Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements
Abstract
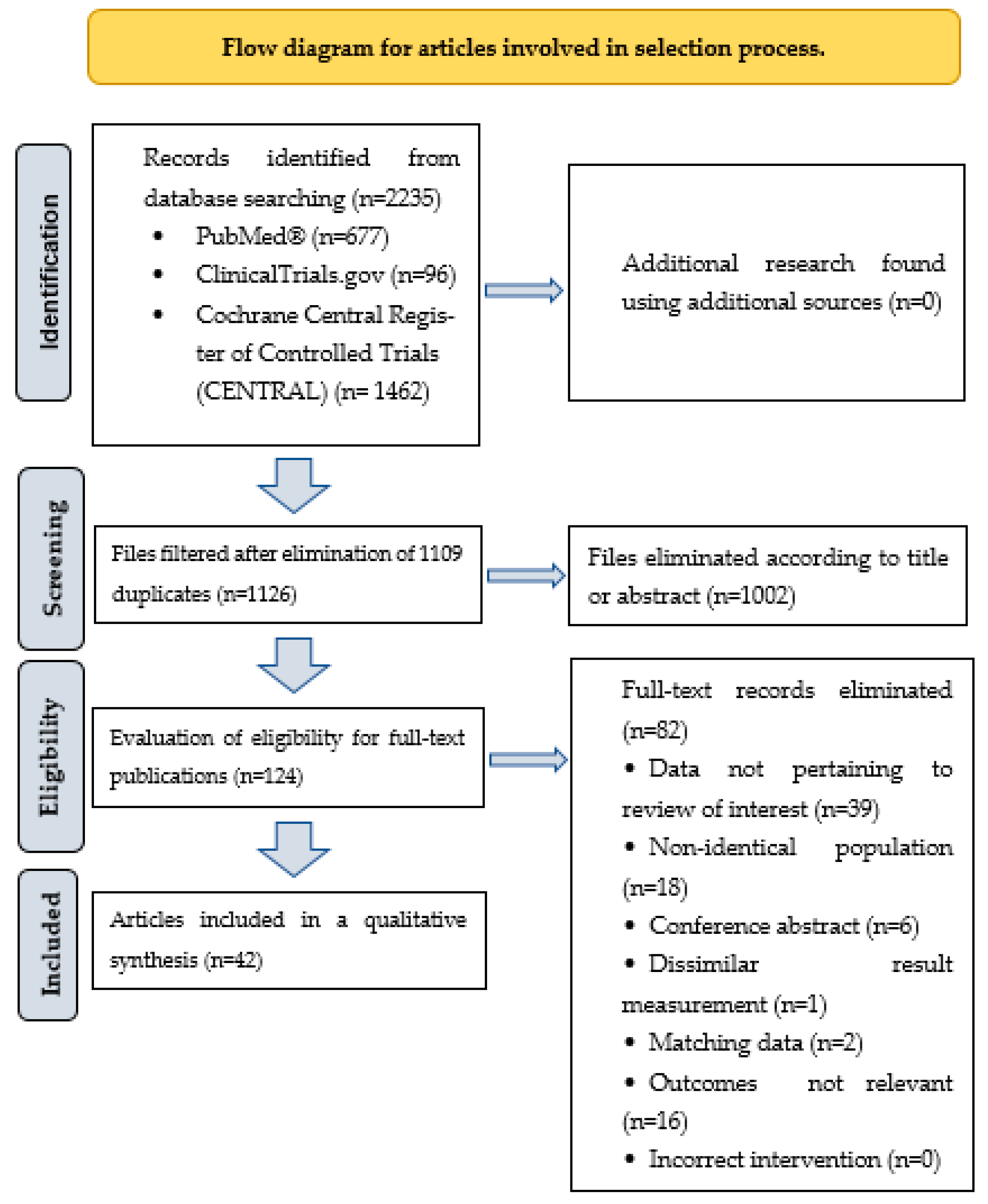
:1. Introduction
2. Methods
3. Results
3.1. Vitamin A Supplementation
3.2. Vitamin B Supplementation and Cognitive Function
3.3. Results on Antioxidants and Cognitive Function
3.4. Vitamin D Supplementation and Cognitive Function
3.5. Omega-3 Dietary Supplements and Cognitive Function
4. Discussion
4.1. Vitamin B
4.2. Vitamin C, Vitamin E and Other Antioxidants
4.3. Vitamin D
4.4. Vitamin K
4.5. Omega-3 Polyunsaturated Fatty Acids
4.6. Mineral Supplementation
5. Practical Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Li, L.; Hu, L.; Ji, J.; McKendrick, K.; Moreno, J.; Kelley, A.S.; Mazumdar, M.; Aldridge, M. Determinants of Total End-of-Life Health Care Costs of Medicare Beneficiaries: A Quantile Regression Forests Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Andersen, S.L.; Sweigart, B.; Du, M.; Cosentino, S.; Thyagarajan, B.; Christensen, K.; Schupf, N.; Perls, T.T. Patterns of multi-domain cognitive aging in participants of the Long Life Family Study. Geroscience 2020, 42, 1335–1350. [Google Scholar] [CrossRef]
- Corrada, M.M.; Brookmeyer, R.; Paganini-Hill, A.; Berlau, D.; Kawas, C.H. Dementia incidence continues to increase with age in the oldest old: The 90+ study. Ann. Neurol. 2010, 67, 114–121. [Google Scholar] [CrossRef]
- Stephan, Y.; Sutin, A.R.; Luchetti, M.; Terracciano, A. Subjective age and risk of incident dementia: Evidence from the National Health and Aging Trends survey. J. Psychiatr. Res. 2018, 100, 1–4. [Google Scholar] [CrossRef]
- Valsdottir, V.; Magnusdottir, B.B.; Chang, M.; Sigurdsson, S.; Gudnason, V.; Launer, L.J.; Jonsdottir, M.K. Cognition and brain health among older adults in Iceland: The AGES-Reykjavik study. Geroscience 2022, 44, 2785–2800. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, L.; Rosset, I.; Roriz-Cruz, M. Global epidemiology of dementia: Alzheimer’s and vascular types. BioMed Res. Int. 2014, 2014, 908915. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.M.; Copeland, J.R.; Dartigues, J.F.; Jagger, C.; Martinez-Lage, J.; et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000, 54 (Suppl. 5), S4–S9. [Google Scholar] [PubMed]
- Shin, J.H. Dementia Epidemiology Fact Sheet 2022. Ann. Rehabil. Med. 2022, 46, 53–59. [Google Scholar] [CrossRef]
- Scott, K.R.; Barrett, A.M. Dementia syndromes: Evaluation and treatment. Expert Rev. Neurother. 2007, 7, 407–422. [Google Scholar] [CrossRef]
- Gumus, M.; Multani, N.; Mack, M.L.; Tartaglia, M.C.; Alzheimer’s Disease Neuroimaging, I. Progression of neuropsychiatric symptoms in young-onset versus late-onset Alzheimer’s disease. Geroscience 2021, 43, 213–223. [Google Scholar] [CrossRef]
- Van der Willik, K.D.; Licher, S.; Vinke, E.J.; Knol, M.J.; Darweesh, S.K.L.; van der Geest, J.N.; Schagen, S.B.; Ikram, M.K.; Luik, A.I.; Ikram, M.A. Trajectories of Cognitive and Motor Function Between Ages 45 and 90 Years: A Population-Based Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 297–306. [Google Scholar] [CrossRef]
- Bendayan, R.; Zhu, Y.; Federman, A.D.; Dobson, R.J.B. Multimorbidity Patterns and Memory Trajectories in Older Adults: Evidence From the English Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 867–875. [Google Scholar] [CrossRef]
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J./Rev. Pharm. Du Can. 2017, 150, 118–129. [Google Scholar] [CrossRef]
- Czakó, C.; Kovács, T.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; Csipo, T.; Lipecz, A.; Horváth, H.; Sándor, G.L.; et al. Retinal biomarkers for Alzheimer’s disease and vascular cognitive impairment and dementia (VCID): Implication for early diagnosis and prognosis. Geroscience 2020, 42, 1499–1525. [Google Scholar] [CrossRef]
- D’Arbeloff, T. Cardiovascular fitness and structural brain integrity: An update on current evidence. Geroscience 2020, 42, 1285–1306. [Google Scholar] [CrossRef]
- Levit, A.; Hachinski, V.; Whitehead, S.N. Neurovascular unit dysregulation, white matter disease, and executive dysfunction: The shared triad of vascular cognitive impairment and Alzheimer disease. Geroscience 2020, 42, 445–465. [Google Scholar] [CrossRef]
- Lopez, M.E.; Turrero, A.; Cuesta, P.; Rodriguez-Rojo, I.C.; Barabash, A.; Marcos, A.; Maestu, F.; Fernandez, A. A multivariate model of time to conversion from mild cognitive impairment to Alzheimer’s disease. Geroscience 2020, 42, 1715–1732. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Roman, R.J.; Fan, F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? Geroscience 2022, 44, 1879–1883. [Google Scholar] [CrossRef]
- Istvan, L.; Czako, C.; Elo, A.; Mihaly, Z.; Sotonyi, P.; Varga, A.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience 2021, 43, 1703–1723. [Google Scholar] [CrossRef]
- Verheggen, I.C.M.; de Jong, J.J.A.; van Boxtel, M.P.J.; Gronenschild, E.; Palm, W.M.; Postma, A.A.; Jansen, J.F.A.; Verhey, F.R.J.; Backes, W.H. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience 2020, 42, 1183–1193. [Google Scholar] [CrossRef]
- Verheggen, I.C.M.; de Jong, J.J.A.; van Boxtel, M.P.J.; Postma, A.A.; Jansen, J.F.A.; Verhey, F.R.J.; Backes, W.H. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience 2020, 42, 1751–1764. [Google Scholar] [CrossRef]
- Kerkhofs, D.; Wong, S.M.; Zhang, E.; Uiterwijk, R.; Hoff, E.I.; Jansen, J.F.A.; Staals, J.; Backes, W.H.; van Oostenbrugge, R.J. Blood-brain barrier leakage at baseline and cognitive decline in cerebral small vessel disease: A 2-year follow-up study. Geroscience 2021, 43, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Barnes, S.R.; Nation, D.A.; Kisler, K.; Toga, A.W.; Zlokovic, B.V. Imaging subtle leaks in the blood-brain barrier in the aging human brain: Potential pitfalls, challenges, and possible solutions. Geroscience 2022, 44, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Roman, R.J. Reversal of cerebral hypoperfusion: A novel therapeutic target for the treatment of AD/ADRD? Geroscience 2021, 43, 1065–1067. [Google Scholar] [CrossRef]
- Wang, S.; Lv, W.; Zhang, H.; Liu, Y.; Li, L.; Jefferson, J.R.; Guo, Y.; Li, M.; Gao, W.; Fang, X.; et al. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience 2020, 42, 1387–1410. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Balasubramanian, P.; Delfavero, J.; Csipo, T.; Yabluchanskiy, A.; Kiss, T.; Nyul-Toth, A.; Mukli, P.; Toth, P.; Ahire, C.; et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience 2021, 43, 2427–2440. [Google Scholar] [CrossRef]
- Vestergaard, M.B.; Lindberg, U.; Knudsen, M.H.; Urdanibia-Centelles, O.; Bakhtiari, A.; Mortensen, E.L.; Osler, M.; Fagerlund, B.; Benedek, K.; Lauritzen, M.; et al. Subclinical cognitive deficits are associated with reduced cerebrovascular response to visual stimulation in mid-sixties men. Geroscience 2022, 44, 1905–1923. [Google Scholar] [CrossRef]
- Sabayan, B.; Westendorp, R.G.J. Neurovascular-glymphatic dysfunction and white matter lesions. Geroscience 2021, 43, 1635–1642. [Google Scholar] [CrossRef]
- Szczesniak, D.; Rymaszewska, J.; Zimny, A.; Sasiadek, M.; Poltyn-Zaradna, K.; Smith, E.E.; Zatonska, K.; Zatonski, T.; Rangarajan, S.; Yusuf, S.; et al. Cerebral small vessel disease and other influential factors of cognitive impairment in the middle-aged: A long-term observational cohort PURE-MIND study in Poland. Geroscience 2021, 43, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Jacob, M.A.; Norris, D.G.; de Leeuw, F.E.; Tuladhar, A.M. Longitudinal Relation Between Structural Network Efficiency, Cognition, and Gait in Cerebral Small Vessel Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Montgomery, P.S.; Wang, M.; Shen, B.; Casanegra, A.I.; Silva-Palacios, F.; Ungvari, Z.; Yabluchanskiy, A.; Csiszar, A.; Waldstein, S.R. Cognitive decrement in older adults with symptomatic peripheral artery disease. Geroscience 2021, 43, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; le Cessie, S.; Blauw, G.J.; Franceschi, C.; Noordam, R.; van Heemst, D. Systemic inflammatory markers in relation to cognitive function and measures of brain atrophy: A Mendelian randomization study. Geroscience 2022, 44, 2259–2270. [Google Scholar] [CrossRef] [PubMed]
- Lazar, G., Jr.; Varga, J.; Lazar, G.; Duda, E.; Takacs, T.; Balogh, A.; Lonovics, J. The effects of glucocorticoids and a glucocorticoid antagonist (RU 38486) on experimental acute pancreatitis in rat. Acta Chir. Hung. 1997, 36, 190–191. [Google Scholar] [PubMed]
- Boutzoukas, E.M.; O’Shea, A.; Kraft, J.N.; Hardcastle, C.; Evangelista, N.D.; Hausman, H.K.; Albizu, A.; Van Etten, E.J.; Bharadwaj, P.K.; Smith, S.G.; et al. Higher white matter hyperintensity load adversely affects pre-post proximal cognitive training performance in healthy older adults. Geroscience 2022, 44, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Hausman, H.K.; Hardcastle, C.; Albizu, A.; Kraft, J.N.; Evangelista, N.D.; Boutzoukas, E.M.; Langer, K.; O’Shea, A.; Van Etten, E.J.; Bharadwaj, P.K.; et al. Cingulo-opercular and frontoparietal control network connectivity and executive functioning in older adults. Geroscience 2022, 44, 847–866. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.W.; Pieruccini-Faria, F.; Witt, S.T.; Rockwood, K.; Bartha, R.; Doherty, T.J.; Nagamatsu, L.S.; Almeida, Q.J.; Liu-Ambrose, T.; Middleton, L.E.; et al. Frailty and functional brain connectivity (FBC) in older adults with mild cognitive impairment (MCI): Baseline results from the SYNERGIC Trial. Geroscience 2023, 45, 1033–1048. [Google Scholar] [CrossRef]
