A Population-Based Cross-Sectional Study of Paediatric Coeliac Disease in Catalonia Showed a Downward Trend in Prevalence Compared to the Previous Decade
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cross-Sectional Study (2013–2019): Subjects and Study Design
2.2. Antibody Detection
2.3. Genetic Markers
2.4. Duodenal Biopsy by Histopathology and Flow Cytometry
2.5. Diagnosis of CD
2.6. Ethical Considerations
2.7. Previous Cross-Sectional Study (2004–2007)
2.8. Factors with Potential Impact on CD Prevalence
2.9. Statistical Analyses
3. Results
3.1. CD Prevalence in Both Cross-Sectional Studies
3.2. Evolution of Factors with a Potential Impact on CD Prevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Coeliac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; deBruyn, J.; Ronksley, P.E.; Shaheen, A.A.; et al. Incidence of Coeliac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- Mariné, M.; Farre, C.; Alsina, M.; Vilar, P.; Cortijo, M.; Salas, A.; Fernández-Bañares, F.; Rosinach, M.; Santaolalla, R.; Loras, C.; et al. The prevalence of coeliac disease is significantly higher in children compared with adults. Aliment. Pharmacol. Ther. 2011, 33, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Myléus, A.; Ivarsson, A.; Webb, C.; Danielsson, L.; Hernell, O.; Högberg, L.; Karlsson, E.; Lagerqvist, C.; Norström, F.; Rosén, A.; et al. Coeliac disease revealed in 3% of Swedish 12-year-olds born during an epidemic. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 170–176. [Google Scholar] [CrossRef]
- Matysiak-Budnik, T.; Malamut, G.; de Serre, N.P.; Grosdidier, E.; Seguier, S.; Brousse, N.; Caillat-Zucman, S.; Cerf-Bensussan, N.; Schmitz, J.; Cellier, C. Long-term follow-up of 61 coeliac patients diagnosed in childhood: Evolution toward latency is possible on a normal diet. Gut 2007, 56, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- IDESCAT. Institut D’estadística de Catalunya. Available online: http://www.idescat.cat/territ/BasicTerr?TC=5&V0=3&V1=3&V3=669&V4=498&P=N&PARENT=1&CTX=B&ALLINFO=TRUE&ANYS=2003&x=10&y=5 (accessed on 13 January 2023).
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘coeliac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Rostami, K.; Kerckhaert, J.P.; Tiemessen, R.; Meijer, J.W.; Mulder, C.J. The relationship between anti-endomysium antibodies and villous atrophy in coeliac disease using both monkey and human substrate. Eur. J. Gastroenterol. Hepatol. 1999, 11, 439–442. [Google Scholar] [CrossRef]
- Hayat, M.; Cairns, A.; Dixon, M.F.; O’Mahony, S. Quantitation of intraepithelial lymphocytes in human duodenum: What is normal? J. Clin. Pathol. 2002, 55, 393–394. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, P.; Carreras, G.; Fajardo, I.; Tristán, E.; Carrasco, A.; Salvador, I.; Zabana, Y.; Andújar, X.; Ferrer, C.; Horta, D.; et al. Intraepithelial Lymphocyte Cytometric Pattern Is a Useful Diagnostic Tool for Coeliac Disease Diagnosis Irrespective of Degree of Mucosal Damage and Age-A Validation Cohort. Nutrients 2021, 13, 1684. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Granmo V7.11 y Ene 3.0. Available online: http://www.imim.cat/ofertadeserveis/softwarep_blic.html (accessed on 5 November 2023).
- Fay, M.P.; Feuer, E.J. Confidence intervals for directly standardized rates: A method based on the gamma distribution. Stat. Med. 1997, 16, 791–801. [Google Scholar] [CrossRef]
- IDESCAT. Institut d’estadística de Catalunya. Available online: http://www.idescat.cat/pub/?id=ep&n=9123 (accessed on 21 February 2021).
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Lehmann, E.L.; Romano, J.P. Testing Statistical Hypotheses, 3rd ed.; Springer: New York, NY, USA, 2005; pp. 110–149. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 5 November 2023).
