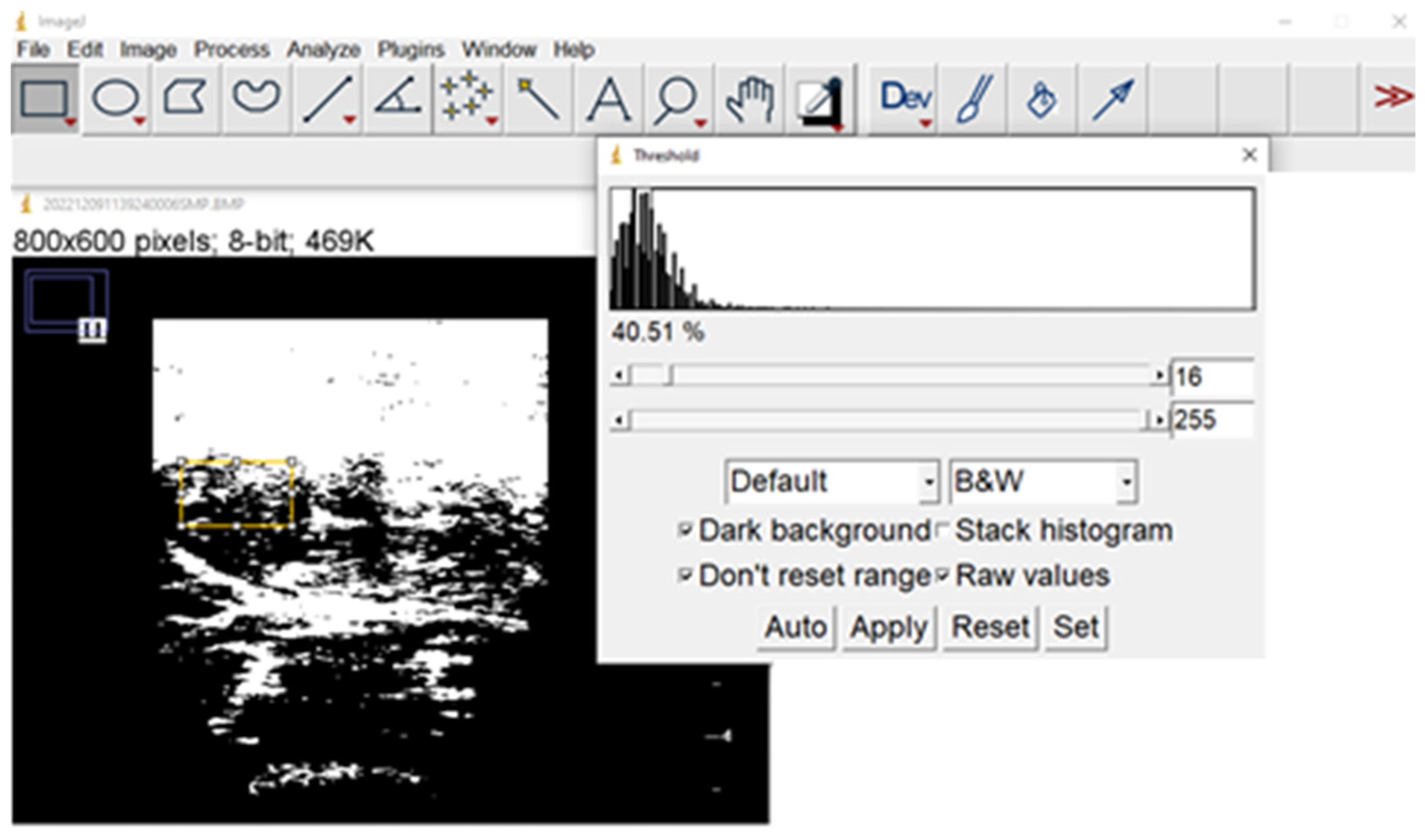
3.3.6. Ultrasound Imaging
Standard Nutritional Ultrasound® to Assess Malnutrition/Sarcopenia
Nutritional Ultrasound® to Assess Visceral Adiposity
Nutritional Ultrasound® to Assess Muscle Quality, including Ectopic Fat
- evaluate cardiometabolic risk, as fat infiltration of muscle is a kind of ectopic fat deposition, which is related to cardiometabolic and other complications, as shown above;
- evaluate muscle functionality, which deteriorates in senescence, frailty, and muscle diseases.
3.3.7. Comparison of Techniques to Evaluate Quantity and Quality of Adipose and Muscle Tissues in Patients with PKU
3.4. Cardiometabolic Risk in PKU Patients
3.4.1. Why Cardiometabolic Risk May Be Important in PKU Patients
3.4.2. Causes of Potential Increased Cardiometabolic Risk in Patients with PKU
Nutrition Therapy: Increased Intake of Carbohydrates and Body Composition
Nutrition Therapy: Chronic Exposition to Amino Acids
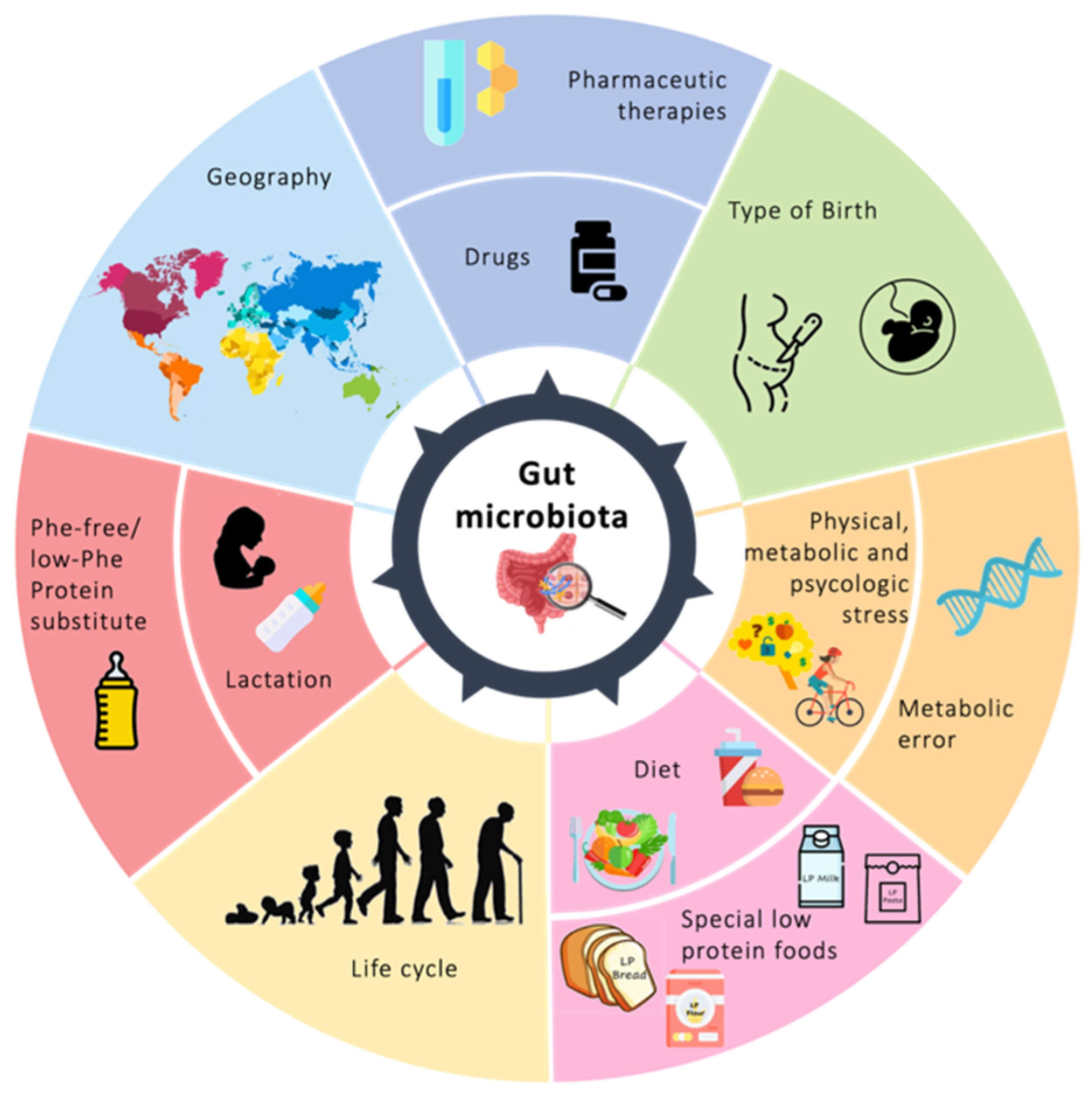
Nutrition Therapy: How It Modulates Microbiota and Microbioma Modulates Metabolism
3.4.3. Cardiometabolic Risk Factors in Patients with PKU
Body Composition
| Age Group | Method | Study | Findings |
|---|---|---|---|
| Pediatric/Mixed | BIA | Camatta 2020 (94, Brazil, 10–20, 14.0) [219] | +fat in: ♀, overweight, less protein intake |
| Tummolo 2022 (36, Italy, 11.4) [217] | +fat in non-compliant patients | ||
| Tummolo 2021 (30, Italy, 4.1–18, 10.8) [240] | fat in prepubertal > postpubertal | ||
| Evans 2017 (37/21, Australia, 0.6–18, 8.8) [222], Huemer 2007 (34, Austria, 8.7) [224], Dobbelaere 2003 (20, France, 0.6–7) [241] | Fat mass: PKU = general population | ||
| Bushueva 2015 (257, Russia, <18) [221] | Fat mass: PKU < general population | ||
| Sailer 2020 (30/30, USA, 5–18) [226] | Fat mass: PKU > general population | ||
| Pena 2021 (11, Portugal, 15–43, 28) [189], Pinto 2017 (11, Portugal, 27.0) [190], | Fat mass 25.5%−28.9%, phase angle 6.7–6.8 | ||
| Rocha 2013 (89/78, Portugal, 14.4) [245], Weng 2020 (22/22, Taiwan, 8–27, 15.2) [231] | Fat mass: PKU ≈ control group (20.74% vs. 18.67%) | ||
| DXA | Daly 2021 (48, UK, 5–15, 11.1) [242] | Fat mass 28−35% | |
| Adamczyk 2011 (45, Poland, 5–18, 13.8) [243] | Fat mass: PKU ≈ healthy population | ||
| Doulgeraki 2014 (80/50, Greece, 10.88) [232] | Fat mass: PKU = control group; + in high [Phe] | ||
| Jani 2017 (27, USA, 4–50, 16) [236] | FMI: children 5.0, adults 9.1 | ||
| Plethysmography | Albersen 2010 (20/20, Netherland, 6–16, 10.0) [228] | Fat mass: PKU 25.2%, control group 18.4% | |
| Neutron capture anal | Allen 1995 (30/65, Australia, 4–17, 9.6) [186], Allen 1996 (37/27, Australia, 3.9–11, 7.3) [244] | Protein: PKU < control, Fat mass: PKU = control | |
| Adults | BIA | Dios-Fuentes 2022 (90, Spain, 16–56, 29) [238] | Mean fat mass 24.6% (19.3–31%), obesity 34% |
| Barta 2022 (50/40, Hungary, 28.97) [235] | Fat mass: PKU ♂ + fat mass,—muscle,—protein and—mineral vs. controls | ||
| Deuterium | Alghamdi 2021 (16/15, UK, >10, 25.5) [181] | Fat mass: PKU 39.4% vs. control group 34.3% |
Metabolic Disturbances
| Age Group | Study | Findings |
|---|---|---|
| Pediatric/Mixed | de Almeida 2020 (84, Brazil, 2.4–19.9, 10.7) [216] | Low HDL 65.5%, 51.9% hypertriglyceridemia. More prevalent in Ow. |
| Tummolo 2022 (36, Italy, 11.4) [217] | Normal fasting glucose, triglycerides, and HDL | |
| Silveira 2022 (101, Brazil, 10–20, 14.8) [220] | -HDL, +triglycerides, +C-reactive protein in ow. HOMA-IR increased: 64.3% Ow. vs. 35.7% non-Ow. | |
| Kanufre 2015 (58, Brazil, 4–15, 9.1) [230] | -HDL, +triglycerides, +insulinemia in Ow. Basal insulinemia 3.8 μUI/mL in non-Ow. | |
| Rocha 2012 (89/79, Portugal, 3–30, 14.4) [182], Couce 2016 (141, Spain, 0.5–50, 15.5) [234], Couce 2018 (83/68, Spain, 4–52, 19.27) [233] | -HDL, +triglycerides, +BP in PKU vs. HPA. +homocysteine (+in ow.), +peptide C, +HOMA-IR,—QUICK index in PKU vs. HPA and control. Correlation with BMI, WC, and age. | |
| Adults | Azabdaftari 2019 (33/28, Germany, 18–47, 30.8) [237] | +BP, +heart rate, +cholesterol (total, LDL/HDL ratio), +inflammation, +oxidative stress, -HDL in PKU vs. control. Worse in poorly controlled. |
| Dios-Fuentes 2022 (90, Spain, 16–56, 29) [238] | High BP 7.9%, DM2 2.2%, hypercholesterolemia 15.6%, hypertriglyceridemia 17.8%, hyperhomocysteinemia 18.2% |
3.4.4. The Challenges of Analyzing Scientific Evidence in Rare Diseases
4. Discussion and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.; Pinto, A.; Faria, A.; Teixeira, D.; van Wegberg, A.M.J.; Ahring, K.; Feillet, F.; Calhau, C.; MacDonald, A.; Moreira-Rosário, A.; et al. Is the Phenylalanine-Restricted Diet a Risk Factor for Overweight or Obesity in Patients with Phenylketonuria (PKU)? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3443. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Evans, S.; Pinto, A.; Ashmore, C.; MacDonald, A. Protein Substitutes in PKU; Their Historical Evolution. Nutrients 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Almeida, M.F.; van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Gokmen-Ozel, H.; Lammardo, A.M.; MacDonald, A.; Robert, M.; et al. Special low protein foods for phenylketonuria: Availability in Europe and an examination of their nutritional profile. Orphanet J. Rare Dis. 2015, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Primers 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.M. Metabolic syndrome and cardiovascular risk. J. Fam. Community Med. 2010, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Oda, E. Historical perspectives of the metabolic syndrome. Clin. Dermatol. 2018, 36, 3–8. [Google Scholar] [CrossRef]
- Berg, G.; Miksztowicz, V.; Morales, C.; Barchuk, M. Epicardial Adipose Tissue in Cardiovascular Disease. Adv. Exp. Med. Biol. 2019, 1127, 131–143. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Pérez-Sousa, M.Á.; Izquierdo, M.; Cano-Gutierrez, C.A.; González-Jiménez, E.; Schmidt-RioValle, J.; González-Ruíz, K.; Correa-Rodríguez, M. Validation of Surrogate Anthropometric Indices in Older Adults: What Is the Best Indicator of High Cardiometabolic Risk Factor Clustering? Nutrients 2019, 11, 1701. [Google Scholar] [CrossRef]