- Chino, B.; Cuesta, P.; Pacios, J.; de Frutos-Lucas, J.; Torres-Simon, L.; Doval, S.; Marcos, A.; Bruna, R.; Maestu, F. Episodic memory dysfunction and hypersynchrony in brain functional networks in cognitively intact subjects and MCI: A study of 379 individuals. Geroscience 2023, 45, 477–489. [Google Scholar] [CrossRef]
- Sanchez-Roman, I.; Ferrando, B.; Holst, C.M.; Mengel-From, J.; Rasmussen, S.H.; Thinggaard, M.; Bohr, V.A.; Christensen, K.; Stevnsner, T. Molecular markers of DNA repair and brain metabolism correlate with cognition in centenarians. Geroscience 2022, 44, 103–125. [Google Scholar] [CrossRef]
- Jiang, J.; Sheng, C.; Chen, G.; Liu, C.; Jin, S.; Li, L.; Jiang, X.; Han, Y.; Alzheimer’s Disease Neuroimaging, I. Glucose metabolism patterns: A potential index to characterize brain ageing and predict high conversion risk into cognitive impairment. Geroscience 2022, 44, 2319–2336. [Google Scholar] [CrossRef]
- Lu, W.H.; Giudici, K.V.; Morley, J.E.; Guyonnet, S.; Parini, A.; Aggarwal, G.; Nguyen, A.D.; Li, Y.; Bateman, R.J.; Vellas, B.; et al. Investigating the combination of plasma amyloid-beta and geroscience biomarkers on the incidence of clinically meaningful cognitive decline in older adults. Geroscience 2022, 44, 1489–1503. [Google Scholar] [CrossRef]
- Uleman, J.F.; Melis, R.J.F.; Quax, R.; van der Zee, E.A.; Thijssen, D.; Dresler, M.; van de Rest, O.; van der Velpen, I.F.; Adams, H.H.H.; Schmand, B.; et al. Mapping the multicausality of Alzheimer’s disease through group model building. Geroscience 2021, 43, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.A. Pharmacotherapy of neuropsychiatric symptoms of dementia. Pharm. Ther. 2015, 40, 284–287. [Google Scholar]
- Chaudhari, K.; Reynolds, C.D.; Yang, S.H. Metformin and cognition from the perspectives of sex, age, and disease. Geroscience 2020, 42, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Balazs, N.; Bereczki, D.; Ajtay, A.; Oberfrank, F.; Kovacs, T. Cholinesterase inhibitors for the treatment of dementia: Real-life data in Hungary. Geroscience 2022, 44, 253–263. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Wang, H. Nexus between cognitive reserve and modifiable risk factors of dementia. Int. Psychogeriatr. 2020, 32, 559–562. [Google Scholar] [CrossRef]
- Dhana, K.; Franco, O.H.; Ritz, E.M.; Ford, C.N.; Desai, P.; Krueger, K.R.; Holland, T.M.; Dhana, A.; Liu, X.; Aggarwal, N.T. Healthy lifestyle and life expectancy with and without Alzheimer’s dementia: Population based cohort study. BMJ 2022, 377, e068390. [Google Scholar] [CrossRef]
- Berg-Weger, M.; Stewart, D.B. Non-pharmacologic interventions for persons with dementia. Mo. Med. 2017, 114, 116. [Google Scholar]
- Choi, H. Healthy lifestyles and more life years without dementia. BMJ 2022, 377, o885. [Google Scholar] [CrossRef] [PubMed]
- Poddar, J.; Pradhan, M.; Ganguly, G.; Chakrabarti, S. Biochemical deficits and cognitive decline in brain aging: Intervention by dietary supplements. J. Chem. Neuroanat. 2019, 95, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, A.; Gulej, R.; Csik, B.; Mukli, P.; Negri, S.; Tarantini, S.; Yabluchanskiy, A.; Benyo, Z.; Csiszar, A.; Ungvari, Z. The Role of Methionine-Rich Diet in Unhealthy Cerebrovascular and Brain Aging: Mechanisms and Implications for Cognitive Impairment. Nutrients 2023, 15, 4662. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Fazekas-Pongor, V.; Csiszar, A.; Kunutsor, S.K. The multifaceted benefits of walking for healthy aging: From Blue Zones to molecular mechanisms. Geroscience 2023, 45, 3211–3239. [Google Scholar] [CrossRef]
- Azhar, G.; Wei, J.Y.; Schutzler, S.E.; Coker, K.; Gibson, R.V.; Kirby, M.F.; Ferrando, A.A.; Wolfe, R.R. Daily Consumption of a Specially Formulated Essential Amino Acid-Based Dietary Supplement Improves Physical Performance in Older Adults With Low Physical Functioning. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1184–1191. [Google Scholar] [CrossRef]
- Dalle, S.; Van Roie, E.; Hiroux, C.; Vanmunster, M.; Coudyzer, W.; Suhr, F.; Bogaerts, S.; Van Thienen, R.; Koppo, K. Omega-3 Supplementation Improves Isometric Strength But Not Muscle Anabolic and Catabolic Signaling in Response to Resistance Exercise in Healthy Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 406–414. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef] [PubMed]
- Cristina, N.M.; Lucia, D. Nutrition and Healthy Aging: Prevention and Treatment of Gastrointestinal Diseases. Nutrients 2021, 13, 4337. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W. Proton pump inhibitors, H2-receptor antagonists, metformin, and vitamin B-12 deficiency: Clinical implications. Adv. Nutr. 2018, 9, 511S–518S. [Google Scholar] [CrossRef] [PubMed]
- Suliburska, J.; Chmurzynska, A.; Kocylowski, R.; Skrypnik, K.; Radziejewska, A.; Baralkiewicz, D. Effect of iron and folic acid supplementation on the level of essential and toxic elements in young women. Int. J. Environ. Res. Public Health 2021, 18, 1360. [Google Scholar] [CrossRef]
- Mooldijk, S.S.; Licher, S.; Vernooij, M.W.; Ikram, M.K.; Ikram, M.A. Seasonality of cognitive function in the general population: The Rotterdam Study. Geroscience 2022, 44, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a Shield against Aging. Int. J. Mol. Sci. 2023, 24, 4546. [Google Scholar] [CrossRef]
- Fekete, M.; Csípő, T.; Fazekas-Pongor, V.; Fehér, Á.; Szarvas, Z.; Kaposvári, C.; Horváth, K.; Lehoczki, A.; Tarantini, S.; Varga, J.T. The Effectiveness of Supplementation with Key Vitamins, Minerals, Antioxidants and Specific Nutritional Supplements in COPD-A Review. Nutrients 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S. Effects of omega-3 polyunsaturated fatty acids on brain functions: A systematic review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- De Magalhães, J.P.; Müller, M.; Rainger, G.E.; Steegenga, W. Fish oil supplements, longevity and aging. Aging 2016, 8, 1578. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Lehoczki, A.; Tarantini, S.; Varga, J.T. Effects of omega-3 supplementation on quality of life, nutritional status, inflammatory parameters, lipid profile, exercise tolerance and inhaled medications in chronic obstructive pulmonary disease. Ann. Palliat. Med. 2022, 11, 2819–2829. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Kim, J.S.; Thomashow, M.A.; Yip, N.H.; Burkart, K.M.; Lo Cascio, C.M.; Shimbo, D.; Barr, R.G. Randomization to Omega-3 Fatty Acid Supplementation and Endothelial Function in COPD: The COD-Fish Randomized Controlled Trial. Chronic Obs. Pulm. Dis. 2021, 8, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Leitão, C.; Mignano, A.; Estrela, M.; Fardilha, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. The Effect of Nutrition on Aging-A Systematic Review Focusing on Aging-Related Biomarkers. Nutrients 2022, 14, 554. [Google Scholar] [CrossRef]
- Lane, K.; Derbyshire, E.; Li, W.; Brennan, C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature. Crit. Rev. Food Sci. Nutr. 2014, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Refsum, H.; Oulhaj, A.; de Jager, C.A.; Jerneren, F. Beneficial Interactions Between B Vitamins and Omega-3 Fatty Acids in the Prevention of Brain Atrophy and of Cognitive Decline in Early Stage Alzheimer’s Disease. FASEB J. 2016, 30, 407.6. [Google Scholar] [CrossRef]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimer’s Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Q.; Zhou, X.; Zhao, J.; Song, A.; Li, W.; Liu, H.; Xu, W.; Huang, G. Effects of folic acid supplementation on cognitive function and Aβ-related biomarkers in mild cognitive impairment: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 345–356. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Q.; Li, S.; Dai, F.; Qian, W.; Hewlings, S.; Yan, T.; Wang, Y. A Magtein(®), Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults. Nutrients 2022, 14, 5235. [Google Scholar] [CrossRef]
- Bai, D.; Fan, J.; Li, M.; Dong, C.; Gao, Y.; Fu, M.; Huang, G.; Liu, H. Effects of Folic Acid Combined with DHA Supplementation on Cognitive Function and Amyloid-β-Related Biomarkers in Older Adults with Mild Cognitive Impairment by a Randomized, Double Blind, Placebo-Controlled Trial. J. Alzheimer’s Dis. 2021, 81, 155–167. [Google Scholar] [CrossRef]
- Ma, F.; Zhou, X.; Li, Q.; Zhao, J.; Song, A.; An, P.; Du, Y.; Xu, W.; Huang, G. Effects of Folic Acid and Vitamin B12, Alone and in Combination on Cognitive Function and Inflammatory Factors in the Elderly with Mild Cognitive Impairment: A Single-blind Experimental Design. Curr. Alzheimer Res. 2019, 16, 622–632. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Gao, Y.; Chen, Y.; Bai, D.; Weng, J.; Du, Y.; Ma, F.; Wang, X.; Liu, H.; et al. Effect of folic acid combined with docosahexaenoic acid intervention on mild cognitive impairment in elderly: A randomized double-blind, placebo-controlled trial. Eur. J. Nutr. 2021, 60, 1795–1808. [Google Scholar] [CrossRef]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. A randomized placebo-controlled trial of using B vitamins to prevent cognitive decline in older mild cognitive impairment patients. Clin. Nutr. 2020, 39, 2399–2405. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.; Wong, R.H. Long-term effects of resveratrol on cognition, cerebrovascular function and cardio-metabolic markers in postmenopausal women: A 24-month randomised, double-blind, placebo-controlled, crossover study. Clin. Nutr. 2021, 40, 820–829. [Google Scholar] [CrossRef]
