Peanut Allergy and Component-Resolved Diagnostics Possibilities—What Are the Benefits?
Abstract
1. Introduction
2. Peanuts-Allergens, Food Allergy Diagnosing and Component-Resolved Diagnostic
3. Early Peanut Exposure Prevention and Component-Resolved Diagnostic
4. Peanut-Induced Anaphylaxis and Component-Resolved Diagnostic
5. Peanut Immunotherapy and Component-Resolved Diagnostics
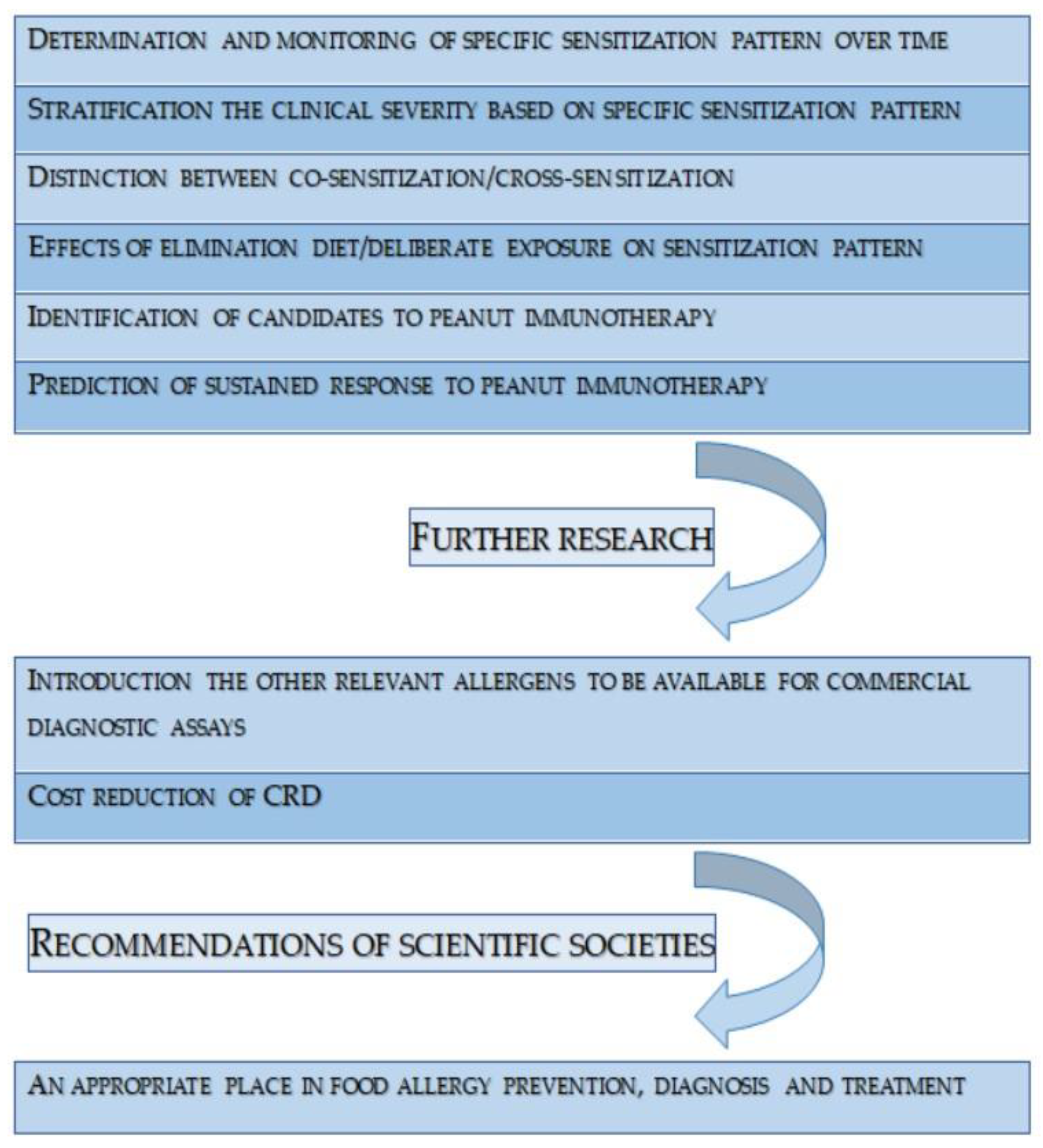
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lange, L.; Klimek, L.; Beyer, K.; Blümchen, K.; Novak, N.; Hamelmann, E.; Bauer, A.; Merk, H.; Rabe, U.; Jung, K.; et al. White paper on peanut allergy—Part 1: Epidemiology, burden of disease, health economic aspects. Allergo J. Int. 2021, 30, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Kim, E.H.; Nadeau, K.C.; Nowak-Wegrzyn, A.; Wood, R.A.; Sampson, H.A.; Scurlock, A.M.; Chinthrajah, S.; Wang, J.; Pesek, R.D.; et al. Immune Tolerance Network. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): A randomised placebo-controlled study. Lancet 2022, 399, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.A.; Clausen, M.; Knulst, A.C.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Fernandez-Perez, C.; Jedrzejczak-Czechowicz, M.; et al. Prevalence of Food Sensitization and Food Allergy in Children Across Europe. J. Allergy Clin. Immunol. Pract. 2020, 8, 2736–2746.e9. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.A.; Burney, P.G.J.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Clausen, M.; Dubakiene, R.; Fernandez-Perez, C.; Fritsche, P.; Jedrzejczak-Czechowicz, M.; et al. Food Allergy in Adults: Substantial Variation in Prevalence and Causative Foods Across Europe. J. Allergy Clin. Immunol. Pract. 2019, 7, 1920–1928.e11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Jobrack, J.; Cerenzia, W.; Tilles, S.; Ryan, R.; Sih-Meynier, R.; Zeitler, S.; Manning, M. A study to assess current approaches of allergists in European countries diagnosing and managing children and adolescents with peanut allergy. PLoS ONE 2020, 15, e0241648. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, V.L.; Koplin, J.J.; Field, M.J.; Sasaki, M.; Dharmage, S.C.; Tang, M.L.K.; Sawyer, S.M.; Peters, R.L.; Allen, K.J.; SchoolNuts Investigators. Self-reported adverse food reactions and anaphylaxis in the SchoolNuts study: A population-based study of adolescents. J. Allergy Clin. Immunol. 2018, 141, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Muñoz-Furlong, A.; Sampson, H.A. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 2001, 107, 191–193. [Google Scholar] [CrossRef]
- Bock, S.A.; Muñoz-Furlong, A.; Sampson, H.A. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J. Allergy Clin. Immunol. 2007, 119, 1016–1018. [Google Scholar] [CrossRef]
- Summers, C.W.; Pumphrey, R.S.; Woods, C.N.; McDowell, G.; Pemberton, P.W.; Arkwright, P.D. Factors predicting anaphylaxis to peanuts and tree nuts in patients referred to a specialist center. J. Allergy Clin. Immunol. 2008, 121, 632–638.e2. [Google Scholar] [CrossRef]
