The Burden of Gastric Cancer Attributable to High Sodium Intake: A Longitudinal Study from 1990 to 2019 in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Data
2.2. Definitions of Gastric Cancer and High Sodium Intake Exposure
2.3. Estimation of High Sodium Intake-Attributed Gastric Cancer Burden
2.4. Statistical Analyses
3. Results
3.1. Burden of Gastric Cancer Attributable to High Sodium Intake from 1990 to 2019
3.2. Burden of Gastric Cancer Being Attributed to High Sodium Intake Separated by Age and Gender
3.3. Burden of Gastric Cancer Attributable to High Sodium Intake Separated by Regions and SDI
3.4. Temporal Trends of Mortality and DALYs of Gastric Cancer Being Attributed to High Sodium Intake from 1990 to 2019
3.5. Temporal Trends of Mortality and DALYs of Gastric Cancer Being Attributed to High Sodium Intake in 1990–2019
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Etemadi, A.; Safiri, S.; Sepanlou, S.G.; Ikuta, K.; Bisignano, C.; Shakeri, R.; Amani, M.; Fitzmaurice, C.; Nixon, M.; Abbasi, N.; et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Yin, X.; He, Q.; Man, J.; Yang, X.; Lu, M. Changing trends of disease burden of gastric cancer in China from 1990 to 2019 and its predictions: Findings from Global Burden of Disease Study. Chin. J. Cancer Res. 2021, 33, 11–26. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Luan, F.; Yu, Z.; Feng, H.; Chen, B.; Chen, W. Chinese and global burdens of gastric cancer from 1990 to 2019. Cancer Med. 2021, 10, 3461–3473. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Lv, J.; Yang, M.; Wang, M.; Zhu, M.; Wang, T.; Yan, C.; Yu, C.; Ding, Y.; Li, G.; et al. Genetic risk, incident gastric cancer, and healthy lifestyle: A meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. 2020, 21, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.X.; Wang, Q.; Wang, C.; Yin, Q.C.; Huo, H.Z.; Yin, B.H. Secular trend of gastric and esophageal cancer attributable to dietary carcinogens from 1990 to 2019 and projections until 2044 in China: A Population-Based Study. JMIR Public Health Surveill. 2023, 9, e48449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Pan, X.F.; Chen, J.; Cao, A.; Zhang, Y.G.; Xia, L.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer 2020, 122, 1085–1093. [Google Scholar] [CrossRef]