- Foroumandi, E.; Javan, R.; Moayed, L.; Fahimi, H.; Kheirabadi, F.; Neamatshahi, M.; Shogofteh, F.; Zarghi, A. The effects of fenugreek seed extract supplementation in patients with Alzheimer’s disease: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2023, 37, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Hong, S.; Jung, S.; Kim, J.S.; Goo, Y.T.; Chun, W.Y.; Shin, D.M. Vitamin C supplementation promotes mental vitality in healthy young adults: Results from a cross-sectional analysis and a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2022, 61, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol. Behav. 2019, 211, 112650. [Google Scholar] [CrossRef]
- Lai, S.; Petramala, L.; Muscaritoli, M.; Cianci, R.; Mazzaferro, S.; Mitterhofer, A.P.; Pasquali, M.; D’Ambrosio, V.; Carta, M.; Ansuini, M.; et al. α-lipoic acid in patients with autosomal dominant polycystic kidney disease. Nutrition 2020, 71, 110594. [Google Scholar] [CrossRef]
- Yoon, J.; Sasaki, K.; Nishimura, I.; Hashimoto, H.; Okura, T.; Isoda, H. Effects of Desert Olive Tree Pearls Containing High Hydroxytyrosol Concentrations on the Cognitive Functions of Middle-Aged and Older Adults. Nutrients 2023, 15, 3234. [Google Scholar] [CrossRef] [PubMed]
- Morató, X.; Marquié, M.; Tartari, J.P.; Lafuente, A.; Abdelnour, C.; Alegret, M.; Jofresa, S.; Buendía, M.; Pancho, A.; Aguilera, N.; et al. A randomized, open-label clinical trial in mild cognitive impairment with EGb 761 examining blood markers of inflammation and oxidative stress. Sci. Rep. 2023, 13, 5406. [Google Scholar] [CrossRef]
- Baker, L.D.; Manson, J.E.; Rapp, S.R.; Sesso, H.D.; Gaussoin, S.A.; Shumaker, S.A.; Espeland, M.A. Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial. Alzheimer’s Dement. 2023, 19, 1308–1319. [Google Scholar] [CrossRef]
- Bell, L.; Whyte, A.R.; Lamport, D.J.; Spencer, J.P.E.; Butler, L.T.; Williams, C.M. Grape seed polyphenol extract and cognitive function in healthy young adults: A randomised, placebo-controlled, parallel-groups acute-on-chronic trial. Nutr. Neurosci. 2022, 25, 54–63. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Maruyama, K.; Hossain, S.; Sumiyoshi, E.; Wakatsuki, H.; Kato, S.; Ohno, M.; Tanabe, Y.; Kuroda, Y.; et al. Perilla seed oil in combination with nobiletin-rich ponkan powder enhances cognitive function in healthy elderly Japanese individuals: A possible supplement for brain health in the elderly. Food Funct. 2022, 13, 2768–2781. [Google Scholar] [CrossRef]
- Zajac, I.T.; Barnes, M.; Cavuoto, P.; Wittert, G.; Noakes, M. The Effects of Vitamin D-Enriched Mushrooms and Vitamin D3 on Cognitive Performance and Mood in Healthy Elderly Adults: A Randomised, Double-Blinded, Placebo-Controlled Trial. Nutrients 2020, 12, 3847. [Google Scholar] [CrossRef]
- Jorde, R.; Kubiak, J.; Svartberg, J.; Fuskevåg, O.M.; Figenschau, Y.; Martinaityte, I.; Grimnes, G. Vitamin D supplementation has no effect on cognitive performance after four months in mid-aged and older subjects. J. Neurol. Sci. 2019, 396, 165–171. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: A randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin D Supplementation Improves Cognitive Function Through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J. Alzheimer’s Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.; Fiedler, N.; Pop, L.C.; Schneider, S.J.; Schlussel, Y.; Sukumar, D.; Hao, L.; Shapses, S.A. Three Doses of Vitamin D and Cognitive Outcomes in Older Women: A Double-Blind Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 835–842. [Google Scholar] [CrossRef]
- Ghaderi, A.; Rasouli-Azad, M.; Farhadi, M.H.; Mirhosseini, N.; Motmaen, M.; Pishyareh, E.; Omidi, A.; Asemi, Z. Exploring the Effects of Vitamin D Supplementation on Cognitive Functions and Mental Health Status in Subjects Under Methadone Maintenance Treatment. J. Addict. Med. 2020, 14, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jia, J.; Zhang, Y.; Miao, R.; Huo, X.; Ma, F. Effects of vitamin D(3) supplementation on cognition and blood lipids: A 12-month randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Schietzel, S.; Fischer, K.; Brugger, P.; Orav, E.J.; Renerts, K.; Gagesch, M.; Freystaetter, G.; Stähelin, H.B.; Egli, A.; Bischoff-Ferrari, H.A. Effect of 2000 IU compared with 800 IU vitamin D on cognitive performance among adults age 60 years and older: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 246–253. [Google Scholar] [CrossRef]
- Byrn, M.A.; Adams, W.; Penckofer, S.; Emanuele, M.A. Vitamin D Supplementation and Cognition in People with Type 2 Diabetes: A Randomized Control Trial. J. Diabetes Res. 2019, 2019, 5696391. [Google Scholar] [CrossRef]
- Beauchet, O.; Launay, C.P.; Galery, K.; Vilcocq, C.; Dontot-Payen, F.; Rousseau, B.; Benoit, V.; Allali, G. Effects of Vitamin D and Calcium Fortified Yogurts on Gait, Cognitive Performances, and Serum 25-Hydroxyvitamin D Concentrations in Older Community-Dwelling Females: Results from the GAit, MEmory, Dietary and Vitamin D (GAME-D2) Randomized Controlled Trial. Nutrients 2019, 11, 2880. [Google Scholar] [CrossRef]
- Owusu, J.E.; Islam, S.; Katumuluwa, S.S.; Stolberg, A.R.; Usera, G.L.; Anwarullah, A.A.; Shieh, A.; Dhaliwal, R.; Ragolia, L.; Mikhail, M.B.; et al. Cognition and Vitamin D in Older African-American Women- Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J. Am. Geriatr. Soc. 2019, 67, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, H.; Brownell, S.; Harris, E.; Duckham, R.L.; O’Connell, S.; Meyer, B.J.; Mirzaee, S.; Daly, R.M. Effects of a 6-Month Multifaceted Diet and Exercise Intervention on Cognition in Older Adults at Risk of Cognitive Decline: The PONDER Double-Blind, Placebo-Controlled Randomized Trial. J. Alzheimer’s Dis. 2022, 89, 247–263. [Google Scholar] [CrossRef]
- Nolan, J.M.; Power, R.; Howard, A.N.; Bergin, P.; Roche, W.; Prado-Cabrero, A.; Pope, G.; Cooke, J.; Power, T.; Mulcahy, R. Supplementation With Carotenoids, Omega-3 Fatty Acids, and Vitamin E Has a Positive Effect on the Symptoms and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 233–249. [Google Scholar] [CrossRef]
- Stavrinou, P.S.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; Patrikios, I.S.; Giannaki, C.D. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 2020, 12, 325. [Google Scholar] [CrossRef]
- Atmadja, T.; Kusharto, C.; Sinaga, T. Supplementation of Catfish (Clarias gariepinus) Oil Enriched with Omega-3 Soft Capsule Improves Oxidative Stress and Cognitive Function in Elderly. J. Nutr. Sci. Vitaminol. 2020, 66, S47–S50. [Google Scholar] [CrossRef]
- Patan, M.J.; Kennedy, D.O.; Husberg, C.; Hustvedt, S.O.; Calder, P.C.; Khan, J.; Forster, J.; Jackson, P.A. Supplementation with oil rich in eicosapentaenoic acid, but not in docosahexaenoic acid, improves global cognitive function in healthy, young adults: Results from randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J. The LipiDiDiet trial: What does it add to the current evidence for Fortasyn Connect in early Alzheimer’s disease? Clin. Interv. Aging 2019, 14, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Cheng, C.; Satyanarayanan, S.K.; Chiu, L.T.; Chien, Y.C.; Chuu, C.P.; Lan, T.H.; Su, K.P. Omega-3 fatty acids and blood-based biomarkers in Alzheimer’s disease and mild cognitive impairment: A randomized placebo-controlled trial. Brain Behav. Immun. 2022, 99, 289–298. [Google Scholar] [CrossRef]
- Giudici, K.V.; de Souto Barreto, P.; Beard, J.; Cantet, C.; Araujo de Carvalho, I.; Rolland, Y.; Vellas, B. Effect of long-term omega-3 supplementation and a lifestyle multidomain intervention on intrinsic capacity among community-dwelling older adults: Secondary analysis of a randomized, placebo-controlled trial (MAPT study). Maturitas 2020, 141, 39–45. [Google Scholar] [CrossRef]
- Mengelberg, A.; Leathem, J.; Podd, J.; Hill, S.; Conlon, C. The effects of docosahexaenoic acid supplementation on cognition and well-being in mild cognitive impairment: A 12-month randomised controlled trial. Int. J. Geriatr. Psychiatry 2022, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leckie, R.L.; Lehman, D.E.; Gianaros, P.J.; Erickson, K.I.; Sereika, S.M.; Kuan, D.C.H.; Manuck, S.B.; Ryan, C.M.; Yao, J.K.; Muldoon, M.F. The effects of omega-3 fatty acids on neuropsychological functioning and brain morphology in mid-life adults: A randomized clinical trial. Psychol. Med. 2020, 50, 2425–2434. [Google Scholar] [CrossRef]
- Sueyasu, T.; Yasumoto, K.; Tokuda, H.; Kaneda, Y.; Obata, H.; Rogi, T.; Izumo, T.; Kondo, S.; Saito, J.; Tsukiura, T.; et al. Effects of Long-Chain Polyunsaturated Fatty Acids in Combination with Lutein and Zeaxanthin on Episodic Memory in Healthy Older Adults. Nutrients 2023, 15, 2825. [Google Scholar] [CrossRef] [PubMed]
- Arellanes, I.C.; Choe, N.; Solomon, V.; He, X.; Kavin, B.; Martinez, A.E.; Kono, N.; Buennagel, D.P.; Hazra, N.; Kim, G.; et al. Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. EBioMedicine 2020, 59, 102883. [Google Scholar] [CrossRef] [PubMed]
- Kuszewski, J.C.; Howe, P.R.C.; Wong, R.H.X. Evaluation of Cognitive Performance following Fish-Oil and Curcumin Supplementation in Middle-Aged and Older Adults with Overweight or Obesity. J. Nutr. 2020, 150, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, S.; Gao, F.; Li, C. Vitamin B6, B9, and B12 Intakes and Cognitive Performance in Elders: National Health and Nutrition Examination Survey, 2011–2014. Neuropsychiatr. Dis. Treat. 2022, 18, 537. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and dementia: An international consensus statement. J. Alzheimer’s Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Kataria, N.; Yadav, P.; Kumar, R.; Kumar, N.; Singh, M.; Kant, R.; Kalyani, V. Effect of vitamin B6, B9, and B12 supplementation on homocysteine level and cardiovascular outcomes in stroke patients: A meta-analysis of randomized controlled trials. Cureus 2021, 13, e14958. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Fazekas-Pongor, V.; Szőllősi, G.; Varga, J.T. A krónikus obstruktív tüdőbetegség metabolikus következményei. Orvosi Hetil. 2021, 162, 185–191. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 931–949. [Google Scholar] [CrossRef]