- Javed, A.; Jumean, M.; Murad, M.H.; Okorodudu, D.; Kumar, S.; Somers, V.K.; Sochor, O.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: A systematic review and meta-analysis. Pediatr. Obes. 2014, 10, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Weig, T.; Irlbeck, T.; Frey, L.; Paprottka, P.; Irlbeck, M. Jenseits des BMI: Alternative Methoden zur Erfassung von Körperfett und Muskelmasse in der Intensivmedizin und deren klinischer Stellenwert [Above and beyond BMI: Alternative methods of measuring body fat and muscle mass in critically ill patients and their clinical significance]. Anaesthesist 2016, 65, 655–662. (In German) [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Tanamas, S.K.; Lean, M.E.J.; Combet, E.; Vlassopoulos, A.; Zimmet, P.Z.; Peeters, A. Changing guards: Time to move beyond body mass index for population monitoring of excess adiposity. QJM 2016, 109, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Frost, G.; Taylor-Robinson, S.D.; Bell, J.D. Excess body fat in obese and normal-weight subjects. Nutr. Res. Rev. 2012, 25, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Magliano, D.; Matsuzawa, Y.; Alberti, G.; Shaw, J. The metabolic syndrome: A global public health problem and a new definition. J. Atheroscler. Thromb. 2005, 12, 95–300. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Correia, M.I.T.D.; Heymsfield, S.B. A requiem for BMI in the clinical setting. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 314–321. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- Wei, M.; Gaskill, S.P.; Haffner, S.M.; Stern, M.P. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican American. A 7-year prospective study. Obes. Res. 1997, 5, 16–23. [Google Scholar] [CrossRef]
- Van der Kooy, K.; Leenen, R.; Seidell, J.C.; Deurenberg, P.; Visser, M. Abdominal diameters as indicators of visceral fat: Comparison between magnetic resonance imaging and anthropometry. Br. J. Nutr. 1993, 70, 47–58. [Google Scholar] [CrossRef] [PubMed]
- López De La Torre, M.; Bellido Guerrero, D.; Vidal Cortada, J.; Soto González, A.; García Malpartida, K.; Hernandez-Mijares, A. Distribución de la circunferencia de la cintura y de la relación circunferencia de la cintura con respecto a la talla según la categoría del índice de masa corporal en los pacientes atendidos en consultas de endocrinología y nutrición [Distribution of waist circumference and waist-to-height ratio by categories of body mass index in patients attended in endocrinology and nutrition units]. Endocrinol. Nut. 2010, 57, 479–485. (In Spanish) [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Chen, Y.; Yao, Y.; Zhang, Y.; Wang, N.; Liu, T.; Fu, C. Visceral adiposity measures are strongly associated with cardiovascular disease among female participants in Southwest China: A population-based prospective study. Front. Endocrinol. 2022, 13, 969753. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, A.M.; Rachubińska, K.; Skonieczna-Żydecka, K.; Drozd, A.; Pawlik, J.; Stachowska, E.; Cymbaluk-Płoska, A.; Grochans, E. Correlations between Fatty Acid Profile and Body Fat Distribution in Postmenopausal Women—A Cross Sectional Study. Nutrients 2022, 14, 3865. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Luengo Pérez, L.M.; Urbano Gálvez, J.M.; Pérez Miranda, M. Validación de índices antropométricos alternativos como marcadores del riesgo cardiovascular [Validation of alternative anthropometric indexes as cardiovascular risk markers]. Endocrinol. Nut. 2009, 56, 439–446. (In Spanish) [Google Scholar] [CrossRef]
- Browning, L.; Hsieh, S.; Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef]
- Rodea-Montero, E.R.; Evia-Viscarra, M.L.; Apolinar-Jiménez, E. Waist-to-Height Ratio Is a Better Anthropometric Index than Waist Circumference and BMI in Predicting Metabolic Syndrome among Obese Mexican Adolescents. Int. J. Endocrinol. 2014, 2014, 195407. [Google Scholar] [CrossRef]
- Hwaung, P.; Heo, M.; Kennedy, S.; Hong, S.; Thomas, D.M.; Shepherd, J.; Heymsfield, S.B. Optimum waist circumference-height indices for evaluating adult adiposity: An analytic review. Obes. Rev. 2020, 21, e12947. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, S.; Feng, Z.; Li, T.; Chu, J.; Hu, W.; Chen, X.; Han, Q.; Sun, N.; Sun, H.; et al. Association between the visceral adiposity index and risks of all-cause and cause-specific mortalities in a large cohort: Findings from the UK biobank. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Kalapur, S.; Singh, C. Correlation of Visceral Adiposity Index with Visceral Fat in Obese Patients with and without Type 2 Diabetes Mellitus. J. Assoc. Physicians. India 2022, 70, 11–12. [Google Scholar] [PubMed]
- Amato, M.C.; Giordano, C. Visceral adiposity index: An indicator of adipose tissue dysfunction. Int. J. Endocrinol. 2014, 2014, 730827. [Google Scholar] [CrossRef] [PubMed]
- Woolcott, O.O.; Bergman, R.N. Relative fat mass (RFM) as a new estimator of whole-body fat percentage—A cross-sectional study in American adult individuals. Sci. Rep. 2018, 8, 10980. [Google Scholar] [CrossRef] [PubMed]
- Senkus, K.E.; Crowe-White, K.M.; Locher, J.L.; Ard, J.D. Relative fat mass assessment estimates changes in adiposity among female older adults with obesity after a 12-month exercise and diet intervention. Ann. Med. 2022, 54, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Criminisi, A.; Sorek, N.; Heymsfield, S.B. Normalized sensitivity of multi-dimensional body composition biomarkers for risk change prediction. Sci. Rep. 2022, 12, 12375. [Google Scholar] [CrossRef]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A better index of body adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef]
- Bilgin Göçer, D.; Baş, M.; Çakır Biçer, N.; Hajhamidiasl, L. Predicting metabolic syndrome by visceral adiposity index, body roundness index, dysfunctional adiposity index, lipid accumulation product index, and body shape index in adults. Nutr. Hosp. 2022, 39, 794–802. (In English) [Google Scholar] [CrossRef]
- Zaragoza-García, O.; Gutiérrez-Pérez, I.A.; Delgado-Floody, P.; Parra-Rojas, I.; Jerez-Mayorga, D.; Campos-Jara, C.; Guzmán-Guzmán, I.P. Emergent Anthropometric Indices in Differential Prediction of Prehypertension and Hypertension in Mexican Population: Results according to Age and Sex. Int. J. Hypertens. 2022, 2022, 4522493. [Google Scholar] [CrossRef]
- Cerqueira, M.S.; Santos, C.A.D.; Silva, D.A.S.; Amorim, P.R.D.S.; Marins, J.C.B.; Franceschini, S.D.C.C. Validity of the Body Adiposity Index in Predicting Body Fat in Adults: A Systematic Review. Adv. Nutr. 2018, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Durnin, J.V.G.A.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 Years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Ros, F.; Moreira, A.C.; Vaquero-Cristóbal, R.; Barrigas, C.; Albaladejo-Saura, M.; Vieira, F. Differences between Four Skinfold Calipers in the Assessment of Adipose Tissue in Young Adult Healthy Population. Nutrients 2022, 14, 2085. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Velázquez Alva, M.C.; Irigoyen Camacho, M.E.; Lazarevich, I.; Delgadillo-Velázquez, J.; Acosta-Domínguez, P.; Cogordan Ramírez, A. Evaluación de la masa muscular a través de 2 indicadores antropométricos para la determinación de sarcopenia en ancianas. [Estimating muscle mass using two anthropometric indicators for diagnosing sarcopenia in the elderly]. Cienc. Clínicas 2014, 15, 47–54. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wang, Y.; Liu, Z.; Fang, Y.; Peng, Z.; Liu, W. Body Composition Measurement Improved Performance of GLIM Criteria in Diagnosing Malnutrition Compared to PG-SGA in Ambulatory Cancer Patients: A Prospective Cross-Sectional Study. Nutrients 2021, 13, 2744. [Google Scholar] [CrossRef] [PubMed]