- Peleteiro, B.; Barros, S.; Castro, C.; Ferro, A.; Morais, S.; Lunet, N. Worldwide burden of gastric cancer in 2010 attributable to high sodium intake in 1990 and predicted attributable burden for 2030 based on exposures in 2010. Br. J. Nutr. 2016, 116, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, B.; Lopes, C.; Figueiredo, C.; Lunet, N. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br. J. Cancer 2011, 104, 198–207. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, M.; Zhang, W.; Zhang, X.; Zhao, Z.; Huang, Z.; Li, C.; Wang, L. Salt-Related Knowledge, Behaviors, and Associated Factors among Chinese Adults—China, 2015. China CDC Wkly. 2020, 2, 678–683. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Giovannucci, E. Potential Impact of Time Trend of Lifestyle Risk Factors on Burden of Major Gastrointestinal Cancers in China. Gastroenterology 2021, 161, 1830–1841.e8. [Google Scholar] [CrossRef]
- Hipgrave, D.B.; Chang, S.; Li, X.; Wu, Y. Salt and Sodium Intake in China. JAMA 2016, 315, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [CrossRef] [PubMed]
- Li, M.; Li, X.; Zhao, Y.; Zhang, L.; Yang, J.; Zhou, M.; Wang, Z. The burden of ischemic heart disease and type 2 diabetes mellitus attributable to diet high in sugar-sweetened beverages in China: An analysis for the Global Burden of Disease Study 2017. J. Diabetes 2021, 13, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Park, Y.; Wu, J.W.; Ren, J.S.; Goldstein, A.M.; Taylor, P.R.; Hollenbeck, A.R.; Freedman, N.D.; Abnet, C.C. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol. Hepatol. 2013, 11, 1130–1136.e1132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, J.; Wu, X.; Cao, W.; Sun, S. Global burden attributable to high sodium intake from 1990 to 2019. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, L.; Yin, W.; Wang, J.; Zuo, X. Global, regional, and national burden of chronic kidney disease attributable to high sodium intake from 1990 to 2019. Front. Nutr. 2023, 10, 1078371. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Hankey, B.F.; Ries, L.A.; Kosary, C.L.; Feuer, E.J.; Merrill, R.M.; Clegg, L.X.; Edwards, B.K. Partitioning linear trends in age-adjusted rates. Cancer Causes Control 2000, 11, 31–35. [Google Scholar] [CrossRef]