- Stiefel, G.; Anagnostou, K.; Boyle, R.J.; Brathwaite, N.; Ewan, P.; Fox, A.T.; Huber, P.; Luyt, D.; Till, S.J.; Venter, C.; et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin. Exp. Allergy 2017, 47, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Greenhawt, M.; Shaker, M.; Wang, J.; Oppenheimer, J.J.; Sicherer, S.; Keet, C.; Swaggart, K.; Rank, M.; Portnoy, J.M.; Bernstein, J.; et al. Peanut allergy diagnosis: A 2020 practice parameter update, systematic review, and GRADE analysis. J. Allergy Clin. Immunol. 2020, 146, 1302–1334. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Gerth van Wijk, R.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [PubMed]
- Hemmings, O.; Kwok, M.; McKendry, R.; Santos, A.F. Basophil activation test: Old and new applications in allergy. Curr. Allergy Asthma Rep. 2018, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Douiri, A.; Bécares, N.; Wu, S.Y.; Stephens, A.; Radulovic, S.; Chan, S.M.; Fox, A.T.; Du Toit, G.; Turcanu, V.; et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J. Allergy Clin. Immunol. 2014, 134, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Briceno Noriega, D.; Teodorowicz, M.; Savelkoul, H.; Ruinemans-Koerts, J. The Basophil Activation Test for Clinical Management of Food Allergies: Recent Advances and Future Directions. J. Asthma Allergy 2021, 14, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Du Toit, G.; O’Rourke, C.; Becares, N.; Couto-Francisco, N.; Radulovic, S.; Khaleva, E.; Basting, M.; Harris, K.M.; Larson, D.; et al. Biomarkers of severity and threshold of allergic reactions during oral peanut challenges. J. Allergy Clin. Immunol. 2020, 146, 344–355. [Google Scholar] [CrossRef]
- Santos, A.F.; Bergmann, M.; Brough, H.A.; Couto-Francisco, N.; Kwok, M.; Panetta, V.; Haddad, D.; Lack, G.; Eigenmann, P.; Caubet, J.C. Basophil Activation Test Reduces Oral Food Challenges to Nuts and Sesame. J. Allergy Clin. Immunol. Pract. 2021, 9, 2016–2027.e6. [Google Scholar] [CrossRef]
- WHO/IUIS Allergen Nomenclature. Allergen Nomenclature. 2021. Available online: http://www.allergen.org/search.php?allergenname=&allergensource=Arachis+hypogaea&TaxSource=&TaxOrder=&foodallerg=all&bioname= (accessed on 12 September 2023).
- Hoffmann-Sommergruber, K.; de las Vecillas, L.; Dramburg, S.; Hilger, C.; Santos, A. Molecular Allergology User’s Guide 2.0; The European Academy of Allergy and Clinical Immunology (EAACI): Zurich, Switzerland, 2022. [Google Scholar]
- Kiewiet, M.B.G.; Lupinek, C.; Vrtala, S.; Wieser, S.; Baar, A.; Kiss, R.; Kull, I.; Melén, E.; Wickman, M.; Porta, D.; et al. A molecular sensitization map of European children reveals exposome- and climate-dependent sensitization profiles. Allergy 2023, 78, 2007–2018. [Google Scholar] [CrossRef]