- Roberts, S.E.; Morrison-Rees, S.; Thapar, N.; Benninga, M.A.; Borrelli, O.; Broekaert, I.; Dolinsek, J.; Martin-de-Carpi, J.; Mas, E.; Miele, E.; et al. Systematic review and meta-analysis: The incidence and prevalence of paediatric coeliac disease across Europe. Aliment. Pharmacol. Ther. 2021, 54, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Almazán, M.V.; Ortega, E.; Moreno Torres, R.; Tovar, M.; Romero, J.; López-Casado, M.Á.; Jáimez, L.; Jiménez-Jáimez, J.; Ballesteros, A.; Caballero-Villarraso, J.; et al. Diagnostic screening for subclinical celiac disease using a rapid test in children aged 2–4. Pediatr. Res. 2015, 78, 280–285. [Google Scholar] [CrossRef]
- Cilleruelo, M.L.; Fernández-Fernández, S.; Jiménez-Jiménez, J.; Rayo, A.I.; de Larramendi, C.H. Prevalence and Natural History of Celiac Disease in a Cohort of At-risk Children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 739–745. [Google Scholar] [CrossRef]
- Levinson-Castiel, R.; Eliakim, R.; Shinar, E.; Perets, T.T.; Layfer, O.; Levhar, N.; Schvimer, M.; Marderfeld, L.; Ben-Horin, S.; Shamir, R. Rising prevalence of coeliac disease is not universal and repeated testing is needed for population screening. United Eur. Gastroenterol. J. 2019, 7, 412–418. [Google Scholar] [CrossRef]
- Ivarsson, A.; Myléus, A.; Norström, F.; van der Pals, M.; Rosén, A.; Högberg, L.; Danielsson, L.; Halvarsson, B.; Hammarroth, S.; Hernell, O.; et al. Prevalence of childhood coeliac disease and changes in infant feeding. Paediatrics 2013, 131, e687–e694. [Google Scholar] [CrossRef]
- Stahl, M.; Li, Q.; Lynch, K.; Koletzko, S.; Mehta, P.; Gragert, L.; Norris, J.M.; Andrén Aronsson, C.; Lindfors, K.; Kurppa, K.; et al. Incidence of Paediatric Coeliac Disease Varies by Region. Am. J. Gastroenterol. 2023, 118, 539–545. [Google Scholar] [CrossRef]
- Makharia, G.K.; Chauhan, A.; Singh, P.; Ahuja, V. Review article: Epidemiology of coeliac disease. Aliment. Pharmacol. Ther. 2022, 56 (Suppl. S1), S3–S17. [Google Scholar] [CrossRef]
- Díez-Domingo, J.; Garcés-Sánchez, M.; Giménez-Sánchez, F.; Colomina-Rodríguez, J.; Martinón-Torres, F. ¿Qué hemos aprendido sobre rotavirus en España en los últimos 10 años? [What have we learnt about rotavirus in Spain in the last 10 years?]. An. Pediatría (Engl. Ed.) 2019, 91, 166–179. [Google Scholar]
- Hemming-Harlo, M.; Lähdeaho, M.L.; Mäki, M.; Vesikari, T. Rotavirus Vaccination Does Not Increase Type 1 Diabetes and May Decrease Coeliac Disease in Children and Adolescents. Pediatr. Infect. Dis. J. 2019, 38, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Shamir, R.; Chmielewska, A.; Pieścik-Lech, M.; Auricchio, R.; Ivarsson, A.; Kolacek, S.; Koletzko, S.; Korponay-Szabo, I.; Mearin, M.L.; et al. Systematic review with meta-analysis: Early infant feeding and coeliac disease—Update 2015. Aliment. Pharmacol. Ther. 2015, 41, 1038–1054. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Shamir, R.; Chmielewska, A.; Stróżyk, A.; Zalewski, B.M.; Auricchio, R.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, L.; Meijer, C.; et al. Early Feeding Practices and Coeliac Disease Prevention: Protocol for an Updated and Revised Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1040. [Google Scholar] [CrossRef] [PubMed]
- Andersen, V.; Möller, S.; Jensen, P.B.; Møller, F.T.; Green, A. Caesarean Delivery and Risk of Chronic Inflammatory Diseases (Inflammatory Bowel Disease, Rheumatoid Arthritis, Coeliac Disease, and Diabetes Mellitus): A Population Based Registry Study of 2,699,479 Births in Denmark during 1973–2016. Clin. Epidemiol. 2020, 12, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Dydensborg Sander, S.; Hansen, A.V.; Størdal, K.; Andersen, A.N.; Murray, J.A.; Husby, S. Mode of delivery is not associated with coeliac disease. Clin. Epidemiol. 2018, 10, 323–332. [Google Scholar] [CrossRef]
- La Moncloa. El Porcentaje de Adultos con Estudios Postobligatorios Sube 10 Puntos en una Década, Hasta el 62.9%, Notas de Prensa en Educación y Formación Profesional. 2021. Available online: https://www.lamoncloa.gob.es/serviciosdeprensa/notasprensa/educacion/Paginas/2021/160921-panorama_educacion_ocde_2021.aspx (accessed on 5 November 2023).