- Yan, X.; Lei, L.; Li, H.; Cao, M.; Yang, F.; He, S.; Zhang, S.; Teng, Y.; Li, Q.; Xia, C.; et al. Stomach cancer burden in China: Epidemiology and prevention. Chin. J. Cancer Res. 2023, 35, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Wu, J. National trend of gastric cancer mortality in China (2003–2015): A population-based study. Cancer Commun. 2019, 39, 24. [Google Scholar] [CrossRef]
- Huh, J.H.; Lim, J.S.; Lee, M.Y.; Chung, C.H.; Shin, J.Y. Gender-specific association between urinary sodium excretion and body composition: Analysis of the 2008–2010 Korean National Health and Nutrition Examination Surveys. Metabolism 2015, 64, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kim, E.S.; Lee, J.; Kim, Y. Trends in sodium intake and major contributing food groups and dishes in Korea: The Korea National Health and Nutrition Examination Survey 2013–2017. Nutr. Res. Pract. 2021, 15, 382–395. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International; American Institute for Cancer Research. Stomach Cancer Statistics. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/stomach-cancer-statistics (accessed on 22 March 2022).
- Grobbee, D.E. Methodology of sodium sensitivity assessment. The example of age and sex. Hypertension 1991, 17, I109–I114. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, X.H.; Zhang, Y.; Hu, D.M.; Liu, K. Global burden of gastric cancer in adolescents and young adults: Estimates from GLOBOCAN 2020. Public Health 2022, 210, 58–64. [Google Scholar] [CrossRef] [PubMed]
- McKetta, S.; Keyes, K.M. Heavy and binge alcohol drinking and parenting status in the United States from 2006 to 2018: An analysis of nationally representative cross-sectional surveys. PLoS Med. 2019, 16, e1002954. [Google Scholar] [CrossRef]
