A Correlation Study of Plasma and Breast Milk Retinol Concentrations in Breastfeeding Women in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Data and Sample Collection
2.3. Sample Analysis
2.4. Statistical Analysis
3. Results
3.1. Maternal and Infant Characteristics
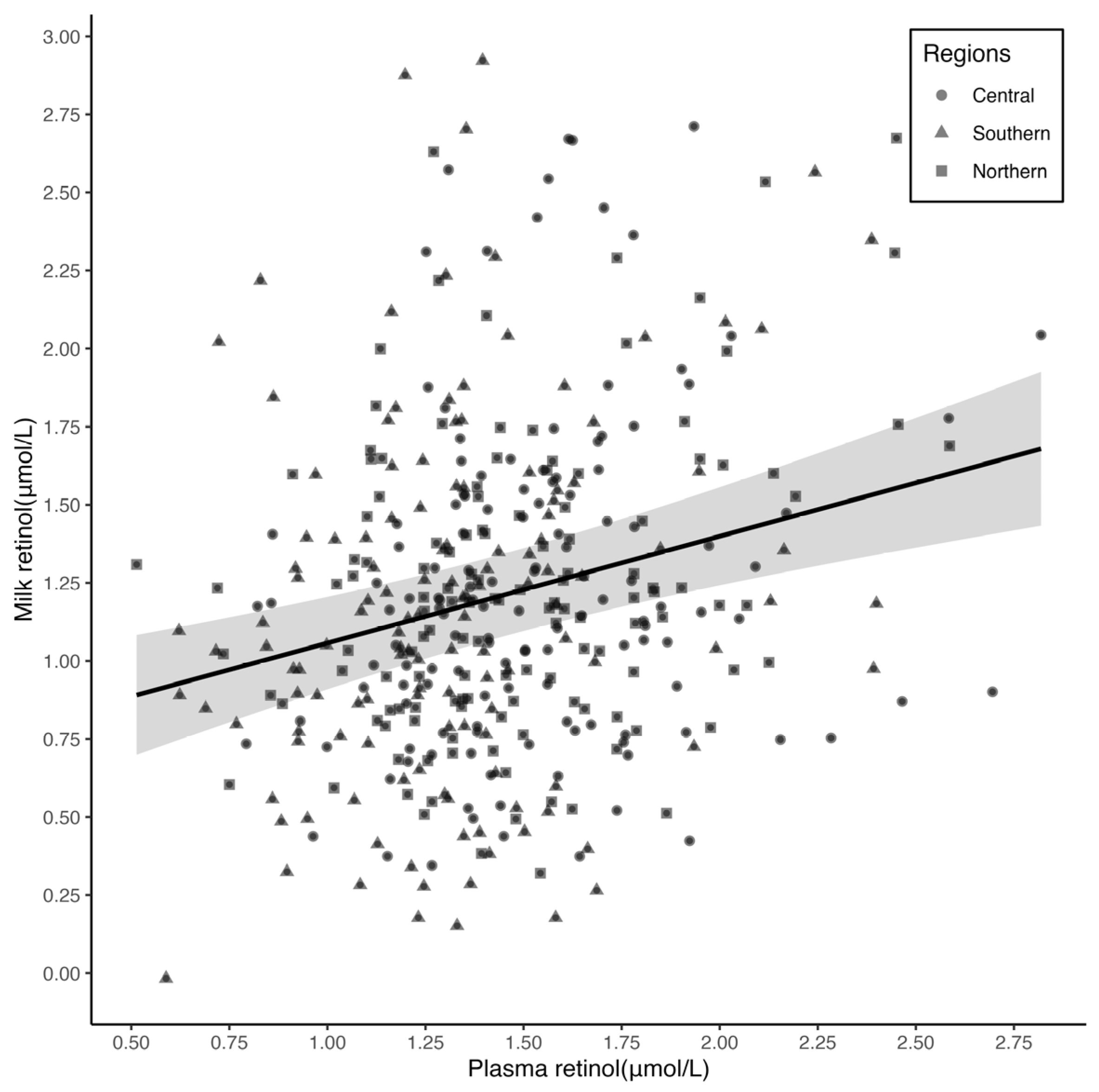
3.2. Relationship between Plasma and Breast Milk Retinol Concentrations
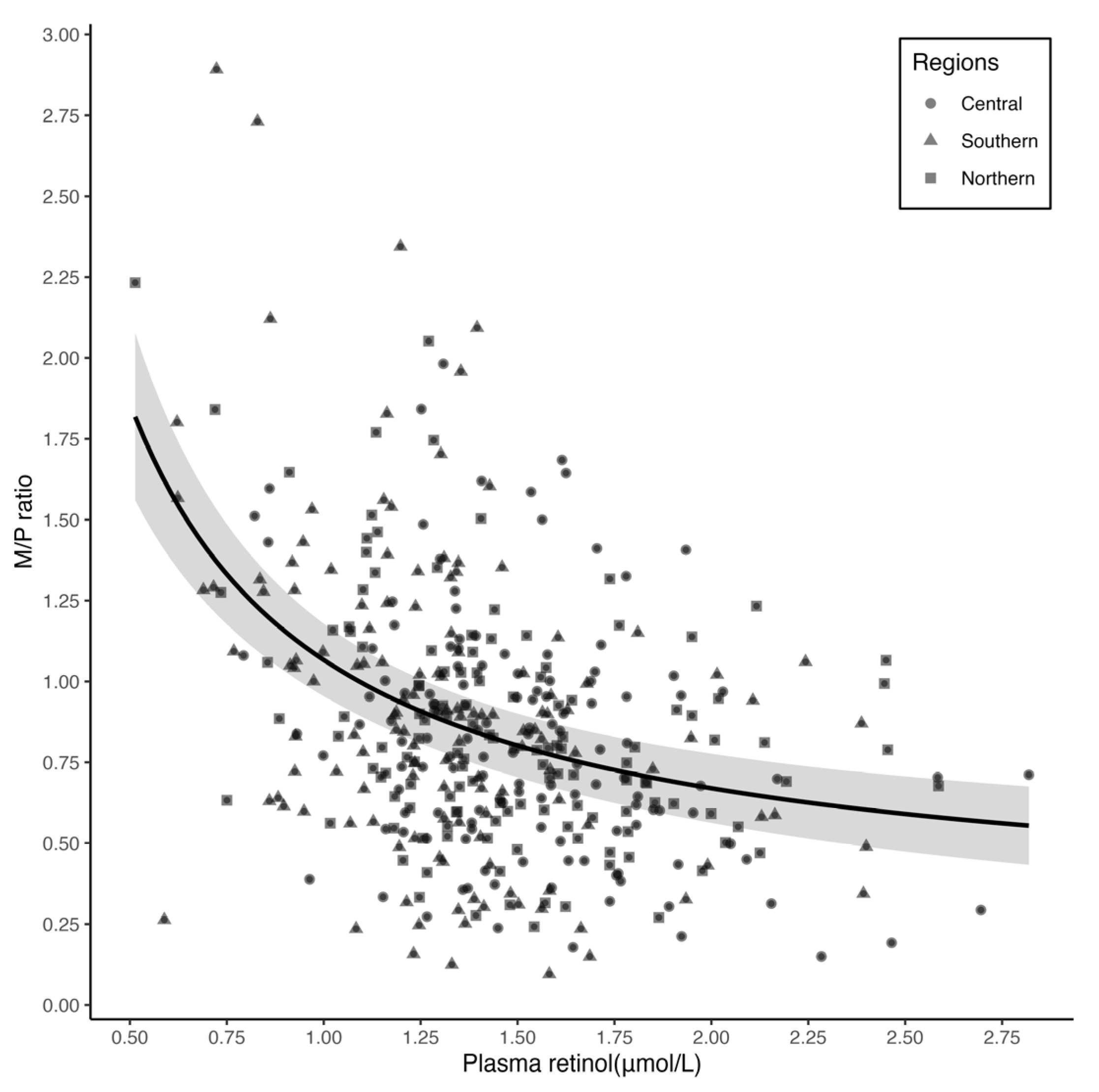
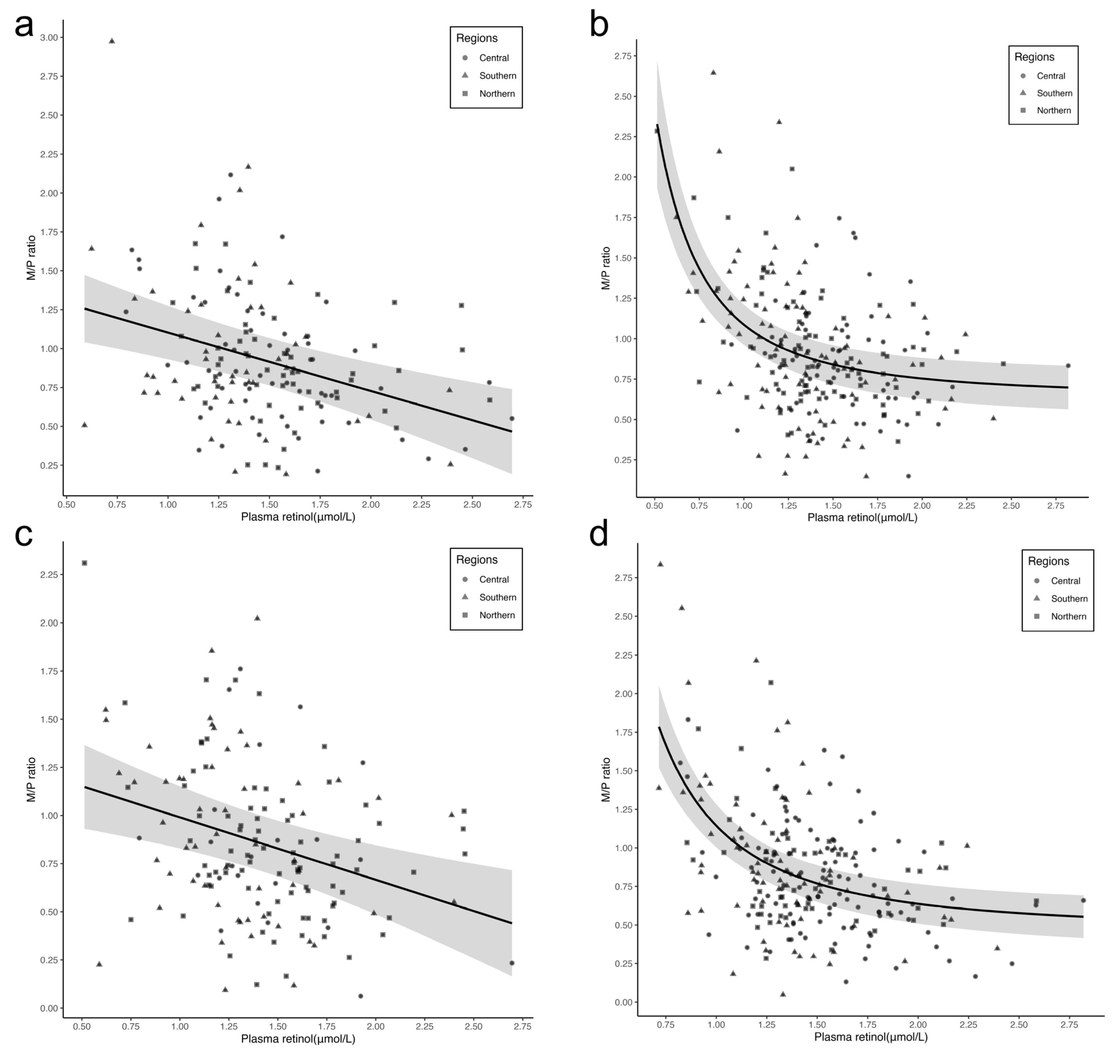
3.3. Relationship between Plasma Retinol Concentration and M/P Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Vitamin A Review. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- World Health Organization. Global Strategy for Infant and Young Child Feeding: The Optimal Duration of Exclusive Breastfeeding; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Debier, C.; Larondelle, Y. Vitamins A and E: Metabolism, roles and transfer to offspring. Br. J. Nutr. 2005, 93, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Pasatiempo, A.M.; Green, M.H. Chylomicron margination, lipolysis, and vitamin a uptake in the lactating rat mammary gland: Implications for milk retinoid content. Exp. Biol. Med. 2004, 229, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Noy, N. Vitamin A Transport and Cell Signaling by the Retinol-Binding Protein Receptor STRA6. Sub-Cell. Biochem. 2016, 81, 77–93. [Google Scholar] [CrossRef]
- Gross, R.; Hänsel, H.; Schultink, W.; Shrimpton, R.; Matulessi, P.; Gross, G.; Tagliaferri, E.; Sastroamdijojo, S. Moderate zinc and vitamin A deficiency in breast milk of mothers from East-Jakarta. Eur. J. Clin. Nutr. 1998, 52, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Dijkhuizen, M.A.; Wieringa, F.T.; West, C.E.; Muherdiyantiningsih; Muhilal. Concurrent micronutrient deficiencies in lactating mothers and their infants in Indonesia. Am. J. Clin. Nutr. 2001, 73, 786–791. [Google Scholar] [PubMed]