- Norström, F.; Namatovu, F.; Carlsson, A.; Högberg, L.; Ivarsson, A.; Myléus, A. Family socio-economic status and childhood coeliac disease seem to be unrelated-A cross-sectional screening study. Acta Paediatr. 2021, 110, 1346–1352. [Google Scholar] [CrossRef]
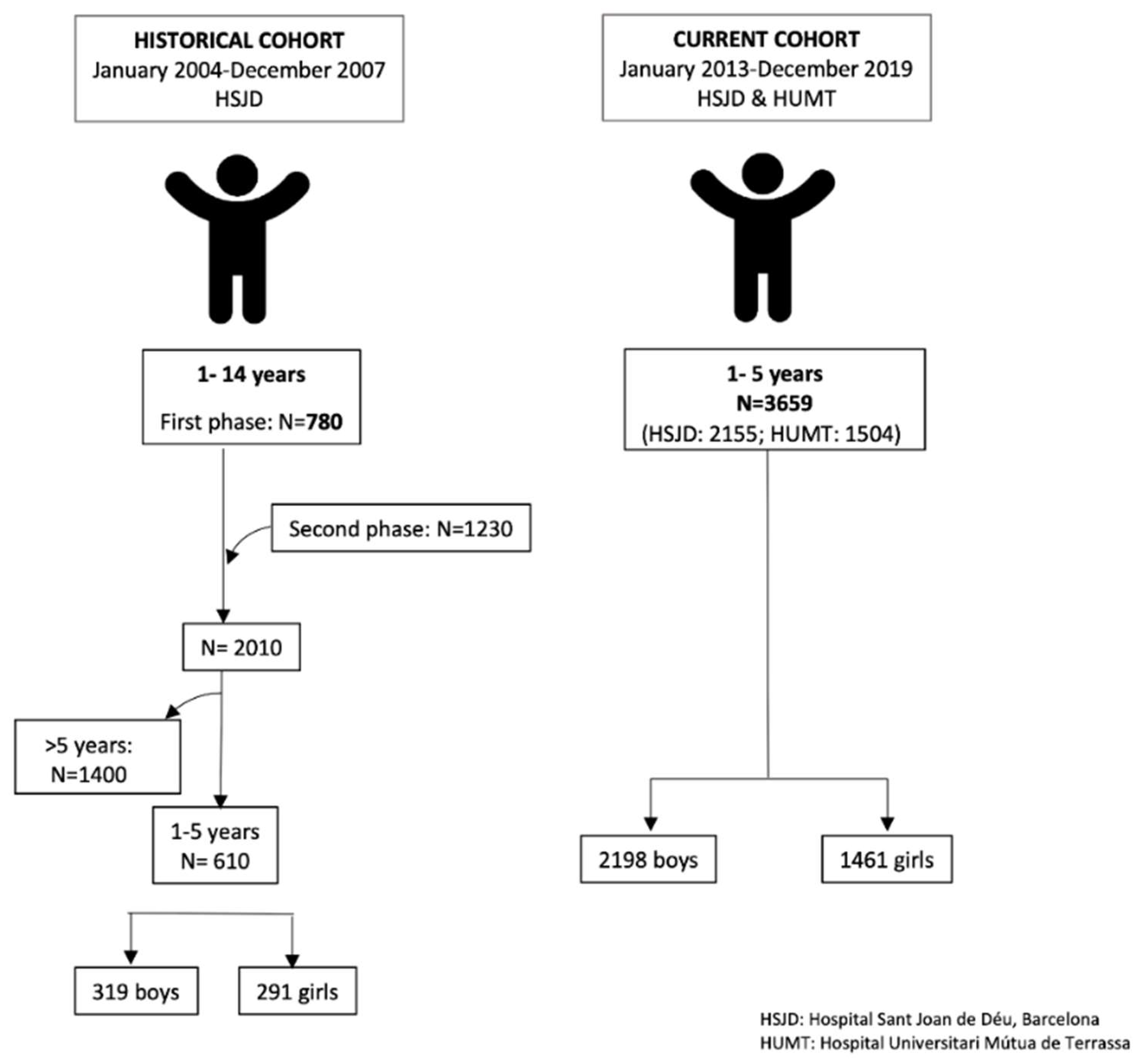
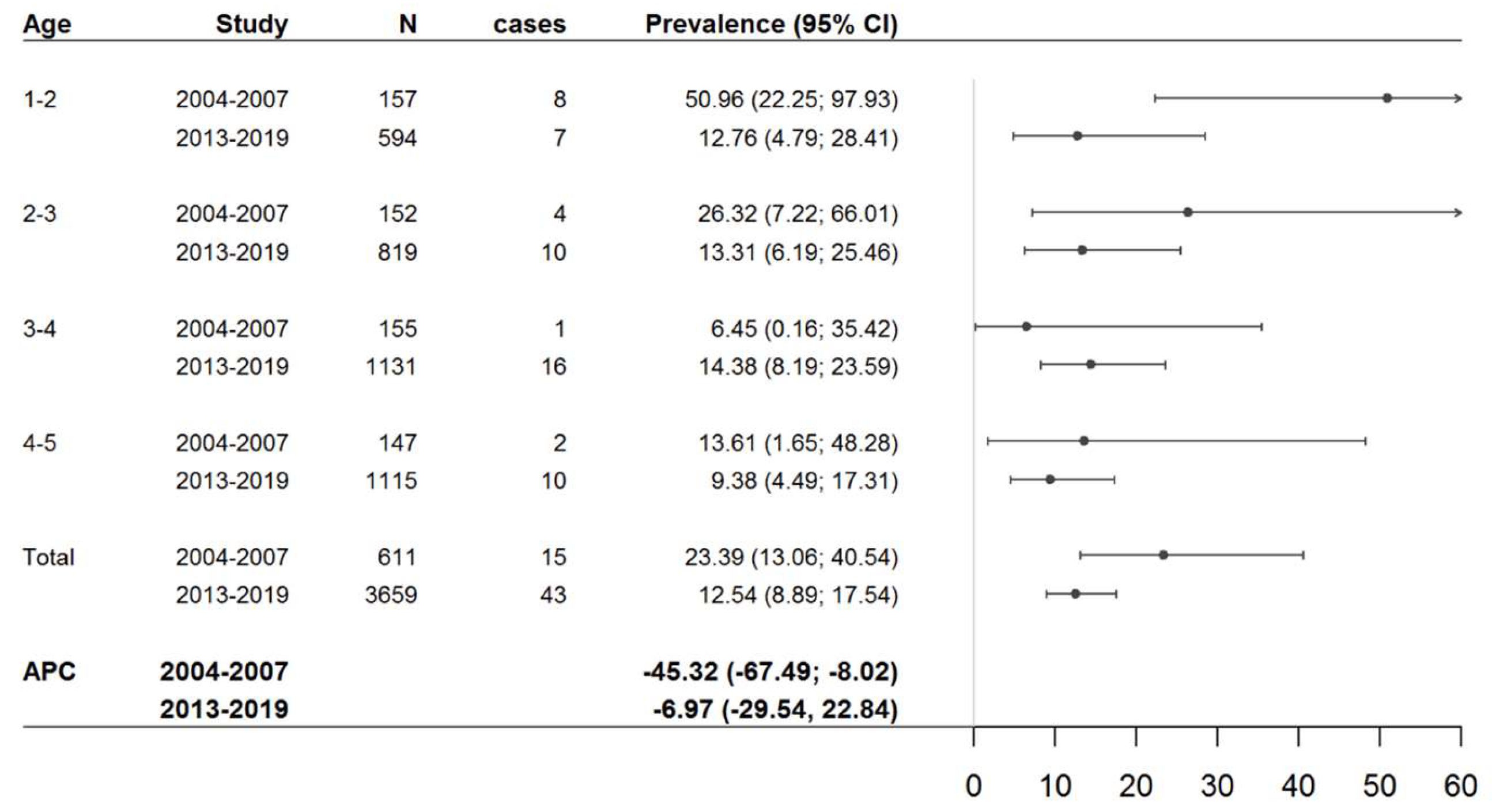
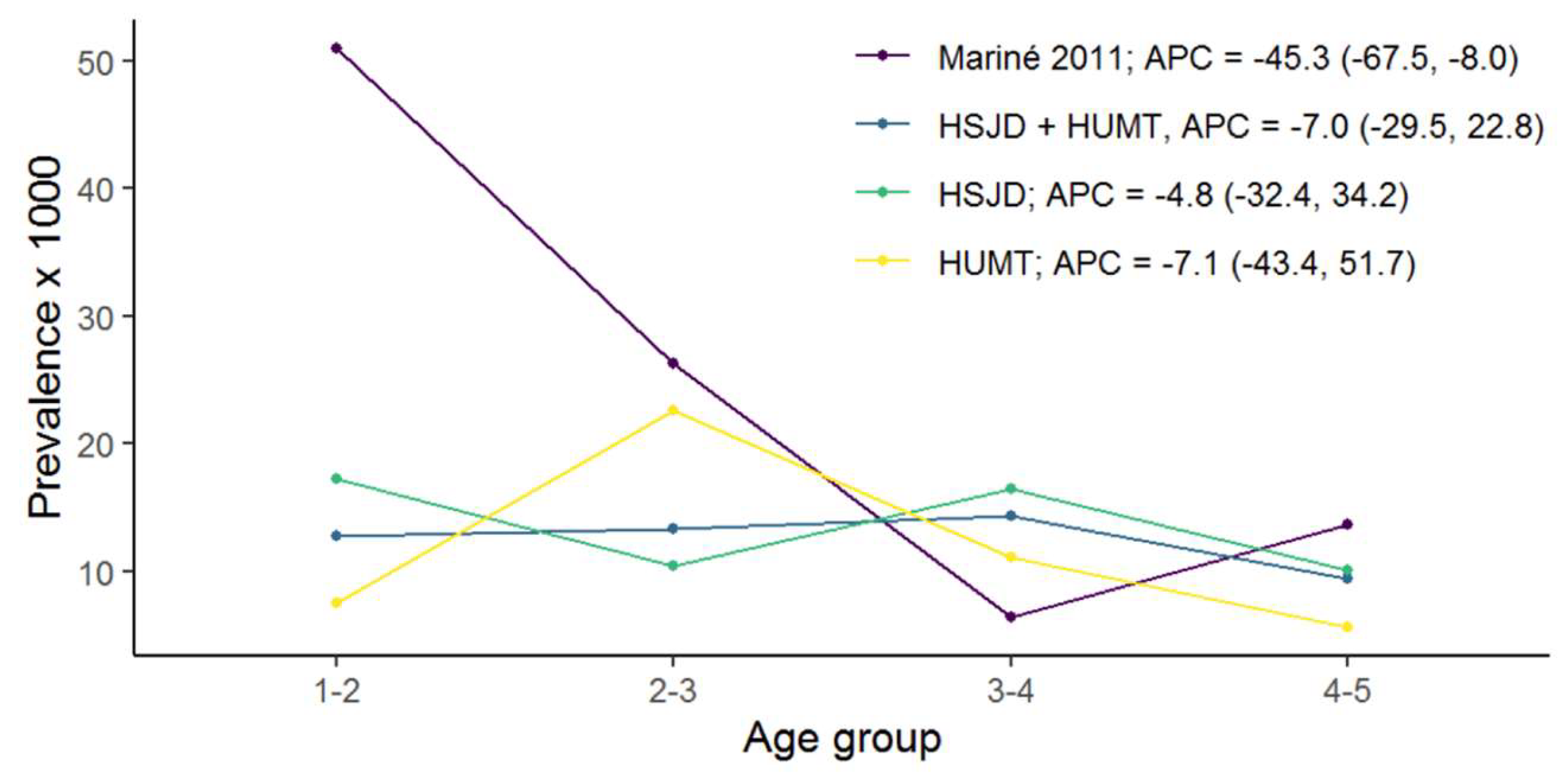
| Historical Study (2004–2007) | Current Study (2013–2019) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HSJD | HUMT | Total | ||||||||||
| Age | CD Cases | N | SP (CI95%) | CD Cases | N | SSP (CI95%) | CD Cases | N | SP (CI95%) | CD Cases | N | SP (CI95%) |
| 1–3 | 12 | 308 | 39.08 (20.19; 68.29) | 12 | 908 | 12.96 (6.55; 23.54) | 5 | 505 | 15.59 (4.79; 37.88) | 17 | 1413 | 13.15 (7.43; 21.81) |
| 3–5 | 3 | 302 | 9.81 (2.02; 29.14) | 18 | 1247 | 14.06 (8.33; 22.35) | 8 | 999 | 8.23 (3.37; 17.38) | 26 | 2246 | 12.00 (7.82; 17.66) |
| Total | 15 | 610 | 23.62 (13.21; 39.40) | 30 | 2155 | 13.61 (9.10; 19.87) | 13 | 1504 | 11.63 (5.47; 22.49) | 43 | 3659 | 12.55 (8.92; 17.40) |
| ID | Sex | Age (Years) * | Anti-tTG2 (IU/mL) * | Duodenal Biopsy | Cytometric Pattern | Genetic Study | Clinical Characteristics | Final Diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 2.5 | 18 | Marsh 0 | Normal | DQ2.5 | Asymptomatic | Non-coeliac |
| 2 | M | 3.4 | 80 | Marsh 1 | ICP | DQ2.5 | Asymptomatic | Non-coeliac |
