Relative Energy Deficiency in Sport (REDs) Indicators in Male Adolescent Endurance Athletes: A 3-Year Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
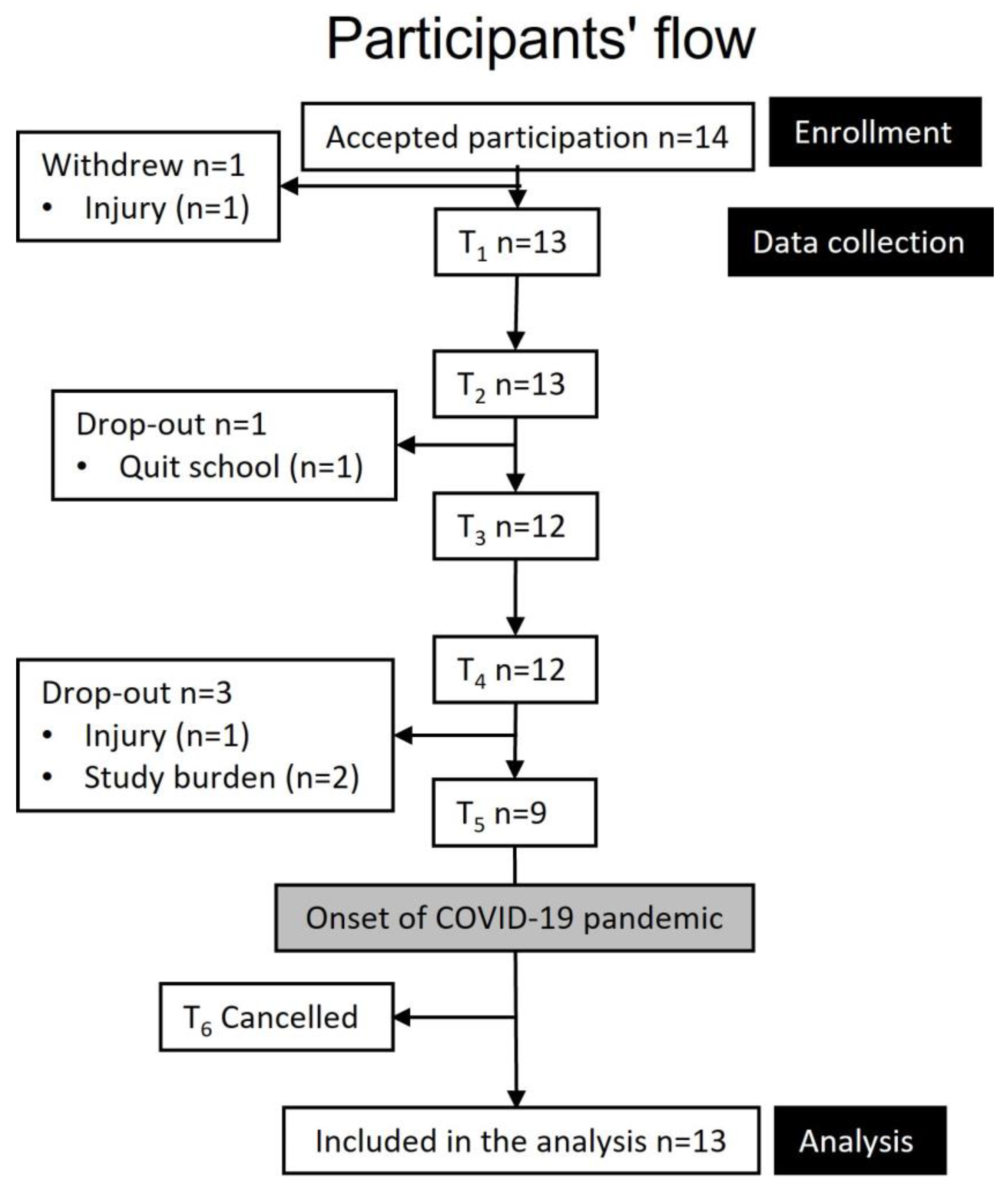
2.1. Study Design and Participants
2.2. Study Protocol
2.3. Athlete Characteristics
2.4. Resting Metabolic Rate
2.5. Body Composition and Bone Health
2.6. Psychological Parameters
2.7. Energy Intake, Exercise Energy Expenditure and Energy Availability
2.8. Performance
2.9. Statistical Analysis
3. Results
3.1. Athlete Characteristics
3.2. Bone Health, Energy Availability and Resting Metabolic Rate
| Fixed Effects Parameter Estimates | Random Components | ||||||
|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | ||||||
| Variable | (SE) | Low–High | p-Value | Variance | ICC | ||
| Body composition | |||||||
| FFM (kg) | Intercept | 57.3 (1.7) | 53.9–60.7 | Intercept | 38.5 | ||
| Slope | 1.1 (0.1) | 0.8–1.4 | <0.001 | Slope | 0.2 | 0.981 | |
| Residual | 0.8 | ||||||
| Bone health | |||||||
| Lumbar BMD (g/cm2) | Intercept | 1.048 (0.019) | 1.039–1.098 | Intercept | 0.001 | ||
| Slope | 0.021 (0.008) | 0.006–0.037 | 0.022 | Slope | 4.9 × 10−4 | 0.362 | |
| Residual | 0.002 | ||||||
| Lumbar BMC (g) | Intercept | 62.0 (2.8) | 55.6–67.4 | Intercept | 58.9 | ||
| Slope | 2.6 (0.9) | 0.8–4.3 | 0.015 | Slope | 5.3 | 0.643 | |
| Residual | 32.7 | ||||||
| Lumbar Z-score | Intercept | −0.77 (0.17) | −1.10 to −0.44 | Intercept | 0.36 | ||
| Slope | 0.04 (0.02) | 0.01–0.07 | 0.029 | Slope | 0.00 | 0.953 | |
| Residual | 0.02 | ||||||
| Femur neck BMD (g/cm2) | Intercept | 1.093 (0.033) | 1.029–1.157 | Intercept | 0.014 | ||
| Slope | 0.012 (0.002) | 0.008–0.017 | <0.001 | Slope | 4.4 × 10−5 | 0.984 | |
| Residual | 2.2 × 10−4 | ||||||
| Femur neck BMC (g) | Intercept | 5.8 (0.3) | 5.2–6.4 | Intercept | 1.21 | ||
| Slope | 0.1 (0.0) | 0.07–0.13 | <0.001 | Slope | 0.00 | 0.992 | |
| Residual | 0.01 | ||||||
| Total hip BMD (g/cm2) | Intercept | 1.123 (0.032) | 1.060–1.185 | Intercept | 0.013 | ||
| Slope | 0.005 (0.002) | 0.001–0.008 | 0.029 | Slope | 3.2 × 10−5 | 0.993 | |
| Residual | 9.6 × 10−5 | ||||||
| Total hip BMC (g) | Intercept | 40.0 (1.9) | 36.3–43.6 | Intercept | 45.2 | ||
| Slope | 0.4 (0.1) | 0.2–0.5 | <0.001 | Slope | 0.05 | 0.996 | |
| Residual | 0.2 | ||||||
| TBLH BMD (g/cm2) | Intercept | 1.157 (0.024) | 1.109–1.204 | Intercept | 0.007 | ||
| Slope | 0.013 (0.003) | 0.008–0.019 | <0.001 | Slope | 7.2 × 10−5 | 0.986 | |
| Residual | 1.1 × 10−4 | ||||||
| TBLH BMC (g) | Intercept | 2719 (131) | 2461–2976 | Intercept | 2.23 × 105 | ||
| Slope | 61 (9) | 43–78 | <0.001 | Slope | 902 | 0.997 | |
| Residual | 731 | ||||||
| Resting metabolic rate and energy availability | |||||||
| RMRratio | Intercept | 1.03 (0.01) | 1.01–1.05 | Intercept | 7.96 × 10−4 | ||