- Fosbøl, M.Ø.; Zerahn, B. Contemporary methods of body composition measurement. Clin. Physiol. Funct. Imaging 2015, 35, 81–97. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Bellido-Castañeda, V.; Bellido-Guerrero, D. Nuevo enfoque de la nutrición. Valoración del estado nutricional del paciente: Función y composición corporal. [A new nutritional approach. Assessment of the patient’s nutritional status: Function and body composition.]. Nutr. Hosp. 2018, 35, 1–14. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Rodríguez-Leal, G.A.; Morán, S.; Gallardo, I.; Milke, P.; Guevara-González, L. Evaluación de la proteína C reactiva ultrasensible (PCR-us) como marcador de inflamación hepática en pacientes con síndrome metabólico [Assessment of high sensitivity C-reactive protein (HS-CRP) as a marker of liver inflammation in patients with metabolic syndrome]. Rev. Gastroenterol. Mex 2006, 71, 39–45. [Google Scholar] [PubMed]
- Koh, K.K.; Han, S.H.; Quon, M.J. Inflammatory markers and the metabolic syndrome: Insights from therapeutic interventions. J. Am. Coll Cardiol. 2005, 46, 1978. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; D’Agostino, R., Jr.; Tracy, R.P.; Haffner, S.M. Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes 2002, 51, 1131. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Zmuda, J.M. Epidemiology of myosteatosis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 260–264. [Google Scholar] [CrossRef]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts. 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Saxton, S.N.; Clark, B.J.; Withers, S.B.; Eringa, E.C.; Heagerty, A.M. Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiol. Rev. 2019, 99, 1701–1763. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Addison, O.; Marcus, R.L.; Lastayo, P.C.; Ryan, A.S. Intermuscular fat: A review of the consequences and causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Cespiati, A.; Meroni, M.; Lombardi, R.; Oberti, G.; Dongiovanni, P.; Fracanzani, A.L. Impact of Sarcopenia and Myosteatosis in Non-Cirrhotic Stages of Liver Diseases: Similarities and Differences across Aetiologies and Possible Therapeutic Strategies. Biomedicines 2022, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, D.W.; Ko, Y.; Ha, J.; Shin, Y.B.; Lee, J.; Sung, Y.S.; Kim, K.W. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia. Ageing Res. Rev. 2021, 70, 101398. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Joo, S.K.; Koo, B.K.; Lin, H.C.; Lee, D.H.; Chang, M.S.; Park, J.H.; So, Y.H.; Kim, W. Innovative Target Exploration of NAFLD (ITEN) Consortium. Myosteatosis, but not Sarcopenia, Predisposes NAFLD Subjects to Early Steatohepatitis and Fibrosis Progression. Clin. Gastroenterol. Hepatol. 2023, 21, 388–397.e10. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Roso, C.; Caviglia, G.P.; Ribaldone, D.G.; Bugianesi, E. The impact of dysmetabolic sarcopenia among insulin sensitive tissues: A narratie review. Front. Endocrinol. 2021, 12, 716533. [Google Scholar] [CrossRef] [PubMed]
- Altajar, S.; Baffy, G. Skeletal Muscle Dysfunction in the Development and Progression of Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2020, 8, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Carda, S.; Cisari, C.; Invernizzi, M. Sarcopenia or muscle modifications in neurologic diseases: A lexical or patophysiological difference? Eur. J. Phys. Rehabil. Med. 2013, 49, 119–130. [Google Scholar]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Srikanthan, P.; Horwich, T.B.; Tseng, C.H. Relation of Muscle Mass and Fat Mass to Cardiovascular Disease Mortality. Am. J. Cardiol. 2016, 117, 1355–1360. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, H.K.; Kim, E.H.; Bae, S.J.; Kim, K.W.; Kim, M.J.; Choe, J. Association Between Muscle Quality Measured by Abdominal Computed Tomography and Subclinical Coronary Atherosclerosis. Arter. Thromb. Vasc. Biol. 2021, 41, e128–e140. [Google Scholar] [CrossRef] [PubMed]
- Terry, J.G.; Shay, C.M.; Schreiner, P.J.; Jacobs, D.R., Jr.; Sanchez, O.A.; Reis, J.P.; Goff, D.C., Jr.; Gidding, S.S.; Steffen, L.M.; Carr, J.J. Intermuscular Adipose Tissue and Subclinical Coronary Artery Calcification in Midlife: The CARDIA Study (Coronary Artery Risk Development in Young Adults). Arter. Thromb. Vasc. Biol. 2017, 37, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, C.H. Quality Matters as Much as Quantity of Skeletal Muscle: Clinical Implications of Myosteatosis in Cardiometabolic Health. Endocrinol. Metab. 2021, 36, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Duren, D.L.; Sherwood, R.J.; Czerwinski, S.A.; Lee, M.; Choh, A.C.; Siervogel, R.M.; Cameron Chumlea, W. Body composition methods: Comparisons and interpretation. J. Diabetes Sci. Technol. 2008, 2, 1139–1146. [Google Scholar] [CrossRef]
- Wells, J.C.; Williams, J.E.; Chomtho, S.; Darch, T.; Grijalva-Eternod, C.; Kennedy, K.; Haroun, D.; Wilson, C.; Cole, T.J.; Fewtrell, M.S. Body-composition reference data for simple and reference techniques and a 4-component model: A new UK reference child. Am. J. Clin. Nutr. 2012, 96, 1316–1326. [Google Scholar] [CrossRef]
- Demerath, E.W.; Fields, D.A. Body composition assessment in the infant. Am. J. Hum. Biol. 2014, 26, 291–304. [Google Scholar] [CrossRef]
- Bianchi, M.L.; Baim, S.; Bishop, N.J.; Gordon, C.M.; Hans, D.B.; Langman, C.B.; Leonard, M.B.; Kalkwarf, H.J. Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr. Nephrol. 2010, 25, 37–47. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Binkley, N.; Morgan, S.L.; Shuhart, C.R.; Camargos, B.M.; Carey, J.J.; Gordon, C.M.; Jankowski, L.G.; Lee, J.K.; Leslie, W.D. International Society for Clinical Densitometry. Best Practices for Dual-Energy X-ray Absorptiometry Measurement and Reporting: International Society for Clinical Densitometry Guidance. J. Clin. Densitom. 2016, 19, 127–140. [Google Scholar] [CrossRef]
- Mazess, R.B.; Barden, H.S.; Bisek, J.P.; Hanson, J. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am. J. Clin. Nutr. 1990, 51, 1106–1112. [Google Scholar] [CrossRef]
- Mazess, R.B.; Barden, H.S.; Hanson, J.A. Body composition by dual-photon absorptiometry and dual-energy X-ray absorptiometry. Basic. Life Sci. 1990, 55, 427–432. [Google Scholar] [CrossRef]
- Wells, J.C.; Fewtrell, M.S.; Williams, J.E.; Haroun, D.; Lawson, M.S.; Cole, T.J. Body composition in normal weight, overweight and obese children: Matched case-control analyses of total and regional tissue masses, and body composition trends in relation to relative weight. Int. J. Obes. 2006, 30, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1993, 9, 480–491; discussion 480, 492. [Google Scholar] [PubMed]