- Gong, X.; Shi, L.; Wu, Y.; Luo, Y.; Kwok, T. B Vitamin Supplementation Slows Cognitive Decline in Mild Cognitive Impairment Patients with Frontal Lobe Atrophy. J. Alzheimer’s Dis. 2022, 89, 1453–1461. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; De Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr. Rev. 1996, 54, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A. The role of natural antioxidants in the prevention of dementia—Where do we stand and future perspectives. Nutrients 2021, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.T.; Chaudhari, K.; Sidhu, A.; Sumien, N. The influence of vitamins E and C and exercise on brain aging. Exp. Gerontol. 2017, 94, 69–72. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Klawitter, J.; Blatchford, P.; Kohrt, W.M. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience 2020, 42, 1699–1714. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Guo, M.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martínez, M.-A. Oxidative stress, the blood–brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants. Acta Pharm. Sin. B 2023, 13, 3988–4024. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of antioxidants supplementation on aging and longevity. BioMed Res. Int. 2014, 2014, 404680. [Google Scholar] [CrossRef]
- Shah, H.; Dehghani, F.; Ramezan, M.; Gannaban, R.B.; Haque, Z.F.; Rahimi, F.; Abbasi, S.; Shin, A.C. Revisiting the Role of Vitamins and Minerals in Alzheimer’s Disease. Antioxidants 2023, 12, 415. [Google Scholar] [CrossRef]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in health and disease: Its role in the metabolism of cells and redox state in the brain. Front. Physiol. 2015, 6, 397. [Google Scholar] [CrossRef]
- McCall, S.J.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T.; Myint, P.K. Plasma vitamin C levels: Risk factors for deficiency and association with self-reported functional health in the European Prospective Investigation into Cancer-Norfolk. Nutrients 2019, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and cardiovascular disease: An update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Conner, T.S.; Fletcher, B.D.; Haszard, J.J.; Pullar, J.M.; Spencer, E.; Mainvil, L.A.; Vissers, M.C. KiwiC for Vitality: Results of a Placebo-Controlled Trial Testing the Effects of Kiwifruit or Vitamin C Tablets on Vitality in Adults with Low Vitamin C Levels. Nutrients 2020, 12, 2898. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Popescu, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Relationship between vitamin C deficiency and cognitive impairment in older hospitalised patients: A cross-sectional study. Antioxidants 2022, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.F.; Pullar, J.M.; Wilson, R.; Spittlehouse, J.K.; Vissers, M.C.; Skidmore, P.M.; Willis, J.; Cameron, V.A.; Carr, A.C. Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: Findings of the CHALICE cohort study. Nutrients 2017, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Travica, N.; Ried, K.; Sali, A.; Hudson, I.; Scholey, A.; Pipingas, A. Plasma vitamin C concentrations and cognitive function: A cross-sectional study. Front. Aging Neurosci. 2019, 11, 72. [Google Scholar] [CrossRef]
- Plevin, D.; Galletly, C. The neuropsychiatric effects of vitamin C deficiency: A systematic review. BMC Psychiatry 2020, 20, 315. [Google Scholar] [CrossRef]
- Yosaee, S.; Keshtkaran, Z.; Abdollahi, S.; Shidfar, F.; Sarris, J.; Soltani, S. The effect of vitamin C supplementation on mood status in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Gen. Hosp. Psychiatry 2021, 71, 36–42. [Google Scholar] [CrossRef]
- Von Arnim, C.A.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G. Dietary antioxidants and dementia in a population-based case-control study among older people in South Germany. J. Alzheimer’s Dis. 2012, 31, 717–724. [Google Scholar] [CrossRef]
- Harrison, F.E. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 29, 711–726. [Google Scholar] [CrossRef]
- Pincemail, J.; Meziane, S. On the Potential Role of the Antioxidant Couple Vitamin E/Selenium Taken by the Oral Route in Skin and Hair Health. Antioxidants 2022, 11, 2270. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157. [Google Scholar] [PubMed]
- Mangialasche, F.; Solomon, A.; Kåreholt, I.; Hooshmand, B.; Cecchetti, R.; Fratiglioni, L.; Soininen, H.; Laatikainen, T.; Mecocci, P.; Kivipelto, M. Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp. Gerontol. 2013, 48, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; D’Angelo, S. Impact of polyphenolic-food on longevity: An elixir of life. An overview. Antioxidants 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; El-Mageed, A.; Taia, A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z. Medicinal properties and functional components of beverages. In Functional and Medicinal Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–284. [Google Scholar]
- Minocha, T.; Birla, H.; Obaid, A.A.; Rai, V.; Sushma, P.; Shivamallu, C.; Moustafa, M.; Al-Shehri, M.; Al-Emam, A.; Tikhonova, M.A. Flavonoids as promising neuroprotectants and their therapeutic potential against Alzheimer’s disease. Oxidative Med. Cell. Longev. 2022, 2022, 6038996. [Google Scholar] [CrossRef]
- Fernandes, L.; Cardim-Pires, T.R.; Foguel, D.; Palhano, F.L. Green tea polyphenol epigallocatechin-gallate in amyloid aggregation and neurodegenerative diseases. Front. Neurosci. 2021, 15, 718188. [Google Scholar] [CrossRef]
- Zheng, T.; Bielinski, D.F.; Fisher, D.R.; Zhang, J.; Shukitt-Hale, B. Protective effects of a polyphenol-rich blueberry extract on adult human neural progenitor cells. Molecules 2022, 27, 6152. [Google Scholar] [CrossRef]
- Lu, W.H.; de Souto Barreto, P.; Rolland, Y.; Bouyahia, A.; Fischer, C.; Mangin, J.F.; Giudici, K.V.; Vellas, B.; Group, M.D. Biological and Neuroimaging Markers as Predictors of 5-Year Incident Frailty in Older Adults: A Secondary Analysis of the MAPT Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, e361–e369. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Sternberg, A.L.; Mitchell, C.M.; Blackford, A.L.; Schrack, J.; Wanigatunga, A.A.; Michos, E.; Juraschek, S.P.; Szanton, S.; Kalyani, R.; et al. Effects of Vitamin D on Physical Function: Results From the STURDY Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S. Nutrition strategies promoting healthy aging: From improvement of cardiovascular and brain health to prevention of age-associated diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Anjum, I.; Jaffery, S.S.; Fayyaz, M.; Samoo, Z.; Anjum, S. The role of vitamin D in brain health: A mini literature review. Cureus 2018, 10, e2960. [Google Scholar] [CrossRef] [PubMed]
- Takács, I.; Dank, M.; Majnik, J.; Nagy, G.; Szabó, A.; Szabó, B.; Szekanecz, Z.; Sziller, I.; Toldy, E.; Tislér, A. Magyarországi konszenzusajánlás a D-vitamin szerepéről a betegségek megelőzésében és kezelésében. Orvosi Hetil. 2022, 163, 575–584. [Google Scholar] [CrossRef]
- Dědečková, E.; Viták, R.; Jirásko, M.; Králová, M.; Topolčan, O.; Pecen, L.; Fürst, T.; Brož, P.; Kučera, R. Vitamin D3 Supplementation: Comparison of 1000 IU and 2000 IU Dose in Healthy Individuals. Life 2023, 13, 808. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D sources, metabolism, and deficiency: Available compounds and guidelines for its treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Meng, X. Vitamin D and neurodegenerative diseases. Heliyon 2023, 9, e12877. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D cell signalling in health and disease. Biochem. Biophys. Res. Commun. 2015, 460, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Mayne, P.E.; Burne, T.H. Vitamin D in synaptic plasticity, cognitive function, and neuropsychiatric illness. Trends Neurosci. 2019, 42, 293–306. [Google Scholar] [CrossRef]
- Roy, N.M.; Al-Harthi, L.; Sampat, N.; Al-Mujaini, R.; Mahadevan, S.; Al Adawi, S.; Essa, M.M.; Al Subhi, L.; Al-Balushi, B.; Qoronfleh, M.W. Impact of vitamin D on neurocognitive function in dementia, depression, schizophrenia and ADHD. Front. Biosci.-Landmark 2020, 26, 566–611. [Google Scholar] [CrossRef]
- Shea, M.K.; Barger, K.; Dawson-Hughes, B.; Leurgans, S.E.; Fu, X.; James, B.D.; Holland, T.M.; Agarwal, P.; Wang, J.; Matuszek, G. Brain vitamin D forms, cognitive decline, and neuropathology in community-dwelling older adults. Alzheimer’s Dement. 2023, 19, 2389–2396. [Google Scholar] [CrossRef]
- Maharjan, R.; Diaz Bustamante, L.; Ghattas, K.N.; Ilyas, S.; Al-Refai, R.; Khan, S. Role of Lifestyle in Neuroplasticity and Neurogenesis in an Aging Brain. Cureus 2020, 12, e10639. [Google Scholar] [CrossRef]
- DeLuca, G.; Kimball, S.; Kolasinski, J.; Ramagopalan, S.; Ebers, G. The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef]
- National Institutes of Heath Office of Dietary Supplements. Vitamin K Fact Sheet for Health Professionals. 2018. Available online: https://ods.od.nih.gov/factsheets/VitaminK-HealthProfessional/ (accessed on 14 November 2023).