- Li, Y.; Ren, N.; Zhang, B.; Yang, C.; Li, A.; Li, X.; Lei, Z.; Fei, L.; Fan, S.; Zhang, J. Gastric cancer incidence trends in China and Japan from 1990 to 2019: Disentangling age-period-cohort patterns. Cancer 2023, 129, 98–106. [Google Scholar] [CrossRef]
- Shao, S.; Hua, Y.; Yang, Y.; Liu, X.; Fan, J.; Zhang, A.; Xiang, J.; Li, M.; Yan, L.L. Salt reduction in China: A state-of-the-art review. Risk Manag. Healthc. Policy 2017, 10, 17–28. [Google Scholar] [CrossRef]
- Hagag, S.; Habib, E.; Tawfik, S. Assessment of Knowledge and Practices toward Salt Intake among Adolescents. Open Access Maced. J. Med. Sci. 2022, 10, 921–925. [Google Scholar] [CrossRef]
- Li, X.; Cao, X.; Guo, M.; Xie, M.; Liu, X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: Systematic analysis for the Global Burden of Disease Study 2017. BMJ 2020, 368, m234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Chen, C.; Pan, X.F.; Guo, J.; Li, Y.; Franco, O.H.; Liu, G.; Pan, A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: Two prospective cohort studies. BMJ 2021, 373, n604. [Google Scholar] [CrossRef] [PubMed]
- Balia, S.; Jones, A.M. Mortality, lifestyle and socio-economic status. J. Health Econ. 2008, 27, 1–26. [Google Scholar] [CrossRef]
- Xu, A.; Ma, J.; Guo, X.; Wang, L.; Wu, J.; Zhang, J.; Bai, Y.; Xu, J.; Lu, Z.; Xu, Z.; et al. Association of a Province-Wide Intervention with Salt Intake and Hypertension in Shandong Province, China, 2011–2016. JAMA Intern. Med. 2020, 180, 877–886. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Chen, S.; Li, B.; Lu, X.; Li, J. Different Changing Patterns for Stroke Subtype Mortality Attributable to High Sodium Intake in China during 1990 to 2019. Stroke 2023, 54, 1078–1087. [Google Scholar] [CrossRef]

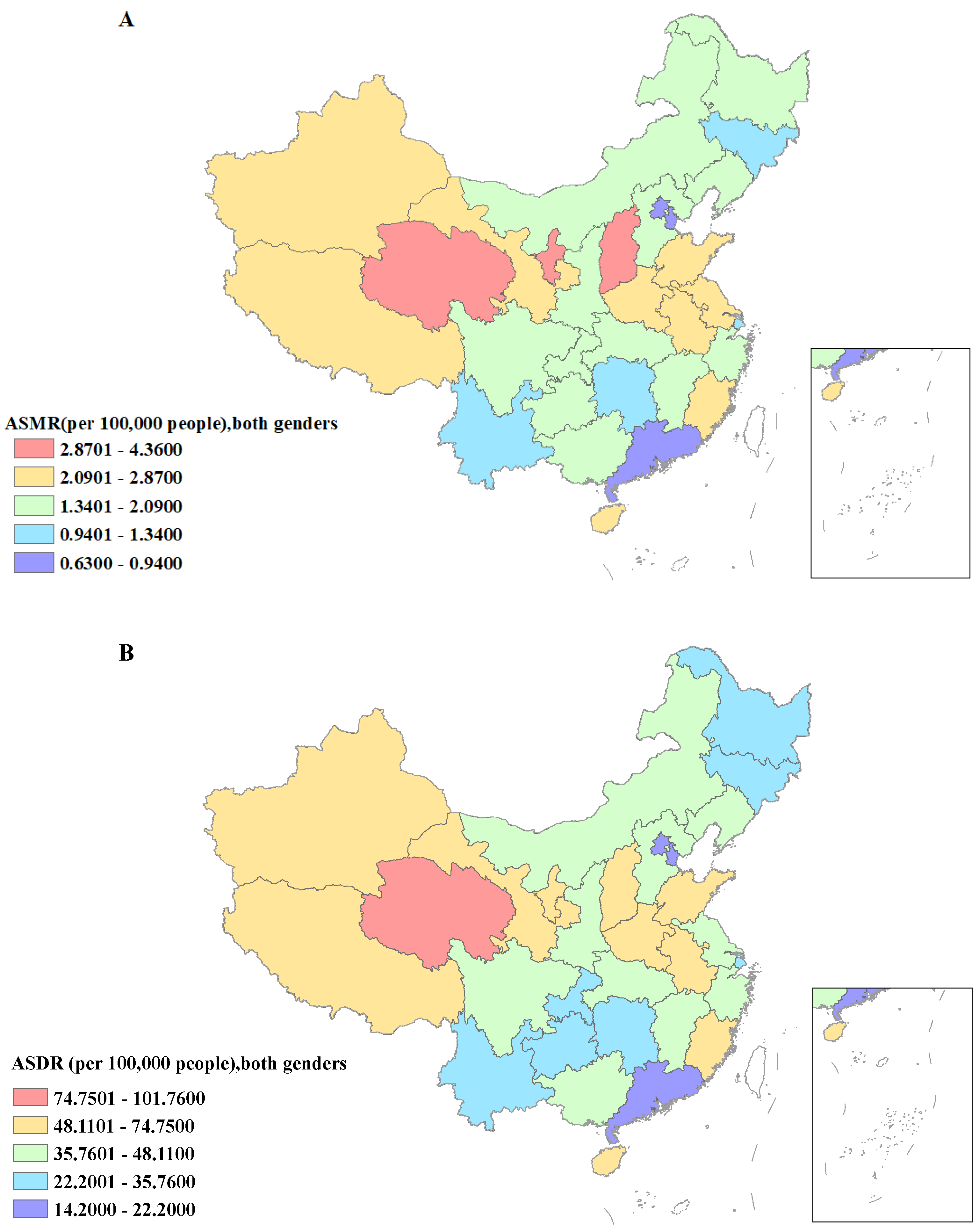
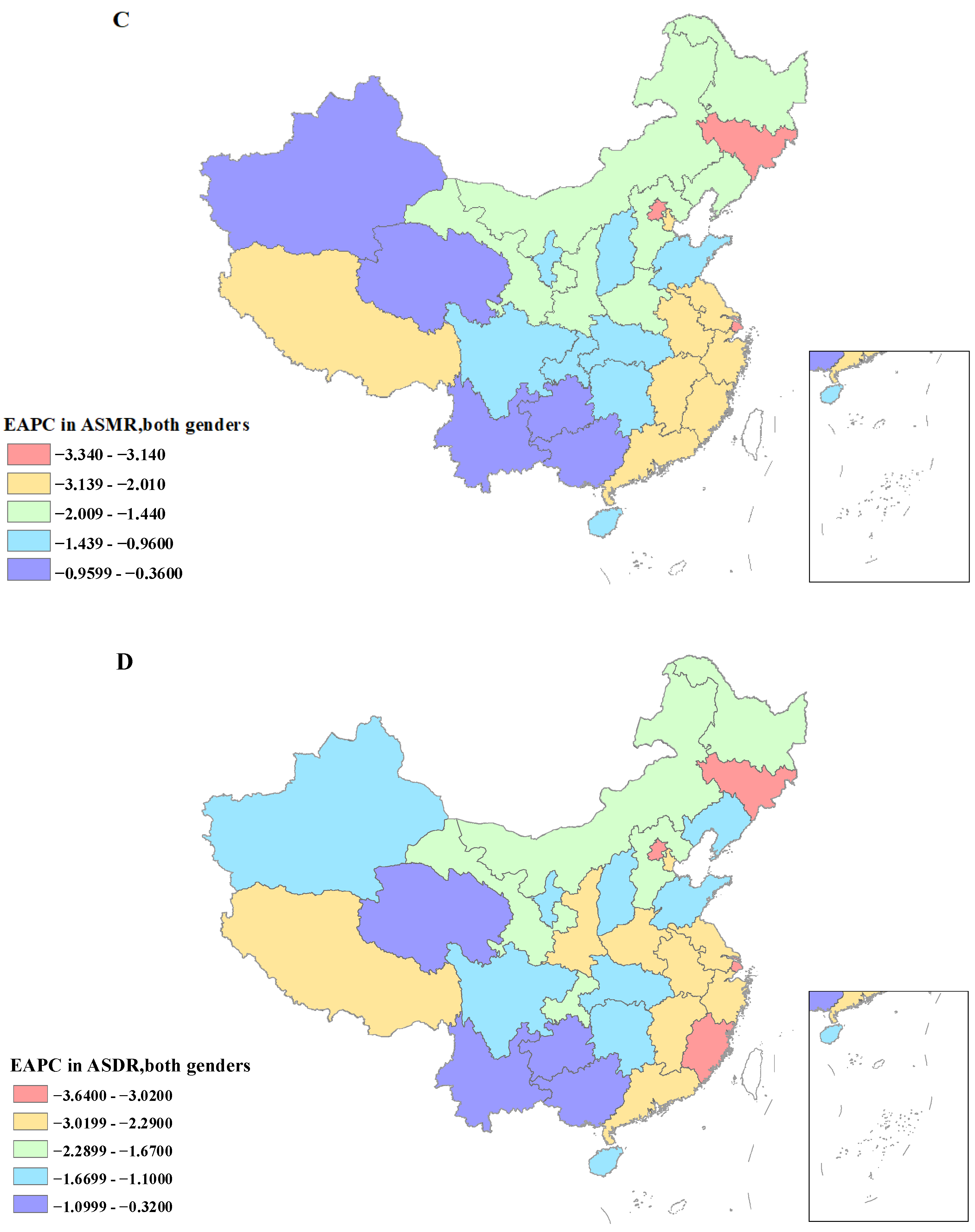




| Characteristics | 1990 | 2019 | 1990–2019 | |||
|---|---|---|---|---|---|---|
| Deaths Cases, No. (95% UI) | ASMR per 100,000 No. (95% UI) | Deaths Cases, No. (95% UI) | ASMR per 100,000 No. (95% UI) | PAFs % (95% UI) | EAPC(%) in ASMR No. (95% CI) | |