- Williams, J.E.; Wells, J.C.; Wilson, C.M.; Haroun, D.; Lucas, A.; Fewtrell, M.S. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am. J. Clin. Nutr. 2006, 83, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Rothney, M.P.; Peters, D.M.; Wacker, W.K.; Davis, C.E.; Shapiro, M.D.; Ergun, D.L. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity 2012, 20, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Snijder, M.B.; Dekker, J.M.; Visser, M.; Bouter, L.M.; Stehouwer, C.D.; Yudkin, J.S.; Heine, R.J.; Nijpels, G.; Seidell, J.C.; Hoorn Study. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: The Hoorn study. Diabetes Care 2004, 27, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, N.J.; Kibirige, M.S.; Fordham, J.N.; Banks, L.M.; Muntoni, F.; Chinn, D.; Boivin, C.M.; Shaw, N.J. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone 2004, 35, 965–972. [Google Scholar] [CrossRef]
- Córdoba-Rodríguez, D.P.; Iglesia, I.; Gomez-Bruton, A.; Rodríguez, G.; Casajús, J.A.; Morales-Devia, H.; Moreno, L.A. Fat-free/lean body mass in children with insulin resistance or metabolic syndrome: A systematic review and meta-analysis. BMC Pediatr. 2022, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Holten, M.K.; Zacho, M.; Gaster, M.; Juel, C.; Wojtaszewski, J.F.; Dela, F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 2004, 53, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Ceniccola, G.D.; Castro, M.G.; Piovacari, S.M.F.; Horie, L.M.; Corrêa, F.G.; Barrere, A.P.N.; Toledo, D.O. Current technologies in body composition assessment: Advantages and disadvantages. Nutrition 2019, 62, 25–31. [Google Scholar] [CrossRef]
- Camm, C.F.; Lacey, B.; Massa, M.S.; Von Ende, A.; Gajendragadkar, P.; Stiby, A.; Valdes-Marquez, E.; Lewington, S.; Wijesurendra, R.; Parish, S.; et al. Independent effects of adiposity measures on risk of atrial fibrillation in men and women: A study of 0.5 million individuals. Int. J. Epidemiol. 2022, 51, 984–995. [Google Scholar] [CrossRef]
- Price, K.L.; Earthman, C.P. Update on body composition tools in clinical settings: Computed tomography, ultrasound, and bioimpedance applications for assessment and monitoring. Eur. J. Clin. Nutr. 2019, 73, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Becroft, L.; Ooi, G.; Forsyth, A.; King, S.; Tierney, A. Validity of multi-frequency bioelectric impedance methods to measure body composition in obese patients: A systematic review. Int. J. Obes. 2019, 43, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Stapel, S.N.; Looijaard, W.G.P.M.; Dekker, I.M.; Girbes, A.R.J.; Weijs, P.J.M.; Oudemans-van Straaten, H.M. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur. J. Clin. Nutr. 2018, 72, 1019–1025. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Vegas-Aguilar, I.M.; García-Almeida, J.M.; Bellido-Guerrero, D.; Talluri, A.; Lukaski, H.; Tinahones, F.J. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: A longitudinal cohort study. Clin. Nutr. 2021, 17, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- de Borba, E.L.; Ceolin, J.; Ziegelmann, P.K.; Bodanese, L.C.; Gonçalves, M.R.; Cañon-Montañez, W.; Mattiello, R. Phase angle of bioimpedance at 50 kHz is associated with cardiovascular diseases: Systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.; Berdin, G.; Virzì, G.M.; Mason, G.; Piccinni, P.; Day, S.; Cruz, D.N.; Wjewodzka, M.; Giuliani, A.; Brendolan, A.; et al. Fluid management in the intensive care unit: Bioelectrical impedance vector analysis as a tool to assess hydration status and optimal fluid balance in critically ill patients. Blood Purif. 2013, 36, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Kusakabe, T.; Arai, H.; Yamamoto, Y.; Nakao, K.; Ikeue, K.; Ishihara, Y.; Tagami, T.; Yasoda, A.; Ishii, K.; et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J. Cachexia Sarcopenia Muscle 2022, 13, 180–189. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Core Leadership Committee, GLIM Working Group. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Amini, B.; Boyle, S.P.; Boutin, R.D.; Lenchik, L. Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography: A Systematic Review. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1671–1678. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Gitto, S.; Ruffo, G.; Guarino, S.; Del Grando, F.; Sconfienza, L.M. Sarcopenia: Imaging assessment and clinical application. Abdom. Radiol. 2022, 47, 3205–3216. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Perez, S.L.; Haus, J.M.; Sheean, P.; Patel, B.; Mar, W.; Chaudhry, V.; McKeever, L.; Braunschweig, C. Measuring Abdominal Circumference and Skeletal Muscle From a Single Cross-Sectional Computed Tomography Image: A Step-by-Step Guide for Clinicians Using National Institutes of Health ImageJ. JPEN J. Parenter Enteral. Nutr. 2016, 40, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.; Matthews, K.; Janssen, I.; Brooks, M.M.; Barinas-Mitchell, E.; Orchard, T.J.; Billheimer, J.; Wang, N.C.; McConnell, D.; Rader, D.J.; et al. Associations of Abdominal and Cardiovascular Adipose Tissue Depots With HDL Metrics in Midlife Women: The SWAN Study. J. Clin. Endocrinol. Metab. 2022, 107, e2245–e2257. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Luo, L.; Fu, H.; Xie, L.; Zhang, W.; Lu, J.; Yang, M. Chest computed tomography-derived muscle mass and quality indicators, in-hospital outcomes, and costs in older inpatients. J. Cachexia Sarcopenia Muscle 2022, 13, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.K. A Comparison of Peripheral Imaging Technologies for Bone and Muscle Quantification: A Mixed Methods Clinical Review. Curr. Osteoporos. Rep. 2016, 14, 359–373. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Hu, H.H.; Chen, J.; Shen, W. Segmentation and quantification of adipose tissue by magnetic resonance imaging. Magn. Reson. Mater. Phys. Biol. Med. 2016, 29, 259–276. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Müller, M.J. Assessment of fat and lean mass by quantitative magnetic resonance: A future technology of body composition research? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 446–451. [Google Scholar] [CrossRef]
- Inoue, T.; Kozawa, E.; Ishikawa, M.; Okada, H. Application of Magnetic Resonance Imaging in the Evaluation of Nutritional Status: A Literature Review with Focus on Dialysis Patients. Nutrients 2021, 13, 2037. [Google Scholar] [CrossRef]
- Lemos, T.; Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J.; Yao, M.; Shypailo, R.J.; Urlando, A.; Wong, W.W.; Heird, W.C. Body-composition assessment in infancy: Air-displacement plethysmography compared with a reference 4-compartment model. Am. J. Clin. Nutr. 2007, 85, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Sainz, R.D.; Urlando, A. Evaluation of a new pediatric air-displacement plethysmograph for body-composition assessment by means of chemical analysis of bovine tissue phantoms. Am. J. Clin. Nutr. 2003, 77, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yao, M.; Liu, Y.; Lin, A.; Zou, H.; Urlando, A.; Wong, W.W.; Nommsen-Rivers, L.; Dewey, K.G. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am. J. Clin. Nutr. 2004, 79, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Allison, D.B. Air-displacement plethysmography pediatric option in 2-6 years old using the four-compartment model as a criterion method. Obesity 2012, 20, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Norris, T.; Ramel, S.E.; Catalano, P.; Caoimh, C.N.; Roggero, P.; Murray, D.; Fields, D.A.; Demerath, E.W.; Johnson, W. New charts for the assessment of body composition, according to air-displacement plethysmography, at birth and across the first 6 mo of life. Am. J. Clin. Nutr. 2019, 109, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Hunter, G.R.; Goran, M.I. Validation of the BOD POD with hydrostatic weighing: Influence of body clothing. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 200–205. [Google Scholar] [CrossRef]