- Nilsson, C.; Berthold, M.; Mascialino, B.; Orme, M.; Sjölander, S.; Hamilton, R. Accuracy of component-resolved diagnostics in peanut allergy: Systematic literature review and meta-analysis. Pediatr. Allergy Immunol. 2020, 31, 303–314. [Google Scholar] [CrossRef]
- Beyer, K.; Grabenhenrich, L.; Härtl, M.; Beder, A.; Kalb, B.; Ziegert, M.; Finger, A.; Harandi, N.; Schlags, R.; Gappa, M.; et al. Predictive values of component-specific IgE for the outcome of peanut and hazelnut food challenges in children. Allergy 2015, 70, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Błażowski, Ł.; Kurzawa, R. ABC Diagnostyki Molekularnej w Alergologii; Część I–IV; Akademia Bebilon: Warsaw, Poland, 2022. [Google Scholar]
- Bublin, M.; Kostadinova, M.; Radauer, C.; Hafner, C.; Szépfalusi, Z.; Varga, E.M.; Maleki, S.J.; Hoffmann-Sommergruber, K.; Breiteneder, H. IgE cross-reactivity between the major peanut allergen Ara h 2 and the nonhomologous allergens Ara h 1 and Ara h 3. J. Allergy Clin. Immunol. 2013, 132, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, O.; Du Toit, G.; Radulovic, S.; Lack, G.; Santos, A.F. Ara h 2 is the dominant peanut allergen despite similarities with Ara h 6. J. Allergy Clin. Immunol. 2020, 146, 621–630.e5. [Google Scholar] [CrossRef] [PubMed]
- Glaumann, S.; Nilsson, C.; Johansson, S.G.; Asarnoj, A.; Wickman, M.; Borres, M.P.; Nopp, A. Evaluation of basophil allergen threshold sensitivity (CD-sens) to peanut and Ara h 8 in children IgE-sensitized to Ara h 8. Clin. Mol. Allergy 2015, 13, 5. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Ruano, L.; Fernández-Lozano, C.; Ferrer, M.; Gómez, F.; de la Hoz, B.; Martínez-Botas, J.; Goikoetxea, M.J. Differences in Linear Epitopes of Ara h 9 Recognition in Peanut Allergic and Tolerant, Peach Allergic Patients. Front. Allergy 2022, 3, 896617. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.T.; Palmer, L.K.; Koppelman, S.J.; Johnson, P.E. Determination of Allergen Levels, Isoforms, and Their Hydroxyproline Modifications Among Peanut Genotypes by Mass Spectrometry. Front. Allergy 2022, 3, 872714. [Google Scholar] [CrossRef] [PubMed]
- Brusca, I.; Barrale, M.; Onida, R.; La Chiusa, S.M.; Gjomarkaj, M.; Uasuf, C.G. The extract, the molecular allergen or both for the in vitro diagnosis of peach and peanut sensitization? Clin. Chim. Acta 2019, 493, 25–30. [Google Scholar] [CrossRef]
- Romano, A.; Scala, E.; Rumi, G.; Gaeta, F.; Caruso, C.; Alonzi, C.; Maggioletti, M.; Ferrara, R.; Palazzo, P.; Palmieri, V.; et al. Lipid transfer proteins: The most frequent sensitizer in Italian subjects with food-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2012, 42, 1643–1653. [Google Scholar] [CrossRef]
- Schwager, C.; Kull, S.; Behrends, J.; Röckendorf, N.; Schocker, F.; Frey, A.; Homann, A.; Becker, W.M.; Jappe, U. Peanut oleosins associated with severe peanut allergy-importance of lipophilic allergens for comprehensive allergy diagnostics. J. Allergy Clin. Immunol. 2017, 140, 1331–1338.e8. [Google Scholar] [CrossRef]