| 3 | M | 1.4 | 55 | Marsh 0 | Normal | DQ2.5 | Asymptomatic | Giardiasis |
| 4 | M | 3.9 | 153 | Marsh 3a | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 5 | M | 2.5 | 80 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 6 | F | 3.2 | 10 | Marsh 3b | CCP | DQ2.5 | Abdominal pain | Coeliac |
| 7 | M | 3.4 | 13 | NA | NA | NA | Asymptomatic | Unknown |
| 8 # | M | 1.8 | 58 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 9 | F | 3 | 80 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 10 | F | 2.9 | 33 | Marsh 3c | CCP | DQ2.5 | Growth retardation | Coeliac |
| 11 # | F | 1.3 | 80 | Marsh 3b | ICP | DQ2.5 | Asymptomatic | Coeliac |
| 12 | M | 4.3 | 80 | Marsh 3c | CCP | DQ2.5 | Growth retardation | Coeliac |
| 13 | F | 4.3 | 80 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 14 | F | 3.1 | 21 | Marsh 1 | ICP | DQ2.5 | Asymptomatic | Non-coeliac |
| 15 | M | 2 | 125 | Marsh 3c | ICP | DQ2.5 | Abdominal pain Iron deficiency | Coeliac |
| 16 | F | 4.7 | 80 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 17 | M | 2.2 | 52 | NA | NA | NA | Asymptomatic | Unknown |
| 18 | F | 1.7 | 103 | Marsh 3c | CCP | DQ2.5 | Growth retardation | Coeliac |
| 19 | M | 3.2 | 25 | Marsh 3c | CCP | DQ2.5 | Iron deficiency | Coeliac |
| 20 | M | 3.1 | 8.4 | Marsh 3a | ICP | DQ2.5 | Asymptomatic | Coeliac |
| 21 # | F | 1.9 | 80 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 22 | F | 2.4 | 15 | NA | NA | NA | Asymptomatic | Unknown |