| Slope | −0.01 (0.01) | −0.02 to −0.0 | 0.016 | Slope | 4.80 × 10−6 | 0.275 | |
| Residual | 0.00210 | ||||||
| EA (kcal/kg FFM/day) | Intercept | 50.1 (3.6) | 43.6–57.1 | Intercept | 122.1 | ||
| Slope | −0.7 (0.9) | −2.6 to 1.2 | 0.475 | Slope | 3.0 | 0.653 | |
| Residual | 64.8 | ||||||
| Disordered eating behavior and exercise addiction | |||||||
| EAI-Y | Intercept | 17.9 (0.7) | 16.7–19.2 | Intercept | 3.1 | ||
| Slope | −0.7 (0.2) | −1.2 to −0.3 | 0.005 | Slope | 0.1 | 0.442 | |
| Residual | 3.9 | ||||||
| Performance | |||||||
| VO2peak (mL/kg/min) | Intercept | 61.3 (1.3) | 58.7–64.0 | Intercept | 16.7 | ||
| Slope | 2.8 (0.4) | 2.1–3.5 | <0.001 | Slope | 0.3 | 0.629 | |
| Residual | 9.9 | ||||||
| Force (Newton) | Intercept | 2464 (106) | 2257–2671 | Intercept | 108,766 | ||
| Slope | 91 (24) | 45–137 | <0.001 | Slope | 70 | 0.649 | |
| Residual | 58,885 | ||||||
| Power (watt) | Intercept | 1115 (49) | 1020–1210 | Intercept | 28,831 | ||
| Slope | 29 (6) | 17–41 | <0.001 | Slope | 146 | 0.912 | |
| Residual | 2797 | ||||||
| Descriptive | Reliable Change a Shown in Absolute Values with % in Brackets | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T1–T3 (n = 12) | T3–T5 (n = 9) | T1–T5 (n = 9) | |||||||
| (n = 13) | (n = 13) | (n = 12) | (n = 12) | (n = 9) | Dec | Stable | Inc | Dec | Stable | Inc | Dec | Stable | Inc | |
| TBLH | ||||||||||||||
| BMC (g) | 2764 ± 459 | 2848 ± 438 | 2952 ± 422 | 2995 ± 422 | 3002 ± 323 | 0 (0%) | 4 (33%) | 8 (67%) | 0 (0%) | 8 (89%) | 1 (11%) | 0 (0%) | 1 (11%) | 8 (89%) |
| BMD (g/cm2) | 1.164 ± 0.080 | 1.189 ± 0.076 | 1.205 ± 0.069 | 1.207 ± 0.066 | 1.215 ± 0.057 | 0 (0%) | 4 (33%) | 8 (67%) | 0 (0%) | 5 (56%) | 4 (44%) | 0 (0%) | 1 (11%) | 8 (89%) |
| Z-score | 0.26 ± 0.84 | 0.32 ± 0.73 | 0.16 ± 0.72 | 0.19 ± 0.90 | −0.05 ± 0.62 | 4 (33%) | 7 (58%) | 1 (8%) | 4 (44%) | 5 (56%) | 0 (0%) | 5 (56%) | 4 (44%) | 0 (0%) |
| L1–L4 | ||||||||||||||
| BMC (g) | 64.1 ± 11.0 | 67.3 ± 12.2 | 71.1 ± 12.6 | 73.7 ± 10.7 | 75.0 ± 9.0 | 4 (33%) | 1 (8%) | 7 (58%) | 0 (0%) | 4 (44%) | 5 (56%) | 4 (44%) | 0 (0%) | 5 (56%) |
| BMD (g/cm2) | 1.066 ± 0.083 | 1.093 ± 0.081 | 1.119 ± 0.087 | 1.139 ± 0.078 | 1.150 ± 0.069 | 5 (42%) | 1 (8%) | 6 (50%) | 0 (0%) | 4 (44%) | 5 (56%) | 4 (44%) | 0 (0%) | 5 (56%) |
| Z-score | −0.70 ± 0.61 | −0.79 ± 0.57 | −0.72 ± 0.62 | −0.59 ± 0.57 | −0.51 ± 0.51 | 4 (33%) | 8 (67%) | 0 (0%) | 0 (0%) | 4 (44%) | 5 (56%) | 1 (11%) | 3 (33%) | 5 (56%) |
| Femur Neck | ||||||||||||||
| BMC (g) | 5.9 ± 1.1 | 6.0 ± 1.1 | 6.2 ± 1.0 | 6.3 ± 1.1 | 6.2 ± 0.6 | 0 (0%) | 9 (75%) | 3 (25%) | 0 (0%) | 9 (100%) | 0 (0%) | 0 (0%) | 4 (44%) | 5 (56%) |
| BMD (g/cm2) | 1.104 ± 0.119 | 1.117 ± 0.121 | 1.141 ± 0.110 | 1.153 ± 0.130 | 1.130 ± 0.088 | 0 (0%) | 7 (58%) | 5 (42%) | 0 (0%) | 7 (78%) | 2 (22%) | 0 (0%) | 4 (44%) | 5 (56%) |