- Majsiak, E.; Choina, M.; Miśkiewicz, K.; Doniec, Z.; Kurzawa, R. Oleosins: A Short Allergy Review. Adv. Exp. Med. Biol. 2021, 1324, 51–55. [Google Scholar]
- Petersen, A.; Kull, S.; Rennert, S.; Becker, W.M.; Krause, S.; Ernst, M.; Gutsmann, T.; Bauer, J.; Lindner, B.; Jappe, U. Peanut defensins: Novel allergens isolated from lipophilic peanut extract. J. Allergy Clin. Immunol. 2015, 136, 1295–1301.e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, L.; Valcour, A.; Holmqvist, M.; Larsson, H.; Lidholm, J. Cyclophilin—A novel cross-reactive determinant in peanut. Clin. Exp. Allergy 2021, 51, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Klemans, R.J.; van Os-Medendorp, H.; Blankestijn, M.; Bruijnzeel-Koomen, C.A.; Knol, E.F.; Knulst, A.C. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: A systematic review. Clin. Exp. Allergy 2015, 45, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, H.; Nolan, R.; Pascoe, E.M.; Cuthbert, P.; Noble, V.; Corderoy, T.; Franzmann, A.; Loh, R.; Prescott, S.L. Skin prick testing and peanut-specific IgE can predict peanut challenge outcomes in preschoolchildren with peanut sensitization. Clin. Exp. Allergy 2011, 41, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- DesRoches, A.; Infante-Rivard, C.; Paradis, L.; Paradis, J.; Haddad, E. Peanut allergy: Is maternal transmission of antigens during pregnancy and breastfeeding a risk factor? J. Investig. Allergol. Clin. Immunol. 2010, 20, 289–294. [Google Scholar]
- Ben-Shoshan, M.; Kagan, R.S.; Alizadehfar, R.; Joseph, L.; Turnbull, E.; St Pierre, Y.; Clarke, A.E. Is the prevalence of peanut allergy increasing? A 5-year follow-up study in children in Montreal. J. Allergy Clin. Immunol. 2009, 123, 783–788. [Google Scholar] [CrossRef]
- Schocker, F.; Jappe, U. Breastfeeding: Maternally Transferred Allergens in Breast Milk: Protective or Sensitizing? Mol. Nutr. Food Res. 2022, 66, e2200066. [Google Scholar] [CrossRef]
- Azad, M.B.; Dharma, C.; Simons, E.; Tran, M.; Reyna, M.E.; Dai, R.; Becker, A.B.; Marshall, J.; Mandhane, P.J.; Turvey, S.E.; et al. Reduced peanut sensitization with maternal peanut consumption and early peanut introduction while breastfeeding. J. Dev. Orig. Health Dis. 2021, 12, 811–818. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. European Academy of Allergy and Clinical Immunology Food Allergy and Anaphylaxis Guidelines Group. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef]
- Kotsapas, C.; Nicolaou, N.; Haider, S.; Kerry, G.; Turner, P.J.; Murray, C.S.; Simpson, A.; Custovic, A. Early-life predictors and risk factors of peanut allergy, and its association with asthma in later-life: Population-based birth cohort study. Clin. Exp. Allergy 2022, 52, 646–657. [Google Scholar] [CrossRef]