- Mladěnka, P.; Macáková, K.; Kujovská Krčmová, L.; Javorská, L.; Mrštná, K.; Carazo, A.; Protti, M.; Remião, F.; Nováková, L.; Researchers, O.; et al. Vitamin K–sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022, 80, 677–698. [Google Scholar] [CrossRef]
- Huang, S.-H.; Fang, S.-T.; Chen, Y.-C. Molecular mechanism of vitamin K2 protection against amyloid-β-induced cytotoxicity. Biomolecules 2021, 11, 423. [Google Scholar] [CrossRef]
- Presse, N.; Belleville, S.; Gaudreau, P.; Greenwood, C.E.; Kergoat, M.J.; Morais, J.A.; Payette, H.; Shatenstein, B.; Ferland, G. Vitamin K status and cognitive function in healthy older adults. Neurobiol. Aging 2013, 34, 2777–2783. [Google Scholar] [CrossRef]
- Chouet, J.; Ferland, G.; Féart, C.; Rolland, Y.; Presse, N.; Boucher, K.; Barberger-Gateau, P.; Beauchet, O.; Annweiler, C. Dietary Vitamin K Intake Is Associated with Cognition and Behaviour among Geriatric Patients: The CLIP Study. Nutrients 2015, 7, 6739–6750. [Google Scholar] [CrossRef]
- Popescu, A.; German, M. Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment. Nutrients 2021, 13, 2206. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Glück, T.; Alter, P. Marine omega-3 highly unsaturated fatty acids: From mechanisms to clinical implications in heart failure and arrhythmias. Vasc. Pharmacol. 2016, 82, 11–19. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements. Nutrients 2018, 10, 1094. [Google Scholar] [CrossRef]
- Fekete, M.; Szőllősi, G.; Németh, A.N.; Varga, J.T. Clinical value of omega-3 polyunsaturated fatty acid supplementation in chronic obstructive pulmonary disease. Orvosi Hetil. 2021, 162, 23–30. [Google Scholar] [CrossRef]
- Witte, A.V.; Kerti, L.; Hermannstädter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex 2014, 24, 3059–3068. [Google Scholar] [CrossRef]
- Fekete, M.; Csípő, T.; Fazekas-Pongor, V.; Bálint, M.; Csizmadia, Z.; Tarantini, S.; Varga, J.T. The Possible Role of Food and Diet in the Quality of Life in Patients with COPD-A State-of-the-Art Review. Nutrients 2023, 15, 3902. [Google Scholar] [CrossRef] [PubMed]
- Marton, J.; Farkas, G.; Takacs, T.; Nagy, Z.; Szasz, Z.; Varga, J.; Jarmay, K.; Balogh, A.; Lonovics, J. Beneficial effects of pentoxifylline treatment of experimental acute pancreatitis in rats. Res. Exp. Med. 1997, 197, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Márton, J.; Farkas, G.; Nagy, Z.; Takacs, T.; Varga, J.; Szasz, Z.; Balogh, A.; Lonovics, J. Plasma levels of TNF and IL-6 following induction of acute pancreatitis and pentoxifylline treatment in rats. Acta Chir. Hung. 1997, 36, 223–225. [Google Scholar] [PubMed]
- Su, K.P.; Tseng, P.T.; Lin, P.Y.; Okubo, R.; Chen, T.Y.; Chen, Y.W.; Matsuoka, Y.J. Association of Use of Omega-3 Polyunsaturated Fatty Acids With Changes in Severity of Anxiety Symptoms: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e182327. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxidative Med. Cell. Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef]
- Ross, B.M.; Seguin, J.; Sieswerda, L.E. Omega-3 fatty acids as treatments for mental illness: Which disorder and which fatty acid? Lipids Health Dis. 2007, 6, 21. [Google Scholar] [CrossRef]
- Hodge, W.; Barnes, D.; Schachter, H.M.; Pan, Y.; Lowcock, E.C.; Zhang, L.; Sampson, M.; Morrison, A.; Tran, K.; Miguelez, M.; et al. Effects of omega-3 fatty acids on eye health. Evid. Rep. Technol. Assess. (Summ.) 2005, 1–6, PMCID:PMC4780934. [Google Scholar] [PubMed]
- Orchard, T.S.; Pan, X.; Cheek, F.; Ing, S.W.; Jackson, R.D. A systematic review of omega-3 fatty acids and osteoporosis. Br. J. Nutr. 2012, 107, S253–S260. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Mandal, C.C. Omega-3 fatty acids in pathological calcification and bone health. J. Food Biochem. 2020, 44, e13333. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, J. Vitamins and minerals: Efficacy and safety. Am. J. Clin. Nutr. 1997, 66, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Klimova, B.; Dziuba, S.; Cierniak-Emerych, A. The effect of healthy diet on cognitive performance among healthy seniors—A mini review. Front. Hum. Neurosci. 2020, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Alateeq, K.; Walsh, E.I.; Cherbuin, N. Dietary magnesium intake is related to larger brain volumes and lower white matter lesions with notable sex differences. Eur. J. Nutr. 2023, 62, 2039–2051. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-Reported Dietary Intake of Potassium, Calcium, and Magnesium and Risk of Dementia in the J apanese: The H isayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 164. [Google Scholar]
- East, P.; Doom, J.R.; Blanco, E.; Burrows, R.; Lozoff, B.; Gahagan, S. Iron deficiency in infancy and neurocognitive and educational outcomes in young adulthood. Dev. Psychol. 2021, 57, 962. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, H.; Wang, D.; Sudfeld, C.R.; Zhao, A.; Xin, Y.; Chen, J.C.; Fawzi, W.W.; Xing, Y.; Li, Z. Effect of Oral Iron Supplementation on Cognitive Function among Children and Adolescents in Low-and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 5332. [Google Scholar] [CrossRef]
- Schieffer, K.M.; Chuang, C.H.; Connor, J.; Pawelczyk, J.A.; Sekhar, D.L. Iron deficiency anemia is associated with hearing loss in the adult population. JAMA Otolaryngol. Head. Neck Surg. 2017, 143, 350. [Google Scholar] [CrossRef]
- Wolters, F.J.; Zonneveld, H.I.; Licher, S.; Cremers, L.G.; Ikram, M.K.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A.; Group, H.B.C.C.R. Hemoglobin and anemia in relation to dementia risk and accompanying changes on brain MRI. Neurology 2019, 93, e917–e926. [Google Scholar] [CrossRef]
- Hong, C.H.; Falvey, C.; Harris, T.B.; Simonsick, E.M.; Satterfield, S.; Ferrucci, L.; Metti, A.L.; Patel, K.V.; Yaffe, K. Anemia and risk of dementia in older adults: Findings from the Health ABC study. Neurology 2013, 81, 528–533. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.D. Oxidative Stress and Antioxidants in Neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Ong, T.P.; Jacob-Filho, W.; Jaluul, O.; Freitas, M.I.d.Á.; Cozzolino, S.M.F. Nutritional status of selenium in Alzheimer’s disease patients. Br. J. Nutr. 2010, 103, 803–806. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, W.; Cao, Z.; Lian, S.; Li, J.; Nie, J.; Huang, Y.; Zhao, K.; He, J.; Liu, C. Association of Selenium Levels with Neurodegenerative Disease: A Systemic Review and Meta-Analysis. Nutrients 2023, 15, 3706. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant therapies for Alzheimer’s disease. Oxidative Med. Cell. Longev. 2012, 2012, 472932. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Li, Y.; Gu, X.; Lei, Z. The correlation between selenium levels and autoimmune thyroid disease: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4398–4408. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium exposure and cancer risk: An updated meta-analysis and meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Takata, Y. Selenium and the prevention of prostate and colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1261–1272. [Google Scholar] [CrossRef]
- Vega-Cabello, V.; Caballero, F.F.; Lana, A.; Arias-Fernandez, L.; Banegas, J.R.; Rodriguez-Artalejo, F.; Lopez-Garcia, E.; Struijk, E.A. Association of Zinc Intake With Risk of Impaired Physical Function and Frailty Among Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2015–2022. [Google Scholar] [CrossRef]
- Ranjbar, E.; Shams, J.; Sabetkasaei, M.; M-Shirazi, M.; Rashidkhani, B.; Mostafavi, A.; Bornak, E.; Nasrollahzadeh, J. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr. Neurosci. 2014, 17, 65–71. [Google Scholar] [CrossRef]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5, 33. [Google Scholar] [CrossRef]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn superoxide dismutase: Not only a dismutase enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef]
- Pal, A.; Cerchiaro, G.; Rani, I.; Ventriglia, M.; Rongioletti, M.; Longobardi, A.; Squitti, R. Iron in Alzheimer’s Disease: From Physiology to Disease Disabilities. Biomolecules 2022, 12, 1248. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Kitala, K.; Tanski, D.; Godlewski, J.; Krajewska-Włodarczyk, M.; Gromadziński, L.; Majewski, M. Copper and Zinc Particles as Regulators of Cardiovascular System Function—A Review. Nutrients 2023, 15, 3040. [Google Scholar] [CrossRef]
- Gunturu, S.; Dharmarajan, T. Copper and zinc. Geriatr. Gastroenterol. 2020, 1–17. [Google Scholar] [CrossRef]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of copper in the onset of Alzheimer’s disease compared to other metals. Front. Aging Neurosci. 2018, 9, 446. [Google Scholar] [CrossRef]
- Varga, J.; Porszasz, J.; Boda, K.; Casaburi, R.; Somfay, A. Felügyelt magas intenzitású folyamatos és intervallum, valamint otthoni tréning hatásának vizsgálata krónikus obstruktív tüdőbetegek rehabilitációjában. Med. Thor. 2008, 61, 135–143. [Google Scholar]
- Pettersson, H.; Alexanderson, H.; Poole, J.L.; Varga, J.; Regardt, M.; Russell, A.-M.; Salam, Y.; Jensen, K.; Mansour, J.; Frech, T. Exercise as a multi-modal disease-modifying medicine in systemic sclerosis: An introduction by The Global Fellowship on Rehabilitation and Exercise in Systemic Sclerosis (G-FoRSS). Best Pract. Res. Clin. Rheumatol. 2021, 35, 101695. [Google Scholar] [CrossRef] [PubMed]
- Csizmadia, Z.; Ács, P.; Szőllősi, G.J.; Tóth, B.; Kerti, M.; Kovács, A.; Varga, J.T. Freedive Training Gives Additional Physiological Effect Compared to Pulmonary Rehabilitation in COPD. Int. J. Environ. Res. Public Health 2022, 19, 1549. [Google Scholar] [CrossRef] [PubMed]
- Gy, B.N.; Balikó, Z.; Kovács, G. Egészségügyi szakmai irányelv a krónikus obstruktív tüdőbetegség (COPD) diagnosztikájáról és kezeléséről, az alap, a szak és a sürgősségi ellátás területén. Med. Thor. 2014, 67, 76112. [Google Scholar]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef]