| Total Hip | ||||||||||||||
| BMC (g) | 40.4 ± 6.6 | 40.7 ± 6.4 | 41.6 ± 6.1 | 41.9± 6.2 | 41.4 ± 3.6 | 0 (0%) | 10 (83%) | 2 (17%) | 0 (0%) | 9 (100%) | 0 (0%) | 0 (0%) | 5 (56%) | 4 (44%) |
| BMD (g/cm2) | 1.129 ± 0.114 | 1.130 ± 0.110 | 1.142 ± 0.101 | 1.147 ± 0.109 | 1.131 ± 0.074 | 1 (8%) | 10 (83%) | 1 (8%) | 0 (0%) | 8 (89%) | 1 (11%) | 0 (0%) | 5 (56%) | 4 (44%) |
4. Discussion
4.1. Bone Health
4.2. Energy Availability
4.3. Resting Metabolic Rate
4.4. Psychological Parameters
4.5. Performance
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mountjoy, M.; Ackerman, K.E.; Bailey, D.M.; Burke, L.M.; Constantini, N.; Hackney, A.C.; Heikura, I.A.; Melin, A.; Pensgaard, A.M.; Stellingwerff, T.; et al. 2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sport. Med. 2023, 57, 1073–1097. [Google Scholar] [CrossRef]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Donnell, S.M.; Corish, C.A. Low Energy Availability in Athletes 2020: An Updated Narrative Review of Prevalence, Risk, Within-Day Energy Balance, Knowledge, and Impact on Sports Performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef]
- Melin, A.K.; Areta, J.L.; Heikura, I.A.; Stellingwerff, T.; Torstveit, M.K.; Hackney, A.C. Direct and indirect impact of low energy availability on sports performance. Scand. J. Med. Sci. Sport. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Gould, R.J.; Ridout, A.J.; Newton, J.L. Relative Energy Deficiency in Sport (RED-S) in Adolescents—A Practical Review. Int. J. Sport. Med. 2022, 44, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Simič, V.; Jevšnik, Š.; Mohorko, N. Low Energy Availability and Carbohydrate Intake in Competitive Adolescent Sport Climbers. Kinesiology 2022, 54, 268–277. [Google Scholar] [CrossRef]
- Matt, S.A.; Barrack, M.T.; Gray, V.B.; Cotter, J.A.; Van Loan, M.D.; Rauh, M.J.; McGowan, R.; Nichols, J.F. Adolescent Endurance Runners Exhibit Suboptimal Energy Availability and Intakes of Key Nutrients. J. Am. Nutr. Assoc. 2022, 41, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Cherian, K.S.; Sainoji, A.; Nagalla, B.; Yagnambhatt, V.R. Energy Balance Coexists with Disproportionate Macronutrient Consumption Across Pretraining, During Training, and Posttraining Among Indian Junior Soccer Players. Pediatr. Exerc. Sci. 2018, 30, 506–515. [Google Scholar] [CrossRef]
- Silva, M.G.; Silva, H.H. Comparison of body composition and nutrients’ deficiencies between Portuguese rink-hockey players. Eur. J. Pediatr. 2017, 176, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.G.; Silva, H.H.; Paiva, T. Sleep duration, body composition, dietary profile and eating behaviours among children and adolescents: A comparison between Portuguese acrobatic gymnasts. Eur. J. Pediatr. 2018, 177, 815–825. [Google Scholar] [CrossRef]