- Fields, D.A.; Goran, M.I.; McCrory, M.A. Body-composition assessment via air-displacement plethysmography in adults and children: A review. Am. J. Clin. Nutr. 2002, 75, 453–467. [Google Scholar] [CrossRef]
- Lockner, D.W.; Heyward, V.H.; Baumgartner, R.N.; Jenkins, K.A. Comparison of air-displacement plethysmography, hydrodensitometry, and dual X-ray absorptiometry for assessing body composition of children 10 to 18 years of age. Ann. N. Y. Acad. Sci. 2000, 904, 72–78. [Google Scholar] [CrossRef]
- Anderson, D.E. Reliability of air displacement plethysmography. J. Strength Cond. Res. 2007, 21, 169–172. [Google Scholar] [CrossRef]
- Noreen, E.E.; Lemon, P.W. Reliability of air displacement plethysmography in a large, heterogeneous sample. Med. Sci. Sports Exerc. 2006, 38, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C. Toward body composition reference data for infants, children, and adolescents. Adv. Nutr. 2014, 5, 320S–329S. [Google Scholar] [CrossRef] [PubMed]
- Pereira-da-Silva, L.; Virella, D. Accurate Direct Measures Are Required to Validate Derived Measures. Neonatology 2018, 113, 266. [Google Scholar] [CrossRef] [PubMed]
- Hassen, A.; Wilson, D.E.; Amin, V.R.; Rouse, G.H.; Hays, C.L. Predicting Percentage of Intramuscular Fat Using Two Types of Real-Time Ultrasound Equipment. J. Anim. Sci. 2001, 79, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bahelka, I.; Oravcová, M.; Peskovicová, J.; Tomka, J.; Hanusová, E.; Lahucký, R.; Demo, P. Comparison of accuracy of intramuscular fat prediction in live pigs using five different ultrasound intensity levels. Animals 2009, 3, 1205–1211. [Google Scholar] [CrossRef]
- Halim, M.H.A.; Buniyamin, N.; Shari, M.A.M.; Kassim, R.A.; Mohamad, Z. The use of ultrasound as a fat measurement sensor for the food industry: A review. In Proceedings of the 2013 IEEE International Conference on Smart Instrumentation, Measurement and Applications (ICSIMA), Kuala Lumpur, Malaysia, 25–27 November 2013; pp. 1–6. [Google Scholar] [CrossRef]
- Ponti, F.; De Cinque, A.; Fazio, N.; Napoli, A.; Guglielmi, G.; Bazzocchi, A. Ultrasound imaging, a stethoscope for body composition assessment. Quant Imaging Med. Surg. 2020, 10, 1699–1722. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, M.; Ballesteros-Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández-Medina, B.; de Luis-Román, D.A.; Bellido-Guerrero, D.; Bretón-Lesmes, I.; Tinahones-Madueño, F.J. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70, S74–S84. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Bellido-Castañeda, V.; Bellido-Guerrero, D. Morphofunctional assessment of patient’s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar] [CrossRef]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwée, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef]
- Utter, A.C.; Hager, M.E. Evaluation of Ultrasound in Assessing Body Composition of High School Wrestlers. Med. Sci. Sports Exerc. 2008, 40, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Sahathevan, S.; Khor, B.H.; Singh, B.K.S.; Sabatino, A.; Fiaccadori, E.; Daud, Z.A.M.; Ali, M.S.; Narayanan, S.S.; Tallman, D.; Chinna, K.; et al. Association of ultrasound-derives metrics of the quadriceps muscle with protein energy wasting in hemodialysis patients: A multicentre cross-sectional study. Nutrients 2020, 12, 3597. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.-Y.; Ong, S.P.; Ng, C.C.; Yap, C.S.L.; Engkasan, J.P.; Barakatun-Nisak, M.Y.; Heyland, D.K.; Hasan, M.S. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: A single-center prospective observational study. Clin. Nutr. 2021, 40, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.T.; Mourtzakis, M.; Day, A.; Leung, R.; Watharkar, S.; Kozar, R.; Earthman, C.; Kuchnia, A.; Dhaliwal, R.; Moisey, L.; et al. Validation of bedside ultrasound of muscle layer thickness of the quadriceps in the critically ill patient (VALIDUM Study). JPEN J. Parenteral. Enteral. Nutr. 2016, 41, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, W.; ter Beek, L.; Hobbelen, J.S.M.; van der Vaart, H.; Wempe, J.B.; van der Schans, C.P.; Jager-Wittenaar, H. The added value of ultrasound muscle measurements in patients with COPD: An exploratory study. Clin. Nutr. ESPEN 2019, 30, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Primo, D.; Izaola, O.; Gómez, J.J.L.; de Luis, D. Correlation of the Phase Angle with Muscle Ultrasound and Quality of Life in Obese Females. Dis. Markers 2022, 2022, 7165126. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Bunout, D.; Barrera, G.; de la Maza, M.P.; Henriquez, S.; Leiva, L.; Hirsch, S. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch. Gerontol. Geriatr. 2015, 61, 33–38. [Google Scholar] [CrossRef]
- Mateos-Angulo, A.; Galán-Mercant, A.; Cuesta-Vargas, A.I. Ultrasound Muscle Assessment and Nutritional Status in Institutionalized Older Adults: A Pilot Study. Nutrients 2019, 11, 1247. [Google Scholar] [CrossRef]
- Lv, S.; Ling, L.; Shi, H.; Chen, X.; Chen, S.; Zhu, S.; Lin, W.; Lv, R.; Ding, G. Application of Muscle Thickness and Quality Measured by Ultrasound in Frailty Assessment in China. Front. Med. 2022, 9, 859555. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.J.; Hur, K.Y.; Choi, S.H.; Ahn, C.W.; Lim, S.K.; Kim, K.R.; Lee, H.C.; Huh, K.B.; Cha, B.S. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am. J. Clin. Nutr. 2004, 79, 593–599. [Google Scholar] [CrossRef]
- Hamagawa, K.; Matsumura, Y.; Kubo, T.; Hayato, K.; Okawa, M.; Tanioka, K.; Yamasaki, N.; Kitaoka, H.; Yabe, T.; Nishinaga, M.; et al. Abdominal visceral fat thickness measured by ultrasonography predicts the presence and severity of coronary artery disease. Ultrasound Med. Biol. 2010, 36, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Alfuraih, A.M.; Tan, A.L.; O’Connor, P.; Emery, P.; Wakefield, R.J. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin. Exp. Res. 2019, 31, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Ruiz-Santana, S. Assessment of Muscle Wasting in Long-Stay ICU Patients Using a New Ultrasound Protocol. Nutrients 2018, 10, 1849. [Google Scholar] [CrossRef] [PubMed]
- Image, J. Image Processing and Analysis in Java. Available online: https://imagej.nih.gov/ij/index.html (accessed on 22 November 2022).