- Varga, J.; Boda, K.; Somfay, A. The effect of controlled and uncontrolled dynamic lower extremity training in the rehabilitation of patients with chronic obstructive pulmonary disease. Orvosi Hetil. 2005, 146, 2249–2255. [Google Scholar]
- Eastman, J.; Bahorik, A.; Kornblith, E.; Xia, F.; Yaffe, K. Sex Differences in the Risk of Dementia in Older Veterans. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1250–1253. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Thyagarajan, B.; Kim, J.K.; Weir, D.; Faul, J. Quest for a summary measure of biological age: The health and retirement study. Geroscience 2021, 43, 395–408. [Google Scholar] [CrossRef]
- Vaccarino, V.; Huang, M.; Wang, Z.; Hui, Q.; Shah, A.J.; Goldberg, J.; Smith, N.; Kaseer, B.; Murrah, N.; Levantsevych, O.M.; et al. Epigenetic Age Acceleration and Cognitive Decline: A Twin Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1854–1863. [Google Scholar] [CrossRef]
- Vetter, V.M.; Sommerer, Y.; Kalies, C.H.; Spira, D.; Bertram, L.; Demuth, I. Vitamin D supplementation is associated with slower epigenetic aging. Geroscience 2022, 44, 1847–1859. [Google Scholar] [CrossRef]
- Syed, M.A.; Aiyegbusi, O.L.; Marston, E.; Lord, J.M.; Teare, H.; Calvert, M. Optimising the selection of outcomes for healthy ageing trials: A mixed methods study. Geroscience 2022, 44, 2585–2609. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Kritchevsky, S.B. Endpoints for geroscience clinical trials: Health outcomes, biomarkers, and biologic age. Geroscience 2022, 44, 2925–2931. [Google Scholar] [CrossRef]
- Amgalan, A.; Maher, A.S.; Ghosh, S.; Chui, H.C.; Bogdan, P.; Irimia, A. Brain age estimation reveals older adults’ accelerated senescence after traumatic brain injury. Geroscience 2022, 44, 2509–2525. [Google Scholar] [CrossRef]
- Dorhout, B.G.; Doets, E.L.; van Dongen, E.J.I.; de Groot, L.; Haveman-Nies, A. In-Depth Analyses of the Effects of a Diet and Resistance Exercise Intervention in Older Adults: Who Benefits Most From ProMuscle in Practice? J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2204–2212. [Google Scholar] [CrossRef]
- Lee-Bravatti, M.A.; O’Neill, H.J.; Wurth, R.C.; Sotos-Prieto, M.; Gao, X.; Falcon, L.M.; Tucker, K.L.; Mattei, J. Lifestyle Behavioral Factors and Integrative Successful Aging Among Puerto Ricans Living in the Mainland United States. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1108–1116. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Song, X.Y.; Pan, X.F.; Feng, L.; Luo, N.; Yuan, J.M.; Pan, A.; Koh, W.P. Association Between Combined Lifestyle Factors and Healthy Ageing in Chinese Adults: The Singapore Chinese Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1796–1805. [Google Scholar] [CrossRef]
- Boumenna, T.; Scott, T.M.; Lee, J.S.; Zhang, X.; Kriebel, D.; Tucker, K.L.; Palacios, N. MIND Diet and Cognitive Function in Puerto Rican Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 605–613. [Google Scholar] [CrossRef]
- Maroto-Rodriguez, J.; Delgado-Velandia, M.; Ortola, R.; Garcia-Esquinas, E.; Martinez-Gomez, D.; Struijk, E.A.; Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Sotos-Prieto, M. A Mediterranean Lifestyle and Frailty Incidence in Older Adults: The Seniors-ENRICA-1 Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1845–1852. [Google Scholar] [CrossRef]
- Merono, T.; Zamora-Ros, R.; Hidalgo-Liberona, N.; Rabassa, M.; Bandinelli, S.; Ferrucci, L.; Fedecostante, M.; Cherubini, A.; Andres-Lacueva, C. Animal Protein Intake Is Inversely Associated With Mortality in Older Adults: The InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Ortola, R.; Garcia-Esquinas, E.; Sotos-Prieto, M.; Struijk, E.A.; Caballero, F.F.; Lopez-Garcia, E.; Rodriguez-Artalejo, F. Mediterranean Diet and Changes in Frequency, Severity, and Localization of Pain in Older Adults: The Seniors-ENRICA Cohorts. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Griswold, M.; Ranadive, R.; Bandeen-Roche, K.; Folsom, A.R.; Petruski-Ivleva, N.; Burgard, S.; Kucharska-Newton, A.; Windham, B.G. Midlife Cardiovascular Health and Robust Versus Frail Late-Life Status: The Atherosclerosis Risk in Communities Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.T.; Jiang, Y.W.; Feng, L.; Pan, A.; Koh, W.P. Dietary Total Antioxidant Capacity and Late-Life Cognitive Impairment: The Singapore Chinese Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 561–569. [Google Scholar] [CrossRef]
- Jung, S.J.; Lee, G.B.; Nishimi, K.; Chibnik, L.; Koenen, K.C.; Kim, H.C. Association between psychological resilience and cognitive function in older adults: Effect modification by inflammatory status. Geroscience 2021, 43, 2749–2760. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tabák, A.G.; Adany, R.; Purebl, G.; Kaposvári, C.; Fazekas-Pongor, V.; Csípő, T.; Szarvas, Z.; Horváth, K.; Mukli, P. The Semmelweis Study: A longitudinal occupational cohort study within the framework of the Semmelweis Caring University Model Program for supporting healthy aging. Geroscience 2023, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, I.; Marston, L.; Mukadam, N. Which components of the Mediterranean diet are associated with dementia? A UK Biobank cohort study. Geroscience 2022, 44, 2541–2554. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Garagnani, P.; Santoro, A.; Giuliani, C.; Ostan, R.; Fabbri, C.; Milazzo, M.; Gentilini, D.; di Blasio, A.M.; Pietruszka, B.; et al. One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: A pilot study from the NU-AGE project. Geroscience 2020, 42, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Madarász, B.; Fazekas-Pongor, V.; Szarvas, Z.; Fekete, M.; Varga, J.T.; Tarantini, S.; Csiszar, A.; Lionetti, V.; Tabák, A.G.; Ungvari, Z.; et al. Survival and longevity of European rulers: Geographical influences and exploring potential factors, including the Mediterranean diet—A historical analysis from 1354 to the twentieth century. Geroscience 2023. [Google Scholar] [CrossRef]
- Hegedűs, B.; Varga, J.; Somfay, A. Az interdiszciplináris rehabilitáció hatása spondylitis ankylopoeticában szenvedő betegekben. Orvosi Hetil. 2016, 157, 1126–1132. [Google Scholar] [CrossRef]
- Varga, J.T.; Munkácsi, A.; Máthe, C.; Somfay, A.; Bálint, B.; Lovász, O.; Várdi, K.; Pesti, A.; Koncz, M.; Szilasi, M. A belégző izmok fizikai tréningjének hatása a betegek fizikai állapotára COPD-ben. Med. Thorac. 2018, 71, 96–102. [Google Scholar]
- Vágvölgyi, A.; Rozgonyi, Z.; Vadász, P.; Varga, J.T. A mellkassebészeti műtéti teherbíró képesség megítélése, perioperatív légzésrehabilitáció. Orvosi Hetil. 2017, 158, 1989–1997. [Google Scholar] [CrossRef]
- Varga, J. A légzésrehabiliáció elméleti és gyakorlati lapjai. Ellátási Színterei. Korányi Bull. 2016, 1, 44–47. [Google Scholar]
- Kraal, A.Z.; Dotterer, H.L.; Sharifian, N.; Morris, E.P.; Sol, K.; Zaheed, A.B.; Smith, J.; Zahodne, L.B. Physical Activity in Early- and Mid-Adulthood Are Independently Associated With Longitudinal Memory Trajectories in Later Life. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1495–1503. [Google Scholar] [CrossRef]
- Yoneda, T.; Lewis, N.A.; Knight, J.E.; Rush, J.; Vendittelli, R.; Kleineidam, L.; Hyun, J.; Piccinin, A.M.; Hofer, S.M.; Hoogendijk, E.O.; et al. The Importance of Engaging in Physical Activity in Older Adulthood for Transitions Between Cognitive Status Categories and Death: A Coordinated Analysis of 14 Longitudinal Studies. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1661–1667. [Google Scholar] [CrossRef]
- Zabetian-Targhi, F.; Srikanth, V.K.; Beare, R.; Breslin, M.; Moran, C.; Wang, W.; Wu, F.; Smith, K.J.; Callisaya, M.L. The Association Between Physical Activity Intensity, Cognition, and Brain Structure in People With Type 2 Diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Palinkas, A.; Lajko, I.; Horváth, I.; Boda, K.; Somfay, A. Pulmonary arterial pressure response during exercise in COPD: A correlation with C-reactive protein (hsCRP). Open Respir. Med. J. 2016, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, C.; Hausman, H.K.; Kraft, J.N.; Albizu, A.; O’Shea, A.; Boutzoukas, E.M.; Evangelista, N.D.; Langer, K.; Van Etten, E.J.; Bharadwaj, P.K.; et al. Proximal improvement and higher-order resting state network change after multidomain cognitive training intervention in healthy older adults. Geroscience 2022, 44, 1011–1027. [Google Scholar] [CrossRef]
- Baciu, M.; Banjac, S.; Roger, E.; Haldin, C.; Perrone-Bertolotti, M.; Loevenbruck, H.; Demonet, J.F. Strategies and cognitive reserve to preserve lexical production in aging. Geroscience 2021, 43, 1725–1765. [Google Scholar] [CrossRef]
- Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Bhat, A.; Bishir, M.; Bolla, S.R.; Yang, J.; Essa, M.M.; Chidambaram, S.B.; Guillemin, G.J.; et al. Sleep, brain vascular health and ageing. Geroscience 2020, 42, 1257–1283. [Google Scholar] [CrossRef]
- Gosalia, J.; Montgomery, P.S.; Zhang, S.; Pomilla, W.A.; Wang, M.; Liang, M.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A.; Proctor, D.N.; et al. Increased pulse wave velocity is related to impaired working memory and executive function in older adults with metabolic syndrome. Geroscience 2022, 44, 2831–2844. [Google Scholar] [CrossRef]
- Aliberti, M.J.R.; Szlejf, C.; Lima-Costa, M.F.; de Andrade, F.B.; Alexandre, T.S.; Ferri, C.P.; Suemoto, C.K. Frailty Modifies the Association of Hypertension With Cognition in Older Adults: Evidence From the ELSI-Brazil. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1134–1143. [Google Scholar] [CrossRef]
- Lennon, M.J.; Lam, B.C.P.; Crawford, J.; Brodaty, H.; Kochan, N.A.; Trollor, J.N.; Numbers, K.; Draper, B.; Thalamuthu, A.; Sachdev, P.S. Does Antihypertensive Use Moderate the Effect of Blood Pressure on Cognitive Decline in Older People? J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 859–866. [Google Scholar] [CrossRef]