| Global | 60,961.34 (1739.22 to 242,439.96) | 1.57 (0.05 to 6.26) | 74,098.60 (2117.22 to 294,837.24) | 0.92 (0.03 to 3.64) | 7.71 (0.23 to 30.82) | −1.83 (−2.02 to −1.65) |
| China | 27,226.5 (612.93 to 101,649.43) | 3.34 (0.08 to 12.56) | 37,131.48 (833.14 to 138,478.72) | 1.90 (0.04 to 7.12) | 8.75 (0.21 to 32.76) | −1.72 (−2.11 to −1.33) |
| Gender | ||||||
| Male | 17,739.88 (383.80 to 66,070.45) | 4.60 (0.10 to 17.09) | 26,497.45 (569.28 to 98,942.04) | 2.92 (0.06 to 10.90) | 8.84 (0.21 to 0.32.76) | −1.22 (−1.60 to −0.83) |
| Female | 9486.62 (217.09 to 35,696.85) | 2.28 (0.05 to 8.60) | 10,634.03 (238.25 to 41,305.44) | 1.05 (0.02 to 4.06) | 8.53 (0.21 to 0.32.36) | −2.66 (−3.05 to −2.27) |
| Socio-demographic Index (SDI) | ||||||
| High-middle SDI | 11,812.80 (260.38 to 44,237.97) | 3.30 (0.07 to 12.46) | 16,331.75 (356.21 to 60,601.55) | 1.73 (0.04 to 6.46) | 8.75 (0.21 to 32.72) | −2.08 (−2.43 to −1.73) |
| High SDI | 86.11 (2.01 to 315.06) | 1.505 (0.03 to 5.49) | 113.07 (2.41 to 432.47) | 0.675 (0.02 to 2.57) | 8.81 (0.21 to 32.93) | −2.80 (−2.9 to −2.59) |
| Low-middle SDI | 1086.65 (24.60 to 4145.30) | 3.54 (0.08 to 13.62) | 1710.31 (38.54 to 6503.53) | 2.25 (0.05 to 8.52) | 8.55 (0.21 to 32.32) | −1.32 (−1.66 to −0.97) |
| Middle SDI | 14,240.96 (313.44 to 52,961.04) | 3.40 (0.08 to 12.79) | 18,976.33 (411.09 to 71,070.87) | 2.12 (0.05 to 8.03) | 8.72 (0.21 to 32.66) | −1.41 (−1.74 to −1.03) |
| Characteristics | 1990 | 2019 | 1990–2019 | |||
|---|---|---|---|---|---|---|
| DALYs, No. (95% UI) | ASDR per 100,000 No. (95% UI) | DALYs, No. (95% UI) | ASDR per 100,000 No. (95% UI) | PAFs % (95% UI) | EAPC (%) in ASDR No. (95% CI) | |
| Global | 1,598,735.69 (44,382.75 to 6,299,148.27) | 38.52 (1.08 to 152.39) | 1,735,810.69 (48,674.79 to 6,804,671.03) | 20.91 (0.59 to 82.11) | 7.79 (0.22 to 30.89) | 0.22 (0.06 to 0.36) |
| China | 734,447.94 (16,388.16 to 2,731,936.05) | 80.72 (1.81 to 301.47) | 873,813.19 (19,283.13 to 3,220,231.82) | 42.52 (0.94 to 157.03) | 8.85 (0.21 to 32.82) | 0.36 (0.08 to 0.68) |
| Gender | ||||||
| Male | 485,849.57 (10,416.51 to 1,808,630.53) | 108.37 (2.36 to 403.47) | 637,200.70 (13,603.95 to 2,377,631.68) | 63.79 (1.38 to 237.25) | 8.91 (0.21 to 32.96) | −1.49 (−1.90 to −1.09) |