- Karapınar, M.; Atilla Ayyıldız, V.; Ünal, M.; Fırat, T. Ultrasound imaging of quadriceps muscle in patients with knee osteoarthritis: The test-retest and inter-rater reliability and concurrent validity of echo intensity measurement. Musculoskelet. Sci. Pract. 2021, 56, 102453. [Google Scholar] [CrossRef]
- Young, H.-J.; Jenkins, N.T.; Zhao, Q.; Mccully, K.K. Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve 2015, 52, 963–971. [Google Scholar] [CrossRef]
- Mirón Mombiela, R.; Vucetic, J.; Monllor, P.; Cárdenas-Herrán, J.S.; Taltavull de la Paz, P.; Borrás, C. Diagnostic Performance of Muscle Echo Intensity and Fractal Dimension for the Detection of Frailty Phenotype. Ultrason 2021, 43, 337–352. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; British Paediatric & Adolescent Bone Group. Bone densitometry in children assessed by dual x ray absorptiometry: Uses and pitfalls. Arch. Dis. Child 2003, 88, 795–798. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Baim, S.; Bilezikian, J.P.; Schousboe, J.T. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on Body Composition. J. Clin. Densitom. 2013, 16, 489–495. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Wang, L.; Fan, B.; Gilsanz, V.; Kalkwarf, H.J.; Lappe, J.; Lu, Y.; Hangartner, T.; Zemel, B.S.; Fredrick, M.; et al. Optimal monitoring time interval between DXA measures in children. J. Bone Miner Res. 2011, 26, 2745–2752. [Google Scholar] [CrossRef]
- van der Sluis, I.M.; de Ridder, M.A.; Boot, A.M.; Krenning, E.P.; de Muinck Keizer-Schrama, S.M. Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch. Dis. Child 2002, 87, 341–347. [Google Scholar] [CrossRef]
- Boot, A.M.; Bouquet, J.; de Ridder, M.A.; Krenning, E.P.; de Muinck Keizer-Schrama, S.M. Determinants of body composition measured by dual-energy X-ray absorptiometry in Dutch children and adolescents. Am. J. Clin. Nutr. 1997, 66, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Going, S.B.; Massett, M.P.; Hall, M.C. Detection of small changes in body composition by dual-energy X-ray absorptiometry. Am. J. Clin. Nutr. 1993, 57, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.G.; Forslund, A.; Sjödin, A.; Mallmin, H.; Hambraeus, L.; Ljunghall, S. Determination of body composition--a comparison of dual-energy X-ray absorptiometry and hydrodensitometry. Am. J. Clin. Nutr. 1993, 57, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Fewtrell, M.S. Is body composition important for paediatricians? Arch. Dis. Child 2008, 93, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Luordi, C.; Maddaloni, E.; Bizzarri, C.; Pedicelli, S.; Zampetti, S.; D’Onofri, L.; Moretti, C.; Cappa, M.; Buzzetti, R. Wrist circumference is a biomarker of adipose tissue dysfunction and cardiovascular risk in children with obesity. J. Endocrinol. Investig. 2020, 43, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K. Waist circumference to height ratio and left ventricular mass in children and adolescents. Cardiol. Young 2016, 26, 658–662. [Google Scholar] [CrossRef]
- Messiah, S.E.; Arheart, K.L.; Natale, R.A.; Hlaing, W.M.; Lipshultz, S.E.; Miller, T.L. BMI, waist circumference, and selected cardiovascular disease risk factors among preschool-age children. Obesity 2012, 20, 1942–1949. [Google Scholar] [CrossRef]
- Daniels, S.R.; Khoury, P.R.; Morrison, J.A. Utility of different measures of body fat distribution in children and adolescents. Am. J. Epidemiol. 2000, 152, 1179–1184. [Google Scholar] [CrossRef]
- Rimm, A.A.; Hartz, A.J.; Fischer, M.E. A weight shape index for assessing risk of disease in 44,820 women. J. Clin. Epidemiol. 1988, 41, 459–465. [Google Scholar] [CrossRef]
- Taylor, R.W.; Jones, I.E.; Williams, S.M.; Goulding, A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3-19 y. Am. J. Clin. Nutr. 2000, 72, 490–495. [Google Scholar] [CrossRef]
- Savva, S.C.; Tornaritis, M.; Savva, M.E.; Kourides, Y.; Panagi, A.; Silikiotou, N.; Georgiou, C.; Kafatos, A. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.D.; Jarrett, K.V.; Crawley, H.F. The development of waist circumference percentiles in British children aged 5.0-16.9 y. Eur. J. Clin. Nutr. 2001, 55, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Zong, X.; Kelishadi, R.; Litwin, M.; Hong, Y.M.; Poh, B.K.; Steffen, L.M.; Galcheva, S.V.; Herter-Aeberli, I.; Nawarycz, T.; et al. International Waist Circumference Percentile Cutoffs for Central Obesity in Children and Adolescents Aged 6 to 18 Years. J. Clin. Endocrinol. Metab. 2020, 105, e1569–e1583. [Google Scholar] [CrossRef] [PubMed]
- Ulijaszek, S.J.; Kerr, D.A. Anthropometric measurement error and the assessment of nutritional status. Br. J. Nutr. 1999, 82, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.J.; Hunger, J.M.; Nguyen-Cuu, J.; Wells, C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. Int. J. Obes. 2016, 40, 883–886. [Google Scholar] [CrossRef]
- Brantlov, S.; Jødal, L.; Lange, A.; Rittig, S.; Ward, L.C. Standardisation of bioelectrical impedance analysis for the estimation of body composition in healthy paediatric populations: A systematic review. J. Med. Eng. Technol. 2017, 41, 460–479. [Google Scholar] [CrossRef]
- Brantlov, S.; Ward, L.C.; Jødal, L.; Rittig, S.; Lange, A. Critical factors and their impact on bioelectrical impedance analysis in children: A review. J. Med. Eng. Technol. 2017, 41, 22–35. [Google Scholar] [CrossRef]
- Houtkooper, L.B.; Going, S.B.; Lohman, T.G.; Roche, A.F.; Van Loan, M. Bioelectrical impedance estimation of fat-free body mass in children and youth: A cross-validation study. J. Appl. Physiol. 1992, 72, 366–373. [Google Scholar] [CrossRef]
- Houtkooper, L.B.; Lohman, T.G.; Going, S.B.; Howell, W.H. Why bioelectrical impedance analysis should be used for estimating adiposity. Am. J. Clin. Nutr. 1996, 64 (Suppl. S3), 436S–448S. [Google Scholar] [CrossRef]
- Kyle, U.G.; Earthman, C.P.; Pichard, C.; Coss-Bu, J.A. Body composition during growth in children: Limitations and perspectives of bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2015, 69, 1298–1305. [Google Scholar] [CrossRef]
- Orsso, C.E.; Gonzalez, M.C.; Maisch, M.J.; Haqq, A.M.; Prado, C.M. Using bioelectrical impedance analysis in children and adolescents: Pressing issues. Eur. J. Clin. Nutr. 2022, 76, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Verney, J.; Schwartz, C.; Amiche, S.; Pereira, B.; Thivel, D. Comparisons of a Multi-Frequency Bioelectrical Impedance Analysis to the Dual-Energy X-Ray Absorptiometry Scan in Healthy Young Adults Depending on their Physical Activity Level. J. Hum. Kinet. 2015, 47, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.E.; Engelke, K.; Zemel, B.S.; Ward, K.A. International Society of Clinical Densitometry. Quantitative computer tomography in children and adolescents: The 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 258–274. [Google Scholar] [CrossRef]
- Ducher, G.; Daly, R.M.; Hill, B.; Eser, P.; Naughton, G.A.; Gravenmaker, K.J.; Seibel, M.J.; Javaid, A.; Telford, R.D.; Bass, S.L. Relationship between indices of adiposity obtained by peripheral quantitative computed tomography and dual-energy X-ray absorptiometry in pre-pubertal children. Ann. Hum. Biol. 2009, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Blew, R.M.; Lee, V.R.; Bea, J.W.; Hetherington-Rauth, M.C.; Galons, J.P.; Altbach, M.I.; Lohman, T.G.; Going, S.B. Validation of Peripheral Quantitative Computed Tomography-Derived Thigh Adipose Tissue Subcompartments in Young Girls Using a 3 T MRI Scanner. J. Clin. Densitom. 2018, 21, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, P.; Bedogni, G.; Moreno, L.A.; Goran, M.I.; Gutin, B.; Fox, K.R.; Peters, D.M.; Barbeau, P.; De Simone, M.; Pietrobelli, A. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int. J. Obes. 2006, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Marunowski, K.; Świętoń, D.; Bzyl, W.; Grzywińska, M.; Kaszubowski, M.; Bandosz, P.; Khrichenko, D.; Piskunowicz, M. MRI-Derived Subcutaneous and Visceral Adipose Tissue Reference Values for Children Aged 6 to Under 18 Years. Front. Nutr. 2021, 757274. [Google Scholar] [CrossRef]
- Rocha, J.C.; MacDonald, A.; Trefz, F. Is overweight an issue in phenylketonuria? Mol. Genet. Metab. 2013, 110, S18–S24. [Google Scholar] [CrossRef]
- Alghamdi, N.; Alfheeaid, H.; Cochrane, B.; Adam, S.; Galloway, P.; Cozens, A.; Preston, T.; Malkova, D.; Gerasimidis, K. Mechanisms of obesity in children and adults with phenylketonuria on contemporary treatment. Clin. Nutr. ESPEN 2021, 46, 539–543. [Google Scholar] [CrossRef]
- Rocha, J.C.; van Spronsen, F.J.; Almeida, M.F.; Soares, G.; Quelhas, D.; Ramos, E.; Guimarães, J.T.; Borges, N. Dietary treatment in phenylketonuria does not lead to increased risk of obesity or metabolic syndrome. Mol. Genet. Metab. 2012, 107, 659–663. [Google Scholar] [CrossRef]