- Li, F.R.; Yang, H.L.; Zhou, R.; Zheng, J.Z.; Chen, G.C.; Wu, X.X.; Zou, M.C.; Wang, J.Y.; Fu, Q.; Wu, X.B. Influence of Diabetes Duration and Glycemic Control on Dementia: A Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2062–2070. [Google Scholar] [CrossRef]
- Marseglia, A.; Darin-Mattsson, A.; Skoog, J.; Ryden, L.; Hadarsson-Bodin, T.; Kern, S.; Rydberg Sterner, T.; Shang, Y.; Zettergren, A.; Westman, E.; et al. Metabolic Syndrome Is Associated With Poor Cognition: A Population-Based Study of 70-Year-Old Adults Without Dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2275–2283. [Google Scholar] [CrossRef]
- Davidson, K.T.; Zhu, Z.; Balabanov, D.; Zhao, L.; Wakefield, M.R.; Bai, Q.; Fang, Y. Beyond conventional medicine-a look at blueberry, a cancer-fighting superfruit. Pathol. Oncol. Res. 2018, 24, 733–738. [Google Scholar] [CrossRef]
- Nicolson, G.L. Lipid replacement/antioxidant therapy as an adjunct supplement to reduce the adverse effects of cancer therapy and restore mitochondrial function. Pathol. Oncol. Res. 2005, 11, 139–144. [Google Scholar] [CrossRef]
- Rusz, O.; Kahán, Z. Bone homeostasis and breast cancer: Implications for complex therapy and the maintenance of bone integrity. Pathol. Oncol. Res. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Farkas, Á.; Szipőcs, A.; Horváth, A.; Horváth, I.; Gálffy, G.; Varga, J.; Galambos, K.; Kugler, S.; Nagy, A.; Szalai, Z. Establishment of relationships between native and inhalation device specific spirometric parameters as a step towards patient tailored inhalation device selection. Respir. Med. 2019, 154, 133–140. [Google Scholar] [CrossRef]
- Zamanzadeh, V.; Jasemi, M.; Valizadeh, L.; Keogh, B.; Taleghani, F. Effective factors in providing holistic care: A qualitative study. Indian. J. Palliat. Care 2015, 21, 214. [Google Scholar] [PubMed]
| Inclusion Criteria | Description |
|---|---|
| Study design | Randomized controlled trial or human clinical trial. |
| Study population | Healthy people or patients admitted with a diagnosis of mild cognitive impairment or Alzheimer’s disease. |
| Intervention | Vitamins, antioxidants, minerals and micronutrients interventions. |
| Language of publication | No language restrictions applied. |
| Published articles | In the PubMed, ClinicalTrials.gov and Cochrane Central Register of Controlled Trials (CENTRAL) databases. |
| Output concepts | Different cognitive functions and their measurement tools, such as validated questionnaires: Full-Scale Intelligence Quotient (FSIQ), Wechsler Adult Intelligence Scale (WAIS), Mini Mental State Examination (MMSE), Stroop Color and Word Test (STROOP), Addenbrooke’s Cognitive Examination-Revised (ACE-R), Verbal Fluency Test. Cognitive index score and different cognitive function tests: attention, calculation, memory, verbal fluency, psychomotor speed, visual-constructional ability, neuropsychological function, reaction time, psychocognitive tests, etc. |
| Exclusion Criteria | |
| Animal experiments. | |
| In vitro studies. | |
| Macronutrients interventions, proteins, carbohydrates, fats, foods, medicines, pharmaceuticals, herbs, essential oils, melatonin and complex diets, such as Dietary Approach to Stop Hypertension (DASH diet), high-protein diet, ketogenic diet, low-fat diet, mediterranean diet, low glycaemic index (GI) diet, Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND). | |
| Interventions for various diseases, such as: tumour diseases, epilepsy, post-traumatic stress disorder, anxiety, depression, stroke, multiple sclerosis, chronic cerebral ischaemia, polycystic kidney disease, opioid patients, psychosis, delirium, schizophrenia, bipolar disorder, frontal lobe atrophy, COVID-19 patients, sepsis, autism, attention deficit hyperactivity disorder (ADHD), Wernicke-Korsakoff syndrome, Fragile X syndrome, and Down syndrome. | |
| Interventions for different ages and conditions, such as: infancy, adolescence, pregnancy and interventions for athletes. | |
| Dietary advice, food and nutrition interventions. | |
| Short-term interventions (<4 weeks). | |
| Intravenous or intramuscular interventions. | |
| Dietary supplement interventions for underweight patients (body mass index (BMI): <18.5 kg/m2). | |
| Study | Design | Mean Follow-Up | Country | Sample Size | Average Age (Year) | Sex Male/Female (%) | Intervention | Main Results |
|---|---|---|---|---|---|---|---|---|
| Zhang C et al. [75] | RCT | 30 days | China | 102 | 41.0 ± 9.1 | 47/53 | Magtein: 400 mg® Vitamin D3: 80 IU Vitamin C: 12 mg Vitamin B6: 4 mg; Phosphatidylserine 50 mg. Total: 2 g/person/day. | Magtein®PS: Significant improvement in memory and cognition in healthy chinese adults (p < 0.001). |
| Ma F et al. [77] | RCT | 6 months | China | 240 | 70.5 ± 9.1 | 35/65 | Four treatment groups: 800 µg FA only, 25 µg vitamin B12 only, FA, and vitamin B12 supplementation or control group. | Supplementation with FA and Vitamin B12: Significant Improvements in FSIQ (d = 0.169, p = 0.024), verbal IQ (d = 0.146, p = 0.033), Information (d = 0.172, p = 0.019), and Digit Span Scores. |
| Gibson GE et al. [73] | RCT | 12 months | USA | 70 | 75.7 ± 7.0 | 41.4/58.6 | Benfotiamine treatment (300 mg/day twice a day) versus placebo group. | Benfotiamine Group vs. Placebo Group: 43% lower increase in ADAS-Cog Scores and 77% less worsening in CDR (p = 0.034) in Benfotiamine Group. |
| Kwok T et al. [79] | RCT | 24 months | Hong Kong | 279 | 78.0 ± 5.3 | 56.1/43.9 | MCI patients administered 500 μg methylcobalamin and 400 μg FA orally once daily. | Supplementation with Vitamin B12 and FA: No reduction in cognitive decline in older individuals with MCI and elevated serum homocysteine. |
| Bai D et al. [76] | RCT | 6 months | China | 138 | 68.3 ± 6.3 | 40/60 | FA (60.0 mg/day) + DHA (8 mg/day), FA (800.0 mg/day), DHA (8 mg/day) versus placebo. | FA, DHA, and FA + DHA vs. placebo: improvements in FSIQ, arithmetic, and picture complement scores with folic acid; FSIQ, information, arithmetic, and digit span scores with DHA; greater enhancements in arithmetic (1.67, 95% CI 1.02 to 2.31) and digit span (1.33, 95% CI 0.24 to 2.43) scores with FA + DHA. |
| Ma F et al. [74] | RCT | 24 months | China | 180 | 74.8 ± 2.8 | 42.7/57.3 | FA 400 µg/day. | FA supplementation linked to improved cognitive function and reduced blood levels of Aβ-related biomarkers in MCI (p < 0.05). |
| Li M et al. [78] | RCT | 6 months | China | 240 | 70.4 ± 6.7 | 42.5/57.5 | Intervention groups: FA + DHA (FA 800 μg/d + DHA 800 mg/d), FA (FA 800 μg/d), DHA (DHA 800 mg/d), and placebo. | Daily oral FA, DHA, and combined use for 6 months: significant improvements in FSIQ and select WAIS subtests compared to placebo (p < 0.05). |
| Study | Design | Mean Follow-Up | Country | Sample Size | Average Age (Year) | Sex Male/Female (%) | Intervention | Main Results |
|---|---|---|---|---|---|---|---|---|
| Sim M et al. [82] | RCT | 4 weeks | Republic of Korea | 214 | 20–39 | 39.3/60.7 | 500 mg vitamin C twice a day. | Vitamin C supplementation notably increased attention and work absorption (p = 0.03), with a clear tendency towards fatigue improvement. |
| Morató X et al. [86] | RCT | 12 months | Spain | 50/50 | 73.1 ± 7.5 | 40/60 | Standardized extract of Ginkgo biloba EGb 761 240 mg tablets were given orally. | No significant differences between groups in MMSE, CDR, NBACE scores, or amnestic profile; higher scores in irritability/lability parameter (p = 0.006) and BDS (p = 0.048) in control group. |
| Thaung Zaw JJ et al. [80] | RCT | 24 months | Australia | 125 | 45–85 | 100% female | 75 mg trans-resveratrol or placebo per day. | Resveratrol supplementation led to 33% improvement in overall cognitive performance (Cohen’s d = 0.170, p = 0.005). |
| Lai S et al. [84] | RCT | 6 months | Italy | 59 | 45.1 ± 10.7 | 55.9/44.1 | ALA 1.6 g/day. | BDI-II, HAM-D, MMSE tests showed significant improvement (p = 0.007, p < 0.001, p < 0.001) in patients treated with ALA compared to control group. |
| Foroumandi E et al. [81] | RCT | 4 months | Iran | 82 | 72.0 ± 2.5 | 34.1/65.9 | Intervention group patients received 500 mg dry extract (5 cc) of fenugreek seed extract. | Positive effects on memory (p < 0.001), quality of life (p < 0.001) and selective oxidative index level. |
| Baker LD et al. [87] | RCT | 3 years | USA | 2262 | 73.0 | 40/60 | Daily administration of cocoa extract (containing 500 mg/day flavanols) versus placebo. | Cocoa extract had no effect on global cognition (mean z-score = 0.03, 95% CI: −0.02–0.08; p = 0.28). |
| Bell L et al. [88] | RCT | 12 weeks | UK | 60 | 18–30 | 10/90 | 400 mg of GSPE extract daily. | 400 mg of GSPE did not consistently improve cognitive function in healthy young adults. |
| Stringham NT et al. [83] | RCT | 6 months | Greece | 59 | 18–25 | 45.7/54.3 | Carotenoids lutein and zeaxanthin, along with the zeaxanthin isomer meso-zeaxanthin (13 mg/day or 27 mg/day total). | For cognitive measures, all scores for composite memory, verbal memory, sustained attention, psychomotor speed, and processing speed improved significantly in treatment groups (p < 0.05 for all) and remained unchanged in the placebo group. |
| Yoon J et al. [85] | RCT | 12 weeks | Japan | 36 + 36 | 69.5 ± 7.6 | 47/53 | Participants received 3 g of DOTP or placebo in olive oil twice daily for 12 weeks. | Among cognitive domains, complex attention had a significant time × group interaction effect (p = 0.049) between the DOTP and placebo groups. Time effects were significant (p < 0.05) for psychomotor speed, reaction time, cognitive flexibility, processing speed, and executive function domains. |
| Hashimoto M et al. [89] | RCT | 12 months | Japan | 44 | 70.2 ± 1.4 | 52.4/47.6 | Randomized participants in the PO group received soft gelatin capsules containing 1.47 mL of PO daily, and those in the PO + POPP group received soft gelatin capsules containing both 1.47 mL of PO and 1.12 g PP daily. | At the end of intervention, the POPP group showed significantly higher cognitive index scores than the PO group, POOP may improve age-related cognitive impairment in healthy elderly people. |