- Santos, L.; Elliott-Sale, K.J.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Olmedillas, H.; Gonzalez-Aguero, A.; Moreno, L.A.; Casajus, J.A.; Vicente-Rodriguez, G. Bone related health status in adolescent cyclists. PLoS ONE 2011, 6, e24841. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, D.; Barker, A.R.; Ubago-Guisado, E.; Williams, C.A.; Gracia-Marco, L. The effect of a high-impact jumping intervention on bone mass, bone stiffness and fitness parameters in adolescent athletes. Arch. Osteoporos. 2018, 13, 128. [Google Scholar] [CrossRef]
- Vlachopoulos, D.; Barker, A.R.; Ubago-Guisado, E.; Williams, C.A.; Gracia-Marco, L. A 9-Month Jumping Intervention to Improve Bone Geometry in Adolescent Male Athletes. Med. Sci. Sport. Exerc. 2018, 50, 2544–2554. [Google Scholar] [CrossRef]
- Barrack, M.T.; Rauh, M.J.; Nichols, J.F. Cross-Sectional Evidence of Suppressed Bone Mineral Accrual Among Female Adolescent Runners. J. Bone Miner. Res. 2010, 25, 1850–1857. [Google Scholar] [CrossRef]
- Wasserfurth, P.; Palmowski, J.; Hahn, A.; Kruger, K. Reasons for and Consequences of Low Energy Availability in Female and Male Athletes: Social Environment, Adaptations, and Prevention. Sport. Med. Open 2020, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Torstveit, M.K.; Ackerman, K.E.; Constantini, N.; Holtzman, B.; Koehler, K.; Mountjoy, M.L.; Sundgot-Borgen, J.; Melin, A. Primary, secondary and tertiary prevention of Relative Energy Deficiency in Sport (REDs): A narrative review by a subgroup of the IOC consensus on REDs. Br. J. Sport. Med. 2023, 57, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sport. Physiol. Perform. 2021, 17, 317–331. [Google Scholar] [CrossRef]
- Sundgot-Borgen, J.; Meyer, N.L.; Lohman, T.G.; Ackland, T.R.; Maughan, R.J.; Stewart, A.D.; Muller, W. How to minimise the health risks to athletes who compete in weight-sensitive sports review and position statement on behalf of the Ad Hoc Research Working Group on Body Composition, Health and Performance, under the auspices of the IOC Medical Commission. Br. J. Sport. Med. 2013, 47, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Júlíusson, P.B.; Roelants, M.; Eide, G.E.; Moster, D.; Juul, A.; Hauspie, R.; Waaler, P.E.; Bjerknes, R. Growth charts for Norwegian children. Tidsskr. Den Nor. Legeforening 2009, 129, 281–286. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L.; Evidence Analysis Working, G. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Nana, A.; Slater, G.J.; Stewart, A.D.; Burke, L.M. Methodology review: Using dual-energy X-ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. Int. J. Sport. Nutr. Exerc. Metab. 2015, 25, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, M.; Holme, I.; Pensgaard, A.M.; Torstveit, M.K.; Sundgot-Borgen, J. The development of the brief eating disorder in athletes questionnaire. Med. Sci. Sport. Exerc. 2014, 46, 1666–1675. [Google Scholar] [CrossRef]