| 23 | M | 1.2 | 143 | Marsh 3c | NA | DQ2.5 | Growth retardation | Coeliac |
| 24 | F | 3.4 | 102 | Marsh 3b | NA | DQ2.5 | Asymptomatic | Coeliac |
| 25 | F | 4.7 | 11 | Marsh 3c | CCP | DQ2.5 DQ8 | Growth retardation | Previously diagnosed coeliac |
| 26 | F | 2.2 | 15 | Marsh 1 | ICP | DQ2.5 | Asymptomatic | Non-coeliac |
| 27 | F | 4.2 | 28 | Marsh 3c | ICP | DQ2.5 | Asymptomatic | Coeliac |
| 28 | M | 2.9 | 9.2 | NA | NA | NA | Asymptomatic | Unknown |
| 29 | M | 4.6 | 109 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 30 | M | 3.3 | 156 | NA | NA | DQ2.5 | Growth retardation | Coeliac & |
| 31 | F | 2.5 | 80 | Marsh 3c | CCP | DQ2.5 | Iron deficiency | Coeliac |
| 32 | F | 4.2 | 80 | Marsh 3c | CCP | DQ8 | Iron deficiency | Coeliac |
| 33 | F | 4.1 | 56 | NA | NA | NA | Asymptomatic | Unknown |
| 34 | F | 3 | 19 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 35 | M | 3.3 | 80 | Marsh 3c | CCP | DQ2.5 | Iron deficiency | Coeliac |
| 36 | M | 1.5 | 16 | NA | NA | NA | Asymptomatic | Unknown |
| 37 | F | 3.8 | 80 | Marsh 3b | NA | NA | Hypertransaminasemia | Coeliac |
| 38 | F | 4.4 | 80 | NA | NA | NA | Iron deficiency | Coeliac & |
| 39 | F | 3.1 | 80 | Marsh 3c | CCP | DQ2.5 | Iron deficiency | Coeliac |
| 40 | M | 2.1 | 80 | NA | NA | DQ2.5 | Iron deficiency | Coeliac & |
| 41 | F | 4.8 | 41 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 42 | F | 3.7 | 33 | Marsh 3c | CCP | DQ2.5 | Asymptomatic | Coeliac |