- Panpanich, R.; Vitsupakorn, K.; Harper, G.; Brabin, B. Serum and breast-milk vitamin A in women during lactation in rural Chiang Mai, Thailand. Ann. Trop. Paediatr. 2002, 22, 321–324. [Google Scholar] [CrossRef]
- Ettyang, G.; Oloo, A.; van Marken Lichtenbelt, W.; Saris, W. Consumption of vitamin A by breastfeeding children in rural Kenya. Food Nutr. Bull. 2004, 25, 256–263. [Google Scholar] [CrossRef]
- Mello-Neto, J.; Rondó, P.H.; Oshiiwa, M.; Morgano, M.A.; Zacari, C.Z.; Domingues, S. The influence of maternal factors on the concentration of vitamin A in mature breast milk. Clin. Nutr. 2009, 28, 178–181. [Google Scholar] [CrossRef]
- Fujita, M.; Lo, Y.J.; Brindle, E. Nutritional, inflammatory, and ecological correlates of maternal retinol allocation to breast milk in agro-pastoral Ariaal communities of northern Kenya. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 2017, 29, e22961. [Google Scholar] [CrossRef]
- Meneses, F.; Trugo, N.M.F. Retinol, β-carotene, and lutein + zeaxanthin in the milk of Brazilian nursing women: Associations with plasma concentrations and influences of maternal characteristics. Nutr. Res. 2005, 25, 443–451. [Google Scholar] [CrossRef]
- de Azeredo, V.B.; Trugo, N.M. Retinol, carotenoids, and tocopherols in the milk of lactating adolescents and relationships with plasma concentrations. Nutrition 2008, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.; de Sousa Rebouças, A.; Mendonça, B.M.A.; Silva, D.; Dimenstein, R.; Ribeiro, K. Relationship between the dietary intake, serum, and breast milk concentrations of vitamin A and vitamin E in a cohort of women over the course of lactation. Matern. Child Nutr. 2019, 15, e12772. [Google Scholar] [CrossRef]