- Smolak, L.; Murnen, S.K. Drive for leanness: Assessment and relationship to gender, gender role and objectification. Body Image 2008, 5, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, M.B.; Griffiths, M.D.; Hemmingsen, S.D.; Stoving, R.K. Exercise addiction in adolescents and emerging adults—Validation of a youth version of the Exercise Addiction Inventory. J. Behav. Addict. 2018, 7, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Crouter, S.E.; Churilla, J.R.; Bassett, D.R., Jr. Accuracy of the Actiheart for the assessment of energy expenditure in adults. Eur. J. Clin. Nutr. 2008, 62, 704–711. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Truax, P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991, 59, 12–19. [Google Scholar] [CrossRef]
- Maassen, G.H.; Bossema, E.; Brand, N. Reliable change and practice effects: Outcomes of various indices compared. J. Clin. Exp. Neuropsychol. 2009, 31, 339–352. [Google Scholar] [CrossRef]
- Nikander, R.; Sievanen, H.; Heinonen, A.; Kannus, P. Femoral neck structure in adult female athletes subjected to different loading modalities. J. Bone Miner. Res. 2005, 20, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Mountjoy, M.; McCluskey, W.T.P.; Ackerman, K.E.; Verhagen, E.; Heikura, I.A. Review of the scientific rationale, development and validation of the International Olympic Committee Relative Energy Deficiency in Sport Clinical Assessment Tool: V.2 (IOC REDs CAT2)-by a subgroup of the IOC consensus on REDs. Br. J. Sport. Med. 2023, 57, 1109–1118. [Google Scholar] [CrossRef]
- Hetland, M.L.; Haarbo, J.; Christiansen, C. Low bone mass and high bone turnover in male long distance runners. J. Clin. Endocr. Metab. 1993, 77, 770–775. [Google Scholar] [CrossRef]
- Scofield, K.L.; Hecht, S. Bone health in endurance athletes: Runners, cyclists, and swimmers. Curr. Sport. Med. Rep. 2012, 11, 328–334. [Google Scholar] [CrossRef]
- Jurov, I.; Keay, N.; Rauter, S. Reducing energy availability in male endurance athletes: A randomized trial with a three-step energy reduction. J. Int. Soc. Sport. Nutr. 2022, 19, 179–195. [Google Scholar] [CrossRef]
- Burke, L.M.; Ackerman, K.E.; Heikura, I.A.; Hackney, A.C.; Stellingwerff, T. Mapping the complexities of Relative Energy Deficiency in Sport (REDs): Development of a physiological model by a subgroup of the International Olympic Committee (IOC) Consensus on REDs. Br. J. Sport. Med. 2023, 57, 1098–1108. [Google Scholar] [CrossRef]