| 43 | M | 3.2 | 59 | Marsh 3b | ICP | DQ8 | Iron deficiency | Coeliac |
| Historical Study (2004–2007) | Current Study (2013–2019) | |||||||
|---|---|---|---|---|---|---|---|---|
| R | NR | NC | p Value | R | NR | NC | p Value | |
| (n = 99) | (n = 202) | (n = 309) | (n = 217) | (n = 262) | (n = 121) | |||
| Age (Years) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| 1–2 | 25 (25.3%) | 59 (29.2%) | 70 (22.7%) | 0.513 | 41 (18.9%) | 44 (16.8%) | 20 (16.5%) | 0.594 |
| 2–3 | 23 (23.2%) | 48(23.8%) | 83 (26.9%) | 51 (23.5%) | 53 (20.2%) | 26 (21.5%) | ||
| 3–4 | 27 (27.3%) | 54 (26.7%) | 74 (23.9%) | 66 (30.4%) | 79 (30.2%) | 30 (24.8%) | ||
| 4–5 | 24 (24.2%) | 41 (20.3%) | 82 (26.5%) | 59 (27.2%) | 86 (32.8%) | 45 (37.2%) | ||
| Sex | ||||||||
| male | 54 (54.5%) | 84 (41.6%) | 153 (49.5%) | 0.071 | 93 (42.9%) | 115 (43.9%) | 52 (43.0%) | 0.971 |
| female | 45 (45.5%) | 118 (58.4%) | 156 (50.5%) | 124 (57.1%) | 147 (56.1%) | 69 (57.0%) | ||
| Coeliac disease | ||||||||
| yes | 5 (5.1%) | 2 (1.0%) | 7 (2.3%) | 0.087 | 2 (0.9%) | 4 (1.5%) | 1 (0.8%) | 0.768 |
| no | 94 (94.9%) | 200 (99.0%) | 302 (97.7%) | 215 (99.1%) | 258 (98.5%) | 120 (99.2%) | ||
| 2004–2007 | 2013–2019 | ||
|---|---|---|---|
| n (%) | n (%) | p Value | |
| Gluten introduction <6 months | |||
| Yes | 33 (32.4%) | 65 (28.6%) | 0.479 |
| No | 58 (56.9%) | 144 (63.4%) | |
| Unknown | 11 (10.8%) | 18 (7.9%) | |
| Antibiotic use before 2 years | |||