| Female | 248,598.36 (5605.48 to 927,208.28) | 54.40 (1.23 to 203.57) | 236,612.49 (5160.81 to 921,927.87) | 22.79 (0.50 to 88.76) | 8.68 (0.21 to 32.51) | −3.07 (−3.44 to −2.69) |
| Socio-demographic Index (SDI) | ||||||
| High-middle SDI | 310,958.47 (6754.15 to 1,163,118.97) | 78.83 (1.73 to 296.67) | 383,445.35(8214.15 to 1,420,311.21) | 38.88 (0.84 to 144.20) | 8.85 (0.21 to 32.85) | −2.27 (−2.64 to −1.89) |
| High SDI | 2041.38 (47.30 to 7501.81) | 18.24 (0.42 to 66.82) | 2100.07(543.96 to 7986.59) | 7.99 (0.17 to 30.29) | 8.84 (0.21 to 32.91) | −2.925 (−3.15 to −3.15) |
| Low-middle SDI | 185,247.99 (5180.19 to 751,627.37) | 27.61 (0.78 to 112.47) | 280,424.65 (8018.74 to 1,137,545.52) | 19.28 (0.55 to 78.31) | 7.42 (0.22 to 30.20) | 0.65 (0.47 to 0.84) |
| Middle SDI | 389,623.72(8469.43 to 1,447,292.53) | 83.72 (1.83 to 313.97) | 446,547.11 (9506.97 to 1,666,429.48) | 47.82 (1.03 to 179.74) | 8.33 (0.21 to 32.78) | −1.77 (−2.08 to −1.45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wang, A.; Yang, S.; Fang, H.; Wang, Q.; Li, H.; Liu, S.; Liu, A. The Burden of Gastric Cancer Attributable to High Sodium Intake: A Longitudinal Study from 1990 to 2019 in China. Nutrients 2023, 15, 5088. https://doi.org/10.3390/nu15245088
Jiang L, Wang A, Yang S, Fang H, Wang Q, Li H, Liu S, Liu A. The Burden of Gastric Cancer Attributable to High Sodium Intake: A Longitudinal Study from 1990 to 2019 in China. Nutrients. 2023; 15(24):5088. https://doi.org/10.3390/nu15245088
Chicago/Turabian StyleJiang, Liying, Anqi Wang, Shuo Yang, Haiqin Fang, Qihe Wang, Huzhong Li, Sana Liu, and Aidong Liu. 2023. "The Burden of Gastric Cancer Attributable to High Sodium Intake: A Longitudinal Study from 1990 to 2019 in China" Nutrients 15, no. 24: 5088. https://doi.org/10.3390/nu15245088
APA StyleJiang, L., Wang, A., Yang, S., Fang, H., Wang, Q., Li, H., Liu, S., & Liu, A. (2023). The Burden of Gastric Cancer Attributable to High Sodium Intake: A Longitudinal Study from 1990 to 2019 in China. Nutrients, 15(24), 5088. https://doi.org/10.3390/nu15245088