- Grinek, S.; Suprun, M.; Raghunathan, R.; Tomalin, L.E.; Getts, R.; Bahnson, T.; Lack, G.; Sampson, H.A.; Suarez-Farinas, M. Epitope-Specific IgE at 1 Year of Age Can Predict Peanut Allergy Status at 5 Years. Int. Arch. Allergy Immunol. 2023, 184, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Ierodiakonou, D.; Garcia-Larsen, V.; Logan, A.; Groome, A.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Reeves, T.; et al. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Sicherer, S.; Greenhawt, M.; Campbell, D.; Chan, E.; Muraro, A.; Halken, S.; Katz, Y.; Ebisawa, M.; Eichenfield, L.; et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. J. Allergy Clin. Immunol. 2015, 136, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef]
- Grimshaw, K.E.; Maskell, J.; Oliver, E.M.; Morris, R.C.; Foote, K.D.; Mills, E.N.; Roberts, G.; Margetts, B.M. Introduction of complementary foods and the relationship to food allergy. Pediatrics 2013, 132, e1529–e1538. [Google Scholar] [CrossRef]
- van Odijk, J.; Bengtsson, U.; Borres, M.P.; Hulthén, L.; Ahlstedt, S. Specific immunoglobulin E antibodies to peanut over time in relation to peanut intake, symptoms and age. Pediatr. Allergy Immunol. 2004, 15, 442–448. [Google Scholar] [CrossRef]
- Flinterman, A.E.; van Hoffen, E.; den Hartog Jager, C.F.; Koppelman, S.; Pasmans, S.G.; Hoekstra, M.O.; Bruijnzeel-Koomen, C.A.; Knulst, A.C.; Knol, E.F. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin. Exp. Allergy 2007, 37, 1221–1228. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy. J. Allergy Clin. Immunol. 2010, 125, S116–S125. [Google Scholar] [CrossRef]
- Antolín-Amérigo, D.; Vidal Albareda, C.; de Olano, D.G.; de la Hoz Caballer, B. Current update on Anaphylaxis: Anaphylaxis management in recent guidelines. Eur. Ann. Allergy Clin. Immunol. 2023. [Google Scholar] [CrossRef]
- Grabenhenrich, L.B.; Dölle, S.; Moneret-Vautrin, A.; Köhli, A.; Lange, L.; Spindler, T.; Ruëff, F.; Nemat, K.; Maris, I.; Roumpedaki, E.; et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J. Allergy Clin. Immunol. 2016, 137, 1128–1137.e1. [Google Scholar] [CrossRef]
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI guidelines: Anaphylaxis (2021 update). Allergy 2022, 77, 357–377. [Google Scholar] [CrossRef]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Burks, A.W.; Sampson, H.A. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics 1998, 102, e6. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Furlong, T.J.; Muñoz-Furlong, A.; Burks, A.W.; Sampson, H.A. A voluntary registry for peanut and tree nut allergy: Characteristics of the first 5149 registrants. J. Allergy Clin. Immunol. 2001, 108, 128–132. [Google Scholar] [CrossRef]
- Peters, R.L.; Allen, K.J.; Dharmage, S.C.; Koplin, J.J.; Dang, T.; Tilbrook, K.P.; Lowe, A.; Tang, M.L.; Gurrin, L.C.; HealthNuts Study. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: A population-based assessment. J. Allergy Clin. Immunol. 2015, 135, 1257–1266.e1–e2. [Google Scholar] [CrossRef]