| Yes | 47 (47.5%) | 111 (48.9%) | 0.956 |
| No | 41 (41.4%) | 90 (39.7%) | |
| Unknown | 11 (11.1%) | 26 (11.5%) | |
| Hospital admission due to infection at <5 years of age | |||
| Yes | 21 (21%) | 59 (26%) | 0.598 |
| No | 78 (78%) | 165 (72.7%) | |
| Unknown | 1 (1%) | 3 (1.3%) | |
| Type of infection | |||
| Urinary | 4 (14.3%) | 5 (6%) | 0.342 |
| Respiratory | 15 (53.6%) | 39 (46.4%) | |
| Enteric | 0 (0%) | 1 (1.2%) | |
| Other | 7 (25%) | 22 (26.2%) | |
| Unknown | 2 (7.1%) | 17 (20.2%) | |
| Caesarean section delivery | |||
| Yes | 18 (18%) | 64 (28.2%) | 0.018 |
| No | 80 (80%) | 163 (71.8%) | |
| Unknown | 2 (2%) | 0 (0%) | |
| Breastfeeding | |||
| Yes | 58 (59.2%) | 165 (73.3%) | 0.032 |
| No | 34 (34.7%) | 48 (21.3%) | |
| Unknown | 6 (6.1%) | 12 (5.3%) | |
| Months of breastfeeding duration (Median and IQR) | |||
| 11 (6; 16) | 24 (12; 36) | <0.001 | |
| Rotavirus vaccination | |||
| Yes | 9 (9%) | 109 (48%) | <0.001 |
| No | 29 (29%) | 73 (32.2%) | |
| Unknown | 62 (62%) | 45 (19.8%) | |
| Educational level of parents | |||
| None | 1 (1%) | 0 (0%) | <0.001 |
| Primary–secondary | 9 (9.1%) | 24 (10.6%) | |
| Professional | 57 (57.6%) | 74 (32.7%) | |
| University | 32 (32.3%) | 128 (56.6%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arau, B.; Dietl, B.; Sudrià-Lopez, E.; Ribes, J.; Pareja, L.; Marquès, T.; Garcia-Puig, R.; Pujalte, F.; Martin-Cardona, A.; Fernández-Bañares, F.; et