- da Silva Ribeiro, K.D.; de Araújo, K.F.; de Souza, H.H.; Soares, F.B.; da Costa Pereira, M.; Dimenstein, R. Nutritional vitamin A status in northeast Brazilian lactating mothers. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2010, 23, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Ribeiro, K.; Araújo, K.; Pires, J.; Azevedo, G.; Dimenstein, R. Alpha-tocopherol concentration in the colostrum of nursing women supplemented with retinyl palmitate and alpha-tocopherol. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2010, 23, 529–534. [Google Scholar] [CrossRef] [PubMed]
- de Lira, L.Q.; Lima, M.S.; de Medeiros, J.M.; da Silva, I.F.; Dimenstein, R. Correlation of vitamin A nutritional status on alpha-tocopherol in the colostrum of lactating women. Matern. Child Nutr. 2013, 9, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Grilo, E.C.; Lima, M.S.; Cunha, L.R.; Gurgel, C.S.; Clemente, H.A.; Dimenstein, R. Effect of maternal vitamin A supplementation on retinol concentration in colostrum. J. Pediatr. 2015, 91, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Li, H.-T.; Yu, L.-X.; Xu, G.-S.; Ge, H.; Wang, L.-L.; Zhang, Y.-L.; Zhou, Y.-B.; Li, Y.; Bai, M.-X. A correlation study of DHA dietary intake and plasma, erythrocyte and breast milk DHA concentrations in lactating women from coastland, lakeland, and inland areas of China. Nutrients 2016, 8, 312. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Schleicher, R.L.; Haynes, B.M.; Rybak, M.E.; Pirkle, J.L. The CDC’s Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population is a valuable tool for researchers and policy makers. J. Nutr. 2013, 143, 938s–947s. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Penniston, K.L. Simplified methodology to determine breast milk retinol concentrations. J. Lipid Res. 2002, 43, 350–355. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Howe, J.A. Twice the amount of alpha-carotene isolated from carrots is as effective as beta-carotene in maintaining the vitamin A status of Mongolian gerbils. J. Nutr. 2005, 135, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Multivariable fractional polynomial method for regression model. Ann. Transl. Med. 2016, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- Muggeo, V.M. Estimating regression models with unknown break-points. Stat. Med. 2003, 22, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Green, M.H.; Green, J.B.; Akohoue, S.A.; Kelley, S.K. Vitamin A intake affects the contribution of chylomicrons vs. retinol-binding protein to milk vitamin A in lactating rats. J. Nutr. 2001, 131, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Retinol-to-Fat Ratio and Retinol Concentration in Human Milk Show Similar Time Trends and Associations with Maternal Factors at the Population Level: A Systematic Review and Meta-Analysis. Adv. Nutr. 2018, 9, 332s–346s. [Google Scholar] [CrossRef] [PubMed]
- Muslimatun, S.; Schmidt, M.K.; West, C.E.; Schultink, W.; Hautvast, J.G.; Karyadi, D. Weekly vitamin A and iron supplementation during pregnancy increases vitamin A concentration of breast milk but not iron status in Indonesian lactating women. J. Nutr. 2001, 131, 2664–2669. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Haskell, M.J.; Nankap, M.; Ndjebayi, A.O.; Brown, K.H. Breast milk retinol and plasma retinol-binding protein concentrations provide similar estimates of vitamin A deficiency prevalence and identify similar risk groups among women in Cameroon but breast milk retinol underestimates the prevalence of deficiency among young children. J. Nutr. 2014, 144, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv. Nutr. 2018, 9, 278s–294s. [Google Scholar] [CrossRef] [PubMed]
- Kaliwile, C.; Michelo, C.; Sheftel, J.; Davis, C.R.; Grahn, M.; Bwembya, P.; Simpungwe, E.; Mwanza, S.; Chileshe, J.; Tanumihardjo, S.A. Breast Milk-Derived Retinol Is a Potential Surrogate for Serum in the 13C-Retinol Isotope Dilution Test in Zambian Lactating Women with Vitamin A Deficient and Adequate Status. J. Nutr. 2021, 151, 255–263. [Google Scholar] [CrossRef]
| Characteristics | Overall (n = 403) | Regions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Central (n = 135) | Southern (n = 134) | Northern (n = 134) | p-Value * | ||||||
| n | % | n | % | n | % | n | % | ||
| Mothers: | |||||||||
| Age (years) | 0.034 | ||||||||
| <25 | 60 | 14.9 | 13 | 9.6 | 29 | 21.6 | 18 | 13.4 | |
| ≥25 to 30 | 251 | 62.3 | 91 | 67.4 | 81 | 60.5 | 79 | 59.0 | |
| ≥30 | 92 | 22.8 | 31 | 23.0 | 24 | 17.9 | 37 | 27.6 | |
| Ethnicity | 0.036 | ||||||||
| Han | 385 | 95.5 | 131 | 97.0 | 131 | 97.8 | 123 | 91.8 | |
| Others | 18 | 4.5 | 4 | 3.0 | 3 | 2.2 | 11 | 8.2 | |
| Education level | 0.264 | ||||||||
| College or above | 270 | 67.0 | 88 | 65.2 | 84 | 62.7 | 98 | 73.1 | |
| High school | 91 | 22.6 | 34 | 25.2 | 31 | 23.1 | 26 | 19.4 | |
| Middle school or less | 42 | 10.4 | 13 | 9.6 | 19 | 14.2 | 10 | 7.5 | |
| BMI (kg/m2) | <0.001 | ||||||||
| <18.5 | 9 | 2.2 | 1 | 0.7 | 7 | 5.2 | 1 | 0.7 | |
| 18.5 to <25 | 273 | 67.7 | 83 | 61.5 | 104 | 77.6 | 86 | 67.7 | |
| ≥25 | 121 | 30.0 | 51 | 37.8 | 23 | 17.2 | 47 | 35.1 | |
| Parity | 0.281 | ||||||||