- Sampson, H.A.; Mendelson, L.; Rosen, J.P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N. Engl. J. Med. 1992, 327, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhsen, S.; Clarke, A.E.; Kagan, R.S. Peanut allergy: An overview. CMAJ 2003, 168, 1279–1285, Erratum in CMAJ 2003, 168, 1529. [Google Scholar] [PubMed]
- Baseggio Conrado, A.; Ierodiakonou, D.; Gowland, M.H.; Boyle, R.J.; Turner, P.J. Food anaphylaxis in the United Kingdom: Analysis of national data, 1998–2018. BMJ 2021, 372, n251. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, P.P.; Kemp, A.S.; Mellis, C.M. Skin test reactivity and clinical allergen sensitivity in infancy. J. Allergy Clin. Immunol. 1984, 73, 381–386. [Google Scholar] [CrossRef]
- Muller, T.; Luc, A.; Adam, T.; Jarlot-Chevaux, S.; Dumond, P.; Schweitzer, C.; Codreanu-Morel, F.; Divaret-Chauveau, A. Relevance of sensitization to legumes in peanut-allergic children. Pediatr. Allergy Immunol. 2022, 33, e13846. [Google Scholar] [CrossRef]
- Haidar, E.; Lakkis, J.; Karam, M.; Koubaa, M.; Louka, N.; Debs, E. Peanut Allergenicity: An Insight into Its Mitigation Using Thermomechanical Processing. Foods 2023, 12, 1253. [Google Scholar] [CrossRef]
- Aalberse, R.C. Assessment of allergen cross-reactivity. Clin. Mol. Allergy 2007, 5, 2. [Google Scholar] [CrossRef]
- Uotila, R.; Kukkonen, A.K.; Blom, W.M.; Remington, B.; Westerhout, J.; Pelkonen, A.S.; Mäkelä, M.J. Component-resolved diagnostics demonstrates that most peanut-allergic individuals could potentially introduce tree nuts to their diet. Clin. Exp. Allergy 2018, 48, 712–721. [Google Scholar] [CrossRef]
- Hebling, C.M.; Ross, M.M.; Callahan, J.H.; McFarland, M.A. Size-selective fractionation and visual mapping of allergen protein chemistry in Arachis hypogaea. J. Proteome Res. 2012, 11, 5384–5395. [Google Scholar] [CrossRef]
- Dreskin, S.C.; Koppelman, S.J.; Andorf, S.; Nadeau, K.C.; Kalra, A.; Braun, W.; Negi, S.S.; Chen, X.; Schein, C.H. The importance of the 2S albumins for allergenicity and cross-reactivity of peanuts, tree nuts, and sesame seeds. J. Allergy Clin. Immunol. 2021, 147, 1154–1163. [Google Scholar] [CrossRef]
- Barre, A.; Sordet, C.; Culerrier, R.; Rance, F.; Didier, A.; Rouge, P. Vicilin allergens ofpeanut and tree nuts (walnut, hazelnut and cashew nut) share structurally related IgE-binding epitopes. Mol. Immunol. 2008, 45, 1231–1240. [Google Scholar] [CrossRef]
- Kamath, S.D.; Bublin, M.; Kitamura, K.; Matsui, T.; Ito, K.; Lopata, A.L. Cross-reactive epitopes and their role in food allergy. J. Allergy Clin. Immunol. 2023, 151, 1178–1190. [Google Scholar] [CrossRef]
- Hourihane, J.O.; Kilburn, S.A.; Dean, P.; Warner, J.O. Clinical characteristics of peanut allergy. Clin. Exp. Allergy 1997, 27, 634–639. [Google Scholar] [CrossRef]
- Mittag, D.; Akkerdaas, J.; Ballmer-Weber, B.K.; Vogel, L.; Wensing, M.; Becker, W.M.; Koppelman, S.J.; Knulst, A.C.; Helbling, A.; Hefle, S.L.; et al. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J. Allergy Clin. Immunol. 2004, 114, 1410–1417. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- DunnGalvin, A.; Chan, C.H.; Crevel, R.; Grimshaw, K.; Poms, R.; Schnadt, S.; Taylor, S.L.; Turner, P.; Allen, K.J.; Austin, M.; et al. Precautionary allergen labelling: Perspectives from key stakeholder groups. Allergy 2015, 70, 1039–1051. [Google Scholar] [CrossRef]
- Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Alviani, C.; Angier, E.; Arasi, S.; Arzt-Gradwohl, L.; Barber, D.; Bazire, R.; Cavkaytar, O.; et al. EAACI Allergen Immunotherapy User’s Guide. Pediatr. Allergy Immunol. 2020, 31, 1–101. [Google Scholar] [CrossRef]
- Patrawala, M.; Shih, J.; Lee, G.; Vickery, B. Peanut Oral Immunotherapy: A Current Perspective. Curr. Allergy Asthma Rep. 2020, 20, 14. [Google Scholar] [CrossRef]
- Oppenheimer, J.J.; Nelson, H.S.; Bock, S.A.; Christensen, F.; Leung, D.Y. Treatment of peanut allergy with rush immunotherapy. J. Allergy Clin. Immunol. 1992, 90, 256–262. [Google Scholar] [CrossRef]
- Nelson, H.S.; Lahr, J.; Rule, R.; Bock, A.; Leung, D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J. Allergy Clin. Immunol. 1997, 99, 744–751. [Google Scholar] [CrossRef]
- Durham, S.R.; Shamji, M.H. Allergen immunotherapy: Past, present and future. Nat. Rev. Immunol. 2023, 23, 317–328. [Google Scholar] [CrossRef]
- Burk, C.M.; Kulis, M.; Leung, N.; Kim, E.H.; Burks, A.W.; Vickery, B.P. Utility of component analyses in subjects undergoing sublingual immunotherapy for peanut allergy. Clin. Exp. Allergy 2016, 46, 347–353. [Google Scholar] [CrossRef]
- Greenhawt, M.; Sindher, S.B.; Wang, J.; O’Sullivan, M.; du Toit, G.; Kim, E.H.; Albright, D.; Anvari, S.; Arends, N.; Arkwright, P.D.; et al. Phase 3 Trial of Epicutaneous Immunotherapy in Toddlers with Peanut Allergy. N. Engl. J. Med. 2023, 388, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Wood, R.A.; French, S.; Fiocchi, A.; Jordana, M.; Waserman, S.; Brożek, J.L.; Schünemann, H.J. Oral immunotherapy for peanut allergy (PACE): A systematic review and meta-analysis of efficacy and safety. Lancet 2019, 393, 2222–2232. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Purington, N.; Andorf, S.; Long, A.; O’Laughlin, K.L.; Lyu, S.C.; Manohar, M.; Boyd, S.D.; Tibshirani, R.; Maecker, H.; et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): A large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2019, 394, 1437–1449. [Google Scholar] [CrossRef]
- Vickery, B.P.; Scurlock, A.M.; Kulis, M.; Steele, P.H.; Kamilaris, J.; Berglund, J.P.; Burk, C.; Hiegel, A.; Carlisle, S.; Christie, L.; et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J. Allergy Clin. Immunol. 2014, 133, 468–475. [Google Scholar] [CrossRef]
- Tsai, M.; Mukai, K.; Chinthrajah, R.S.; Nadeau, K.C.; Galli, S.J. Sustained successful peanut oral immunotherapy associated with low basophil activation and peanut-specific IgE. J. Allergy Clin. Immunol. 2020, 145, 885–896.e6. [Google Scholar] [CrossRef]