| Study | Design | Mean Follow-Up | Country | Sample Size | Average Age (Year) | Sex Male/Female (%) | Intervention | Main Results |
|---|---|---|---|---|---|---|---|---|
| Jia J et al. [93] | RCT | 12 months | China | 210 | 68.0 ± 5.9 | 45/55 | Patients received 800 IU/day of vitamin D. | The FSIQ and cognitive test score were significantly higher in the intervention group than in the control group (p < 0.001). |
| Bischoff-Ferrari HA et al. [92] | RCT | 3 years | Switzerland | 2157 | 74.9 | 38.3/61.7 | 2000 IU/day of vitamin D3, 1 g/day of omega-3 strength-training exercise program. | Among adults aged 70 years or older, treatment with vitamin D3, omega-3, or a strength-training exercise program did not result in statistically significant differences in improving cognitive function. |
| Zajac IT et al. [90] | RCT | 6 months | Australia | 436 | 60–90 | 48.4/51.6 | 600 IU/day of vitamin D3 | No effect. |
| Yang T et al. [94] | RCT | 12 months | China | 183 | 67.2 ± 6.1 | 46/54 | 800 IU/day of vitamin D3 | The ANOVA showed improvements in the FSIQ, information, digit span, vocabulary, block design, and picture arrangement scores in the vitamin D group over the placebo group (p < 0.001). |
| Byrn MA et al. [99] | RCT | 12 weeks | USA | 206 | 55.71 | 17/83 | Administration of either weekly vitamin D3 supplementation (50,000 IU) or 5000 IU cholecalciferol once a week. | No significant differences in cognitive outcomes between participants who received high-dose therapy and those who received low dose. |
| Castle M et al. [95] | RCT | 1 year | USA | 138 | 58 ± 6 | 100% female | Vitamin D3 supplementation (600, 2000, or 4000 IU/day). | The CANTAB test results indicated that the 2000 IU/d group, when compared to other groups, performed better in PAL test parameters (p < 0.05). RTI was slower in the 4000 IU/d compared to 600 IU/d group for the 5-choice test (p < 0.01). |
| Jorde R et al. [91] | RCT | 4 months | Norway | 422 | 52 | 52.9/47.1 | Vitamin D 100,000 IU administered as a bolus dose followed by 20,000 IU per week versus placebo. | Vitamin D supplementation did not improve cognitive function during a four-month intervention. |
| Ghaderi A et al. [96] | RCT | 24 weeks | Iran | 64 | 59.2 ± 11.3 | 53/47 | Administration of either 50,000 IU vitamin D supplements (n = 32) or placebo (n = 32) every 2 weeks. | Subjects who were administered vitamin D had a significant reduction in IGT (β −6.25; 95% CI, −8.60 to −3.90; p < 0.001), and significant increases in VFT (β 2.82; 95% CI, 0.78–4.86; p = 0.007), immediate LM (β 1. 32; 95% CI, 0.27–2.37; p = 0.01), reverse DGS (β 2.06; 95% CI, 1.18–2.94; p < 0.001) and VWM (β 0.75; 95% CI, 0.33–1.16; p = 0.001). |
| Schietzel S et al. [98] | RCT | 2 years | Switzerland | 273 | 70.3 | 46.5/53.5 | 2000 or 800 IU vitamin D3/day | No effect. |
| Beauchet O et al. [100] | RCT | 3 months | Canada | 40 | ≥65 | 100% female | Fortified yogurt (400 IU vitamin D and 800 mg calcium). | Fortified yogurts with vitamin D and calcium maintained global cognitive performance (MMSE score (p = 0.022). Global cognitive performance decreased in the control group. |
| Owusu JE et al. [101] | RCT | 3 years | USA | 260 | 68.2 ± 4.9 | 100% female | Adminsitration of vitamin D (adjusted to achieve a serum level > 30 ng/mL) with calcium (diet and supplement total of 1.200 mg) | There is no evidence that vitamin D intakes above the recommended daily allowance are needed to prevent cognitive decline in this population. |
| Hu J et al. [97] | RCT | 12 months | China | 181 | 67.22 ± 6.1 | 44.5/55.5 | Administration of 800 IU/day of vitamin D. | The mean scores of information, DGS, vocabulary, block design and picture arrangement tests in the vitamin D3 group were significantly higher than that in the placebo group both before and after adjustment. In addition, the performance of FIQ (p < 0.001, d = 0.70), VIQ (p < 0.001, d = 0.77) and PIQ (p < 0.001, d = 0.70) was consistent with the five subtests mentioned above. |
| Macpherson H et al. [102] | RCT | 6 months | Australia | 147 | 70.2 ± 6.1 | 30/70 | Daily vitamin D (1000 IU) and omega-3 (900 mg EPA, 600 mg DHA) and protein (20 g) supplementation. | There were no significant between-group differences in cognition at 6 or 12 months. |
| Study | Design | Mean Follow-Up | Country | Sample Size | Average Age (Year) | Sex Male/Female (%) | Intervention | Main Results |
|---|---|---|---|---|---|---|---|---|
| Lin PY et al. [108] | RCT | 24 months | Taiwan | 163 | 77.8 ± 8.4 | 66.2/33.8 | 163 patients were randomly assigned to DHA (0.7 g/day), EPA (1.6 g/day), or EPA (0.8 g/day) + DHA (0.35 g/day) group for 24 months. | A statistically significant difference in cognitive, functional, and mood status scores, biochemical profiles, and inflammatory cytokines levels was not determined between the placebo and treatment groups. |
| Nolan JM et al. [103] | RCT | 12 months | Ireland | 50 + 27 | ≥65 | 53/47 | Patients consumed 1 g fish oil (of which 500 mg DHA, 150 mg EPA), 22 mg carotenoids (10 mg lutein, 10 mg meso-zeaxanthin, 2 mg zeaxanthin), and 15 mg vitamin E daily. | The active group performed better in objective measures of AD severity (i.e., memory and mood), with a statistically significant difference in the clinical collateral for memory (p < 0.001). |
| Giudici KV et al. [109] | RCT | 3 years | France | 1445 | 75.3 ± 4.4 | 35.8/64.2 | ω-3 (800 mg DHA and 225 mg EPA/day) | No effect. |
| Arellanes IC et al. [113] | RCT | 6 months | USA | 33 | 68 (58–90) | 18.2/81.8 | 33 individuals were provided with a vitamin B complex (1 mg of vitamin B12, 100 mg of vitamin B6 and 800 mcg of folic acid per day) and randomized to 2152 mg of DHA per day or placebo over 6 months. | No effect. |
| Rasmussen J. [107] | RCT | 24 months | Finland, Germany, Netherlands, Sweden | 311 | >65 | 45/55 | Patients were given a combination of DHA, EPA, uridine monophosphate, choline, phospholipids, selenium, folic acid, and vitamins B12 and E (>200% the recommended daily intake). | This intervention had the potential to improve the progression of Alzheimer’s disease (attention, memory, executive function p < 0.05). |
| Stavrinou PS et al. [104] | RCT | 6 months | Cyprus | 36 | 78.8 ± 7.3 | 38.8/61.2 | 20 mL dose of a formula containing a mixture of omega-3 (810 mg EPA and 4140 mg DHA) and omega-6 FA (1800 mg gamma-LA and 3150 mg LA) (1:1 w/w), with 0.6 mg of vitamin A, vitamin E (22 mg) plus pure γ-tocopherol (760 mg). | A significant interaction between supplementation and time was found on cognitive function (MMSE, ACE-R, STROOP) functional capacity (6 min walk test; p = 0.028) fatigue (p < 0.001), physical health (p = 0.007), and daily sleepiness (p = 0.007). |
| Mengelberg A et al. [110] | RCT | 12 months | New Zealand | 30 + 30 | 72.3 ± 6.1 | 69.8/30.2 | 1491 mg of DHA + 351 mg of EPA per day. | No effect. |
| Atmadja T et al. [105] | RCT | 90 days | Indonesia | 29 | 66.1 ± 5.3 | 20.6/79.4 | Subjects were divided into three groups: SO, CFO, and CO with omega-3 (catfish oil enriched with omega-3). The intervention involved 1000 mg of oil/day. | Significant effects on oxidative stress and cognitive function (p < 0.05), and significantly increased MMSE score (p < 0.05). |
| Leckie RL et al. [111] | RCT | 18 weeks | USA | 271 | 30–54 | 43.5/56.5 | Fish oil capsules (1400 mg/day of EPA and DHA). | No effect. |
| Kuszewski JC et al. [114] | RCT | 16 weeks | Australia | 64 | 65.8 ± 1.4 | 44/56 | Randomly assigned to either fish oil (2000 mg/d of DHA + 400 mg/d of EPA), curcumin (160 mg/d), or a combination. | Fish oil improved CVR to a processing speed test (4.4% ± 1.9% vs. −2.2% ± 2.1%; p = 0.023) and processing speed in males only (Z-score: 0.6 ± 0.2 vs. 0.1 ± 0.2; p = 0.043). |
| Patan MJ et al. [106] | RCT | 26 h | UK | 310 | 25–49 | 35/65 | Participants consumed either 900 mg of DHA/d and 270 mg of EPA/d (DHA-rich oil), 360 mg of DHA/d and 900 mg of EPA/d (EPA-rich oil), or 3000 mg/d of refined olive oil (placebo). | EPA supplementation improved global cognitive function, superior to oil enriched with DHA; improved memory accuracy compared with DHA (p = 0.034) |
| Sueyasu T et al. [112] | RCT | 24 weeks | Japan | 71 | 65.7 ± 1.0 | 45.1/54.9 | PUFA in Combination with LZ (containing 120 mg ARA, 300 mg DHA, and 100 mg EPA per day) combined with LZ (containing 10 mg lutein and 2 mg zeaxanthin per day). | LCPUFAs + LZ supplementation did not significantly affect memory function. |
| Vitamins | Minerals | Antioxidants |
|---|---|---|
| Vitamin B Complex | Magnesium | Beta-carotene |
| Vitamin A | Selenium | Lutein |
| Vitamin C | Copper | Lycopene |
| Vitamin D | Iron | Coenzyme Q10 |
| Vitamin E | Zinc | Polyphenols |
| Vitamin K | Potassium | Curcumin (Turmeric) |
| Choline | Calcium | Acetyl-L-Carnitine |
| Omega 3 Polyunsaturated Fatty Acids (fish oils) | ||
| Probiotics, Prebiotics, Synbiotics | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete, M.; Lehoczki, A.; Tarantini, S.; Fazekas-Pongor, V.; Csípő, T.; Csizmadia, Z.; Varga, J.T. Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements. Nutrients 2023, 15, 5116. https://doi.org/10.3390/nu15245116
Fekete M, Lehoczki A, Tarantini S, Fazekas-Pongor V, Csípő T, Csizmadia Z, Varga JT. Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements. Nutrients. 2023; 15(24):5116. https://doi.org/10.3390/nu15245116
Chicago/Turabian StyleFekete, Mónika, Andrea Lehoczki, Stefano Tarantini, Vince Fazekas-Pongor, Tamás Csípő, Zoltán Csizmadia, and János Tamás Varga. 2023. "Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements" Nutrients 15, no. 24: 5116. https://doi.org/10.3390/nu15245116
APA StyleFekete, M., Lehoczki, A., Tarantini, S., Fazekas-Pongor, V., Csípő, T., Csizmadia, Z., & Varga, J. T. (2023). Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements. Nutrients, 15(24), 5116. https://doi.org/10.3390/nu15245116