| Primiparous | 343 | 85.1 | 119 | 88.1 | 109 | 81.3 | 115 | 85.8 | |
| Multiparous | 60 | 14.9 | 16 | 11.9 | 25 | 18.7 | 19 | 14.2 | |
| Gestational age (week) | 0.488 | ||||||||
| <37 | 16 | 4.0 | 6 | 4.4 | 5 | 3.7 | 5 | 3.7 | |
| 37 to <42 | 379 | 94.0 | 124 | 91.9 | 127 | 94.8 | 128 | 95.5 | |
| ≥42 | 8 | 2.0 | 5 | 3.7 | 2 | 1.5 | 1 | 0.7 | |
| Delivery modes | <0.001 | ||||||||
| vaginal delivery | 242 | 60.0 | 112 | 83.0 | 79 | 59.0 | 51 | 38.1 | |
| cesarean section | 161 | 40.0 | 23 | 17.0 | 55 | 41.0 | 83 | 61.9 | |
| Breastfeeding Practice | 0.193 | ||||||||
| Exclusively breastfeeding | 240 | 59.6 | 73 | 54.1 | 87 | 64.9 | 80 | 59.7 | |
| Partially breastfeeding | 163 | 40.4 | 62 | 45.9 | 47 | 35.1 | 54 | 40.3 | |
| Infants: | |||||||||
| Sex | 0.049 | ||||||||
| Male | 212 | 52.6 | 70 | 51.9 | 61 | 45.5 | 81 | 60.4 | |
| Female | 191 | 47.4 | 65 | 48.1 | 73 | 54.5 | 53 | 39.6 | |
| Birth weight (g) | 0.456 | ||||||||
| <2500 | 5 | 1.2 | 1 | 0.7 | 2 | 1.5 | 2 | 1.5 | |
| 2500 to <4000 | 358 | 88.8 | 116 | 85.9 | 123 | 91.8 | 119 | 88.8 | |
| ≥4000 | 40 | 9.9 | 18 | 13.3 | 9 | 6.7 | 13 | 9.7 | |
| Overall | Regions | |||
|---|---|---|---|---|
| Central | Southern | Northern | ||
| Retinol concentration (μmol/L) in plasma | ||||
| Median (IQR) *** | 1.39 (1.21, 1.63) | 1.50 (1.30, 1.71) a | 1.31 (1.10, 1.50) b | 1.42 (1.25, 1.67) a |
| Mean ± SDs | 1.44 ± 0.37 | 1.53 ± 0.35 | 1.32 ± 0.36 | 1.48 ± 0.37 |
| Retinol concentration (μmol/L) in breast milk | ||||
| Median (IQR) ** | 1.15 (0.83, 1.49) | 1.20 (0.91, 1.57) a | 1.20 (0.87, 1.55) ab | 1.03 (0.72, 1.40) b |
| Mean ± SDs | 1.22 ± 0.56 | 1.28 ± 0.54 | 1.26 ± 0.60 | 1.10 ± 0.52 |
| M/P ratio | ||||
| Median (IQR) *** | 0.80 (0.57, 1.12) | 0.79 (0.59, 1.05) ab | 0.97 (0.68, 1.29) a | 0.68 (0.49, 1.00) b |