- Després, J.P. Is visceral obesity the cause of the metabolic syndrome? Ann. Med. 2006, 38, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota-Gut-Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.R.; McCauley, J.C.; Waters, D.L.; O’Connor, J.; Roberts, D.C.; Gaskin, K.J. Resting energy expenditure in children with phenylketonuria. Am. J. Clin. Nutr. 1995, 62, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Daly, A.; Wildgoose, J.; Cochrane, B.; Chahal, S.; Ashmore, C.; Loveridge, N.; MacDonald, A. Growth, Protein and Energy Intake in Children with PKU Taking a Weaning Protein Substitute in the First Two Years of Life: A Case-Control Study. Nutrients 2019, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Moretti, F.; Pellegrini, N.; Salvatici, E.; Rovelli, V.; Banderali, G.; Radaelli, G.; Scazzina, F.; Giovannini, M.; Verduci, E. Dietary glycemic index, glycemic load and metabolic profile in children with phenylketonuria. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Pinto, A.; de Almeida, M.F.; de Sousa Barbosa, C.; Ramos, P.C.; Rocha, S.; Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; et al. Continuous use of glycomacropeptide in the nutritional management of patients with phenylketonuria: A clinical perspective. Orphanet. J. Rare Dis. 2021, 16, 84. [Google Scholar] [CrossRef]
- Pinto, A.; Almeida, M.F.; Ramos, P.C.; Rocha, S.; Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; MacDonald, A.; Rocha, J.C. Nutritional status in patients with phenylketonuria using glycomacropeptide as their major protein source. Eur. J. Clin. Nutr. 2017, 71, 1230–1234. [Google Scholar] [CrossRef]
- MacDonald, A.; Singh, R.H.; Rocha, J.C.; van Spronsen, F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2019, 32, 70–78. [Google Scholar] [CrossRef]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet. J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Kanufre, V.; Almeida, M.F.; Barbosa, C.S.; Carmona, C.; Bandeira, A.; Martins, E.; Rocha, S.; Guimas, A.; Ribeiro, R.; MacDonald, A.; et al. Metabolic Control of Patients with Phenylketonuria in a Portuguese Metabolic Centre Comparing Three Different Recommendations. Nutrients 2021, 13, 3118. [Google Scholar] [CrossRef] [PubMed]
- Hinault, C.; Van Obberghen, E.; Mothe-Satney, I. Role of amino acids in insulin signaling in adipocytes and their potential to decrease insulin resistance of adipose tissue. J. Nutr. Biochem. 2006, 17, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Jiang, L.; Li, T. Aberrant branched-chain amino acid catabolism in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 965899. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Moens de Hase, E.; Van Hul, M. Gut Microbiota and Host Metabolism: From Proof of Concept to Therapeutic Intervention. Microorganisms 2021, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Zmora, N.; Soffer, E.; Elinav, E. Transforming medicine with the microbiome. Sci. Transl. Med. 2019, 11, eaaw1815. [Google Scholar] [CrossRef]
- Marteau, P.; Doré, J. Dysbiosis. Gut Microbiota: A Full-Fledged Organ, 1st ed.; Marteau, P., Doré, J., Eds.; John Libbey Eurotext: Arcueil, France, 2017; pp. 97–101. [Google Scholar]
- Dong, P.; Feng, J.J.; Yan, D.Y.; Lyu, Y.J.; Xu, X. Early-life gut microbiome and cow’s milk allergy—A prospective case—Control 6-month follow-up study. Saudi. J. Biol. Sci. 2018, 25, 875–880. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Timmer, C.; Davids, M.; Nieuwdorp, M.; Levels, J.H.M.; Langendonk, J.G.; Breederveld, M.; Ahmadi Mozafari, N.; Langeveld, M. Differences in faecal microbiome composition between adult patients with UCD and PKU and healthy control subjects. Mol. Genet Metab. Rep. 2021, 29, 100794. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Shadike, Q.; Wang, M.; Jiang, H.; Liu, W.; Liu, J.; Tuerdi, R.; Zhou, W.; Li, L. A low abundance of genus Bacteroides in gut microbiota is negatively correlated with blood phenylalanine levels in Uygur patients with phenylketonuria. Transl. Pediatr. 2021, 10, 2521–2532. [Google Scholar] [CrossRef]
- Montanari, C.; Ceccarani, C.; Corsello, A.; Zuvadelli, J.; Ottaviano, E.; Dei Cas, M.; Banderali, G.; Zuccotti, G.; Borghi, E.; Verduci, E. Glycomacropeptide Safety and Its Effect on Gut Microbiota in Patients with Phenylketonuria: A Pilot Study. Nutrients 2022, 14, 1883. [Google Scholar] [CrossRef]
- Mancilla, V.J.; Mann, A.E.; Zhang, Y.; Allen, M.S. The Adult Phenylketonuria (PKU) Gut Microbiome. Microorganisms 2021, 9, 530. [Google Scholar] [CrossRef]
- Bassanini, G.; Ceccarani, C.; Borgo, F.; Severgnini, M.; Rovelli, V.; Morace, G.; Verduci, E.; Borghi, E. Phenylketonuria Diet Promotes Shifts in Firmicutes Populations. Front. Cell Infect. Microbiol. 2019, 9, 101. [Google Scholar] [CrossRef]
- Pinheiro de Oliveira, F.; Mendes, R.H.; Dobbler, P.T.; Mai, V.; Pylro, V.S.; Waugh, S.G.; Vairo, F.; Refosco, L.F.; Roesch, L.F.; Schwartz, I.V. Phenylketonuria and Gut Microbiota: A Controlled Study Based on Next-Generation Sequencing. PLoS ONE 2016, 11, e0157513. [Google Scholar] [CrossRef]
- McWhorter, N.; Dhillon, J.; Hoffman, J. Preliminary Investigation of Microbiome and Dietary Differences in Patients with Phenylketonuria on Enzyme Substitution Therapy Compared to Traditional Therapies. J. Acad. Nutr. Diet. 2022, 122, 1283–1295. [Google Scholar] [CrossRef]
- White, J.E.; Kronmal, R.A.; Acosta, P.B. Excess weight among children with phenylketonuria. J. Am. Coll. Nutr. 1982, 1, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, Z.D.; Boa-Sorte, N.; Leite, M.E.Q.; Toralles, M.B.P.; Amorim, T. Metabolic control and body composition of children and adolescents with phenylketonuria. Rev. Paul. Pediatr. 2021, 39, e2020095. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, B.N.F.; Laufer, J.A.; Mezzomo, T.R.; Shimada, N.C.; Furtado, I.H.F.; Dias, M.R.M.G.; Pereira, R.M. Nutritional and metabolic parameters of children and adolescents with phenylketonuria. Clin. Nutr. ESPEN 2020, 37, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tummolo, A.; Carella, R.; Paterno, G.; Bartolomeo, N.; Giotta, M.; Dicintio, A.; De Giovanni, D.; Fischetto, R. Body composition in adolescent PKU patients: Beyond fat mass. Children 2022, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, L.; Bueno, M.A.; Couce, M.L.; Lage, S.; Dalmau, J.; Vitoria, I.; Andrade, F.; Llarena, M.; Blasco, J.; Alcalde, C.; et al. Tetrahydrobiopterin therapy vs phenylalanine-restricted diet: Impact on growth in PKU. Mol. Genet Metab. 2013, 109, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Camatta, G.C.; Kanufre, V.C.; Alves, M.R.A.; Soares, R.D.L.; Norton, R.C.; de Aguiar, M.J.B.; Starling, A.L.P. Body fat percentage in adolescents with phenylketonuria and associated factors. Mol. Genet. Metab. Rep. 2020, 23, 100595. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.M.; Lima, P.L.; Alves, M.R.A.; Soares, R.D.L.; Kanufre, V.C.; Rodrigues, V.M.; Starling, A.L.P.; Norton, R.C.; Aguiar, M.J.B. Overweight/obesity in adolescents with phenylketonuria: Protective and predisposing factors. J. Pediatr. 2022, 98, 104–110. [Google Scholar] [CrossRef]
- Bushueva, T.V.; Borovik, T.E.; Ladodo, K.S.; Kuzenkova, L.M.; Maslova, O.I.; Gevorkyan, A.K. [Evaluation of physical development in children with classical phenylketonuria]. Vopr. Pitan. 2015, 84, 34–43. (In Russian) [Google Scholar]
- Evans, M.; Truby, H.; Boneh, A. The relationship between dietary intake, growth and body composition in Phenylketonuria. Mol. Genet Metab. 2017, 122, 36–42. [Google Scholar] [CrossRef]
- Belanger-Quintana, A.; Martínez-Pardo, M. Physical development in patients with phenylketonuria on dietary treatment: A retrospective study. Mol. Genet Metab. 2011, 104, 480–484. [Google Scholar] [CrossRef]
- Huemer, M.; Huemer, C.; Möslinger, D.; Huter, D.; Stöckler-Ipsiroglu, S. Growth and body composition in children with classical phenylketonuria: Results in 34 patients and review of the literature. J. Inherit. Metab. Dis. 2007, 30, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, P.N.; Nalin, T.; Castro, K.; van Rijn, M.; Derks, T.G.J.; Perry, I.D.S.; Mainieri, A.S.; Schwartz, I.V.D. Analysis of body composition and nutritional status in Brazilian phenylketonuria patients. Mol. Genet Metab. Rep. 2016, 6, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Sailer, M.; Elizondo, G.; Martin, J.; Harding, C.O.; Gillingham, M.B. Nutrient intake, body composition, and blood phenylalanine control in children with phenylketonuria compared to healthy controls. Mol. Genet Metab. Rep. 2020, 23, 100599. [Google Scholar] [CrossRef] [PubMed]
- Burrage, L.C.; McConnell, J.; Haesler, R.; O’Riordan, M.A.; Sutton, V.R.; Kerr, D.S.; McCandless, S.E. High prevalence of overweight and obesity in females with phenylketonuria. Mol. Genet Metab. 2012, 107, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Albersen, M.; Bonthuis, M.; de Roos, N.M.; van den Hurk, D.A.; Carbasius Weber, E.; Hendriks, M.M.; de Sain-van der Velden, M.G.; de Koning, T.J.; Visser, G. Whole body composition analysis by the BodPod air-displacement plethysmography method in children with phenylketonuria shows a higher body fat percentage. J. Inherit. Metab. Dis. 2010, 33 (Suppl. S3), S283–S288. [Google Scholar] [CrossRef]