- Nattiv, A.; De Souza, M.J.; Koltun, K.J.; Misra, M.; Kussman, A.; Williams, N.I.; Barrack, M.T.; Kraus, E.; Joy, E.; Fredericson, M. The Male Athlete Triad-A Consensus Statement from the Female and Male Athlete Triad Coalition Part 1: Definition and Scientific Basis. Clin. J. Sport Med. 2021, 31, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Strock, N.C.A.; Koltun, K.J.; Southmayd, E.A.; Williams, N.I.; De Souza, M.J. Indices of Resting Metabolic Rate Accurately Reflect Energy Deficiency in Exercising Women. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 14–24. [Google Scholar] [CrossRef]
- Staal, S.; Sjodin, A.; Fahrenholtz, I.; Bonnesen, K.; Melin, A.K. Low RMRratio as a Surrogate Marker for Energy Deficiency, the Choice of Predictive Equation Vital for Correctly Identifying Male and Female Ballet Dancers at Risk. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 412–418. [Google Scholar] [CrossRef]
- Langan-Evans, C.; Germaine, M.; Artukovic, M.; Oxborough, D.L.; Areta, J.L.; Close, G.L.; Morton, J.P. The Psychological and Physiological Consequences of Low Energy Availability in a Male Combat Sport Athlete. Med. Sci. Sport. Exerc. 2020, 53, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.L.; Garvican-Lewis, L.A.; Lundy, B.; Rice, A.J.; Thompson, K.G. New approaches to determine fatigue in elite athletes during intensified training: Resting metabolic rate and pacing profile. PLoS ONE 2017, 12, e0173807. [Google Scholar] [CrossRef]
- Woods, A.L.; Rice, A.J.; Garvican-Lewis, L.A.; Wallett, A.M.; Lundy, B.; Rogers, M.A.; Welvaert, M.; Halson, S.; McKune, A.; Thompson, K.G. The effects of intensified training on resting metabolic rate (RMR), body composition and performance in trained cyclists. PLoS ONE 2018, 13, e0191644. [Google Scholar] [CrossRef] [PubMed]
- Schofield, K.L.; Thorpe, H.; Sims, S.T. Resting metabolic rate prediction equations and the validity to assess energy deficiency in the athlete population. Exp. Physiol. 2019, 104, 469–475. [Google Scholar] [CrossRef]
- De Souza, M.J.; West, S.L.; Jamal, S.A.; Hawker, G.A.; Gundberg, C.M.; Williams, N.I. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone 2008, 43, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Cherian, K.S.; Shahkar, F.; Sainoji, A.; Balakrishna, N.; Yagnambhatt, V.R. Resting metabolic rate of Indian Junior Soccer players: Testing agreement between measured versus selected predictive equations. Am. J. Hum. Biol. 2018, 30, e23066. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.H.; Kim, G.S.; Park, J.S.; Kim, E.K. Accuracy of predictive equations for resting metabolic rate in Korean athletic and non-athletic adolescents. Nutr. Res. Pract. 2015, 9, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.; Penna-Franca, P.A.; Dias-Silva, C.H.; Bittencourt, V.Z.; Cahue, F.; Fonseca-Junior, S.J.; Pierucci, A. Predictive equations for resting metabolic rate are not appropriate to use in Brazilian male adolescent football athletes. PLoS ONE 2021, 16, e0244970. [Google Scholar] [CrossRef]