- Vickery, B.P.; Lin, J.; Kulis, M.; Fu, Z.; Steele, P.H.; Jones, S.M.; Scurlock, A.M.; Gimenez, G.; Bardina, L.; Sampson, H.A.; et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J. Allergy Clin. Immunol. 2013, 131, 128–134.e1–e3. [Google Scholar] [CrossRef]
- Santos, A.F.; James, L.K.; Bahnson, H.T.; Shamji, M.H.; Couto-Francisco, N.C.; Islam, S.; Houghton, S.; Clark, A.T.; Stephens, A.; Turcanu, V.; et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J. Allergy Clin. Immunol. 2015, 135, 1249–1256. [Google Scholar] [CrossRef]
- LaHood, N.A.; Min, J.; Keswani, T.; Richardson, C.M.; Amoako, K.; Zhou, J.; Marini-Rapoport, O.; Bernard, H.; Hazebrouck, S.; Shreffler, W.G.; et al. Immunotherapy-induced neutralizing antibodies disrupt allergen binding and sustain allergen tolerance in peanut allergy. J. Clin. Investig. 2023, 133, e164501. [Google Scholar] [CrossRef]
- Patil, S.U.; Steinbrecher, J.; Calatroni, A.; Smith, N.; Ma, A.; Ruiter, B.; Virkud, Y.; Schneider, M.; Shreffler, W.G. Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy. J. Allergy Clin. Immunol. 2019, 144, 1310–1319.e4. [Google Scholar] [CrossRef]
- Sztuk, T.K.S.; Rigby, N.M.; Nørskov-Nielsen, L.; Koppelman, S.J.; Sancho, A.I.; Knudsen, N.H.; Marsh, J.; Johnson, P.; Gupta, S.; Mackie, A.R.; et al. Dose and route of administration determine the efficacy of prophylactic immunotherapy for peanut allergy in a Brown Norway rat model. Front. Immunol. 2023, 14, 1121497. [Google Scholar] [CrossRef] [PubMed]
- MacGinnitie, A.J.; Rachid, R.; Gragg, H.; Little, S.V.; Lakin, P.; Cianferoni, A.; Heimall, J.; Makhija, M.; Robison, R.; Chinthrajah, R.S.; et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J. Allergy Clin. Immunol. 2017, 139, 873–881.e8. [Google Scholar] [CrossRef] [PubMed]
| Protein Families of Peanuts Molecules | |
|---|---|
| Ara h 1, Ara h 3 | cupins |
| Ara h 2, Ara h 6, Ara h 7 | conglutins |
| Ara h 5 | profilins |
| Ara h 8 | Bet v 1-like |
| Ara h 9, Ara h 16, Ara h 17 | nsLTP |
| Ara h 10, Ara h 11, Ara h 14, Ara h 15 | oleosins |
| Ara h 12, Ara h 13 | defensins |
| Ara h 18 | cyclophilins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Połomska, J.; Dydak, P.; Sozańska, B.; Sikorska-Szaflik, H. Peanut Allergy and Component-Resolved Diagnostics Possibilities—What Are the Benefits? Nutrients 2023, 15, 5132. https://doi.org/10.3390/nu15245132
Połomska J, Dydak P, Sozańska B, Sikorska-Szaflik H. Peanut Allergy and Component-Resolved Diagnostics Possibilities—What Are the Benefits? Nutrients. 2023; 15(24):5132. https://doi.org/10.3390/nu15245132
Chicago/Turabian StylePołomska, Joanna, Paulina Dydak, Barbara Sozańska, and Hanna Sikorska-Szaflik. 2023. "Peanut Allergy and Component-Resolved Diagnostics Possibilities—What Are the Benefits?" Nutrients 15, no. 24: 5132. https://doi.org/10.3390/nu15245132
APA StylePołomska, J., Dydak, P., Sozańska, B., & Sikorska-Szaflik, H. (2023). Peanut Allergy and Component-Resolved Diagnostics Possibilities—What Are the Benefits? Nutrients, 15(24), 5132. https://doi.org/10.3390/nu15245132