al. A Population-Based Cross-Sectional Study of Paediatric Coeliac Disease in Catalonia Showed a Downward Trend in Prevalence Compared to the Previous Decade. Nutrients 2023, 15, 5100. https://doi.org/10.3390/nu15245100
Arau B, Dietl B, Sudrià-Lopez E, Ribes J, Pareja L, Marquès T, Garcia-Puig R, Pujalte F, Martin-Cardona A, Fernández-Bañares F, et al. A Population-Based Cross-Sectional Study of Paediatric Coeliac Disease in Catalonia Showed a Downward Trend in Prevalence Compared to the Previous Decade. Nutrients. 2023; 15(24):5100. https://doi.org/10.3390/nu15245100
Chicago/Turabian StyleArau, Beatriz, Beatriz Dietl, Emma Sudrià-Lopez, Josefa Ribes, Laura Pareja, Teresa Marquès, Roger Garcia-Puig, Francisco Pujalte, Albert Martin-Cardona, Fernando Fernández-Bañares, and et al. 2023. "A Population-Based Cross-Sectional Study of Paediatric Coeliac Disease in Catalonia Showed a Downward Trend in Prevalence Compared to the Previous Decade" Nutrients 15, no. 24: 5100. https://doi.org/10.3390/nu15245100
APA StyleArau, B., Dietl, B., Sudrià-Lopez, E., Ribes, J., Pareja, L., Marquès, T., Garcia-Puig, R., Pujalte, F., Martin-Cardona, A., Fernández-Bañares, F., Mariné, M., Farré, C., & Esteve, M. (2023). A Population-Based Cross-Sectional Study of Paediatric Coeliac Disease in Catalonia Showed a Downward Trend in Prevalence Compared to the Previous Decade. Nutrients, 15(24), 5100. https://doi.org/10.3390/nu15245100