- Speakman, J.R.; Selman, C. Physical activity and resting metabolic rate. Proc. Nutr. Soc. 2003, 62, 621–634. [Google Scholar] [CrossRef]
- Heikura, I.A.; Stellingwerff, T.; Areta, J.L. Low energy availability in female athletes: From the lab to the field. Eur. J. Sport. Sci. 2021, 22, 709–719. [Google Scholar] [CrossRef]
- Keay, N.; Francis, G.; Hind, K. Low energy availability assessed by a sport-specific questionnaire and clinical interview indicative of bone health, endocrine profile and cycling performance in competitive male cyclists. BMJ Open Sport Exerc. Med. 2018, 4, e000424. [Google Scholar] [CrossRef]
- Khairullah, A.; Klein, L.C.; Ingle, S.M.; May, M.T.; Whetzel, C.A.; Susman, E.J.; Paus, T. Testosterone trajectories and reference ranges in a large longitudinal sample of male adolescents. PLoS ONE 2014, 9, e108838. [Google Scholar] [CrossRef]
| Variables | Athletes (n = 13) |
|---|---|
| Anthropometry | |
| Age (years) | 16.3 ± 0.4 |
| Height (cm) | 179.4 ± 7.6 |
| Weight (kg) | 63.6 ± 8.2 |
| BMI (kg/m2) | 19.7 ± 1.6 |
| FFM (kg) † | 57.4 ± 6.6 |
| Body fat (%) † | 11.5 ± 3.3 |
| Resting metabolic rate and energy availability | |
| RMR (kcal/kg/day) | 31.9 ± 2.7 |
| RMRratio | 1.03 ± 0.07 |
| EA (kcal/kg FFM/day) | 50.7 ± 15.8 |
| Disordered eating behaviors and exercise addiction | |
| BEDA-Q | 0.0 (0.0–0.0) |
| DLS | 3.8 (3.2–4.7) |
| EAI-Y | 18.0 (17.0–20.0) |
| Performance | |
| VO2peak (mL/kg/minute) | 61.5 ± 5.3 |
| Force (Newton) | 2754 ± 566 |
| Power (Watt) | 1167 ± 200 |
| Training volume (h/year) | 531 ± 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenqvist, T.B.; Melin, A.K.; Torstveit, M.K. Relative Energy Deficiency in Sport (REDs) Indicators in Male Adolescent Endurance Athletes: A 3-Year Longitudinal Study. Nutrients 2023, 15, 5086. https://doi.org/10.3390/nu15245086
Stenqvist TB, Melin AK, Torstveit MK. Relative Energy Deficiency in Sport (REDs) Indicators in Male Adolescent Endurance Athletes: A 3-Year Longitudinal Study. Nutrients. 2023; 15(24):5086. https://doi.org/10.3390/nu15245086
Chicago/Turabian StyleStenqvist, Thomas Birkedal, Anna Katarina Melin, and Monica Klungland Torstveit. 2023. "Relative Energy Deficiency in Sport (REDs) Indicators in Male Adolescent Endurance Athletes: A 3-Year Longitudinal Study" Nutrients 15, no. 24: 5086. https://doi.org/10.3390/nu15245086
APA StyleStenqvist, T. B., Melin, A. K., & Torstveit, M. K. (2023). Relative Energy Deficiency in Sport (REDs) Indicators in Male Adolescent Endurance Athletes: A 3-Year Longitudinal Study. Nutrients, 15(24), 5086. https://doi.org/10.3390/nu15245086