- Aldámiz-Echevarría, L.; Bueno, M.A.; Couce, M.L.; Lage, S.; Dalmau, J.; Vitoria, I.; Andrade, F.; Blasco, J.; Alcalde, C.; Gil, D.; et al. Anthropometric characteristics and nutrition in a cohort of PAH-deficient patients. Clin. Nutr. 2014, 33, 702–717. [Google Scholar] [CrossRef] [PubMed]
- Kanufre, V.C.; Soares, R.D.; Alves, M.R.; Aguiar, M.J.; Starling, A.L.; Norton, R.C. Metabolic syndrome in children and adolescents with phenylketonuria. J. Pediatr. 2015, 91, 98–103. [Google Scholar] [CrossRef]
- Weng, H.L.; Yang, F.J.; Chen, P.R.; Hwu, W.L.; Lee, N.C.; Chien, Y.H. Dietary intake and nutritional status of patients with phenylketonuria in Taiwan. Sci. Rep. 2020, 10, 14537. [Google Scholar] [CrossRef]
- Doulgeraki, A.; Skarpalezou, A.; Theodosiadou, A.; Monopolis, I.; Schulpis, K. Body composition profile of young patients with phenylketonuria and mild hyperphenylalaninemia. Int. J. Endocrinol. Metab. 2014, 12, e16061. [Google Scholar] [CrossRef]
- Couce, M.L.; Sánchez-Pintos, P.; Vitoria, I.; de Castro, M.J.; Aldámiz-Echevarría, L.; Correcher, P.; Fernández-Marmiesse, A.; Roca, I.; Hermida, A.; Martínez-Olmos, M.A.; et al. Carbohydrate status in patients with phenylketonuria. Orphanet. J. Rare Dis. 2018, 13, 103. [Google Scholar] [CrossRef]
- Couce, M.L.; Vitoria, I.; Aldámiz-Echevarría, L.; Fernández-Marmiesse, A.; Roca, I.; Llarena, M.; Sánchez-Pintos, P.; Leis, R.; Hermida, A. Lipid profile status and their related factors in patients with Hyperphenylalaninaemia. Orphanet. J. Rare Dis. 2016, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Barta, A.G.; Becsei, D.; Kiss, E.; Sumánszki, C.; Simonová, E.; Reismann, P. The Impact of Phenylketonuria on Body Composition in Adults. Ann. Nutr. Metab. 2022, 78, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Jani, R.; Coakley, K.; Douglas, T.; Singh, R. Protein intake and physical activity are associated with body composition in individuals with phenylalanine hydroxylase deficiency. Mol. Genet Metab. 2017, 121, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Azabdaftari, A.; van der, G.; Schuchardt, M.; Hennermann, J.B.; Plöckinger, U.; Querfeld, U. The cardiovascular phenotype of adult patients with phenylketonuria. Orphanet. J. Rare Dis. 2019, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Dios-Fuentes, E.; Gonzalo Marin, M.; Remón-Ruiz, P.; Benitez Avila, R.; Bueno Delgado, M.A.; Blasco Alonso, J.; Doulatram Gamgaram, V.K.; Olveira, G.; Soto-Moreno, A.; Venegas-Moreno, E. Cardiometabolic and Nutritional Morbidities of a Large, Adult, PKU Cohort from Andalusia. Nutrients 2022, 14, 1311. [Google Scholar] [CrossRef] [PubMed]
- Tankeu, A.T.; Pavlidou, D.C.; Superti-Furga, A.; Gariani, K.; Tran, C. Overweight and obesity in adult patients with phenylketonuria: A systematic review. Orphanet. J. Rare Dis. 2023, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Tummolo, A.; Arico, M.; Pesce, S.; Fantasia, M.L.; Paterno, G.; Melpignano, L.; De Giovanni, D.; Faienza, M.F. Body Composition and Bone Mineral Quality in Phenylketonuria: Influence of Pubertal Development. J. Endocr. Disord. 2021, 7, 1045. Available online: https://austinpublishinggroup.com/endocrinology/fulltext/endocrinology-v7-id1045.pdf (accessed on 19 June 2023).
- Dobbelaere, D.; Michaud, L.; Debrabander, A.; Vanderbecken, S.; Gottrand, F.; Turck, D.; Farriaux, J.P. Evaluation of nutritional status and pathophysiology of growth retardation in patients with phenylketonuria. J. Inherit. Metab. Dis. 2003, 26, 1–11. [Google Scholar] [CrossRef]
- Daly, A.; Högler, W.; Crabtree, N.; Shaw, N.; Evans, S.; Pinto, A.; Jackson, R.; Strauss, B.J.; Wilcox, G.; Rocha, J.C.; et al. Growth and Body Composition in PKU Children-A Three-Year Prospective Study Comparing the Effects of L-Amino Acid to Glycomacropeptide Protein Substitutes. Nutrients 2021, 13, 1323. [Google Scholar] [CrossRef]
- Adamczyk, P.; Morawiec-Knysak, A.; Płudowski, P.; Banaszak, B.; Karpe, J.; Pluskiewicz, W. Bone metabolism and the muscle-bone relationship in children, adolescents and young adults with phenylketonuria. J. Bone Miner Metab. 2011, 29, 236–244. [Google Scholar] [CrossRef]
- Allen, J.R.; Baur, L.A.; Waters, D.L.; Humphries, I.R.; Allen, B.J.; Roberts, D.C.; Gaskin, K.J. Body protein in prepubertal children with phenylketonuria. Eur. J. Clin. Nutr. 1996, 50, 178–186. [Google Scholar] [PubMed]
- Rocha, J.C.; van Spronsen, F.J.; Almeida, M.F.; Ramos, E.; Guimarães, J.T.; Borges, N. Early dietary treated patients with phenylketonuria can achieve normal growth and body composition. Mol. Genet Metab. 2013, 110, S40–S43. [Google Scholar] [CrossRef] [PubMed]
- DeWard, S.J.; Wilson, A.; Bausell, H.; Volz, A.S.; Mooney, K. Practical aspects of recruitment and retention in clinical trials of rare genetic diseases: The phenylketonuria (PKU) experience. J. Genet Couns. 2014, 23, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Tingley, K.; Chow, A.; Pallone, N.; Smith, M.; Rahman, A.; Chakraborty, P.; Geraghty, M.T.; Irwin, J.; Tessier, L.; et al. Canadian Inherited Metabolic Diseases Research Network. Outcomes in pediatric studies of medium-chain acyl-coA dehydrogenase (MCAD) deficiency and phenylketonuria (PKU): A review. Orphanet. J. Rare Dis. 2020, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Tingley, K.; Chow, A.; Pallone, N.; Smith, M.; Chakraborty, P.; Geraghty, M.T.; Irwin, J.K.; Mitchell, J.J.; Stockler, S.; et al. Canadian Inherited Metabolic Diseases Research Network. Core Outcome Sets for Medium-Chain Acyl-CoA Dehydrogenase Deficiency and Phenylketonuria. Pediatrics 2021, 148, e2020037747. [Google Scholar] [CrossRef] [PubMed]
- Gagne, J.J.; Thompson, L.; O’Keefe, K.; Kesselheim, A.S. Innovative research methods for studying treatments for rare diseases: Methodological review. BMJ 2014, 349, g6802. [Google Scholar] [CrossRef] [PubMed]
- Whicher, D.; Philbin, S.; Aronson, N. An overview of the impact of rare disease characteristics on research methodology. Orphanet. J. Rare Dis. 2018, 13, 14. [Google Scholar] [CrossRef]
- Lauer, M.S.; D’Agostino, R.B., Sr. The randomized registry trial--the next disruptive technology in clinical research? N. Engl. J. Med. 2013, 369, 1579–1581. [Google Scholar] [CrossRef]
- MacDonald, A.; Rocha, J.C.; van Rijn, M.; Feillet, F. Nutrition in phenylketonuria. Mol. Genet Metab. 2011, 104, S10–S18. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Braun, W.; Geisler, C.; Norman, K.; Müller, M.J. Body composition and cardiometabolic health: The need for novel concepts. Eur. J. Clin. Nutr. 2018, 72, 638–644. [Google Scholar] [CrossRef]
- Linge, J.; Borga, M.; West, J.; Tuthill, T.; Miller, M.R.; Dumitriu, A.; Thomas, E.L.; Romu, T.; Tunón, P.; Bell, J.D.; et al. Body Composition Profiling in the UK Biobank Imaging Study. Obesity 2018, 26, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.A.; Delmonico, M.J.; Visser, M.; Boudreau, R.M.; Goodpaster, B.H.; Schwartz, A.V.; Simonsick, E.M.; Satterfield, S.; Harris, T.; Newman, A.B. Body composition explains sex differential in physical performance among older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 93–100. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luengo-Pérez, L.M.; Fernández-Bueso, M.; Ambrojo, A.; Guijarro, M.; Ferreira, A.C.; Pereira-da-Silva, L.; Moreira-Rosário, A.; Faria, A.; Calhau, C.; Daly, A.; et al. Body Composition Evaluation and Clinical Markers of Cardiometabolic Risk in Patients with Phenylketonuria. Nutrients 2023, 15, 5133. https://doi.org/10.3390/nu15245133
Luengo-Pérez LM, Fernández-Bueso M, Ambrojo A, Guijarro M, Ferreira AC, Pereira-da-Silva L, Moreira-Rosário A, Faria A, Calhau C, Daly A, et al. Body Composition Evaluation and Clinical Markers of Cardiometabolic Risk in Patients with Phenylketonuria. Nutrients. 2023; 15(24):5133. https://doi.org/10.3390/nu15245133
Chicago/Turabian StyleLuengo-Pérez, Luis M., Mercedes Fernández-Bueso, Ana Ambrojo, Marta Guijarro, Ana Cristina Ferreira, Luís Pereira-da-Silva, André Moreira-Rosário, Ana Faria, Conceição Calhau, Anne Daly, and et al. 2023. "Body Composition Evaluation and Clinical Markers of Cardiometabolic Risk in Patients with Phenylketonuria" Nutrients 15, no. 24: 5133. https://doi.org/10.3390/nu15245133
APA StyleLuengo-Pérez, L. M., Fernández-Bueso, M., Ambrojo, A., Guijarro, M., Ferreira, A. C., Pereira-da-Silva, L., Moreira-Rosário, A., Faria, A., Calhau, C., Daly, A., MacDonald, A., & Rocha, J. C. (2023). Body Composition Evaluation and Clinical Markers of Cardiometabolic Risk in Patients with Phenylketonuria. Nutrients, 15(24), 5133. https://doi.org/10.3390/nu15245133