| Mean ± SDs | 0.88 ± 0.44 | 0.87 ± 0.38 | 1.01 ± 0.51 | 0.77 ± 0.38 |
| Correlation between plasma and breast milk retinol † | ||||
| Crude rs | 0.17 ** | 0.15 | 0.18 * | 0.24 ** |
| Partial rs ¶ | 0.17 ** | 0.23 * | 0.21 * | 0.12 |
| Correlation between plasma retinol and M/P ratio § | ||||
| Crude rs | −0.34 *** | −0.33 *** | −0.38 *** | −0.25 ** |
| Partial rs ¶ | −0.33 *** | −0.25 ** | −0.33 *** | −0.37 *** |
| Outcome | Exposure | β (95% CI) † | SE | R2 |
|---|---|---|---|---|
| Breast milk § | Plasma | 0.34 (0.19, 0.49) | 0.08 | 0.16 *** |
| M/P ratio ¶ | Plasma | −0.40 (−0.51, −0.28) | 0.06 | 0.22 *** |
| M/P ratio ‡ | Plasma ≤ 1.00 μmol/L | −1.69 (−2.75, −0.62) | 0.54 | 0.25 *** |
| Plasma > 1.00 μmol/L | −0.29 (−0.42, −0.16) | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Zhou, Y.; Li, H.; Meng, Y.; Tanumihardjo, S.A.; Liu, J. A Correlation Study of Plasma and Breast Milk Retinol Concentrations in Breastfeeding Women in China. Nutrients 2023, 15, 5085. https://doi.org/10.3390/nu15245085
Qin J, Zhou Y, Li H, Meng Y, Tanumihardjo SA, Liu J. A Correlation Study of Plasma and Breast Milk Retinol Concentrations in Breastfeeding Women in China. Nutrients. 2023; 15(24):5085. https://doi.org/10.3390/nu15245085
Chicago/Turabian StyleQin, Jing, Yubo Zhou, Hongtian Li, Ying Meng, Sherry A. Tanumihardjo, and Jianmeng Liu. 2023. "A Correlation Study of Plasma and Breast Milk Retinol Concentrations in Breastfeeding Women in China" Nutrients 15, no. 24: 5085. https://doi.org/10.3390/nu15245085
APA StyleQin, J., Zhou, Y., Li, H., Meng, Y., Tanumihardjo, S. A., & Liu, J. (2023). A Correlation Study of Plasma and Breast Milk Retinol Concentrations in Breastfeeding Women in China. Nutrients, 15(24), 5085. https://doi.org/10.3390/nu15245085




