Vascular and Platelet Effects of Tomato Soffritto Intake in Overweight and Obese Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics Statement
2.3. Soffritto Samples
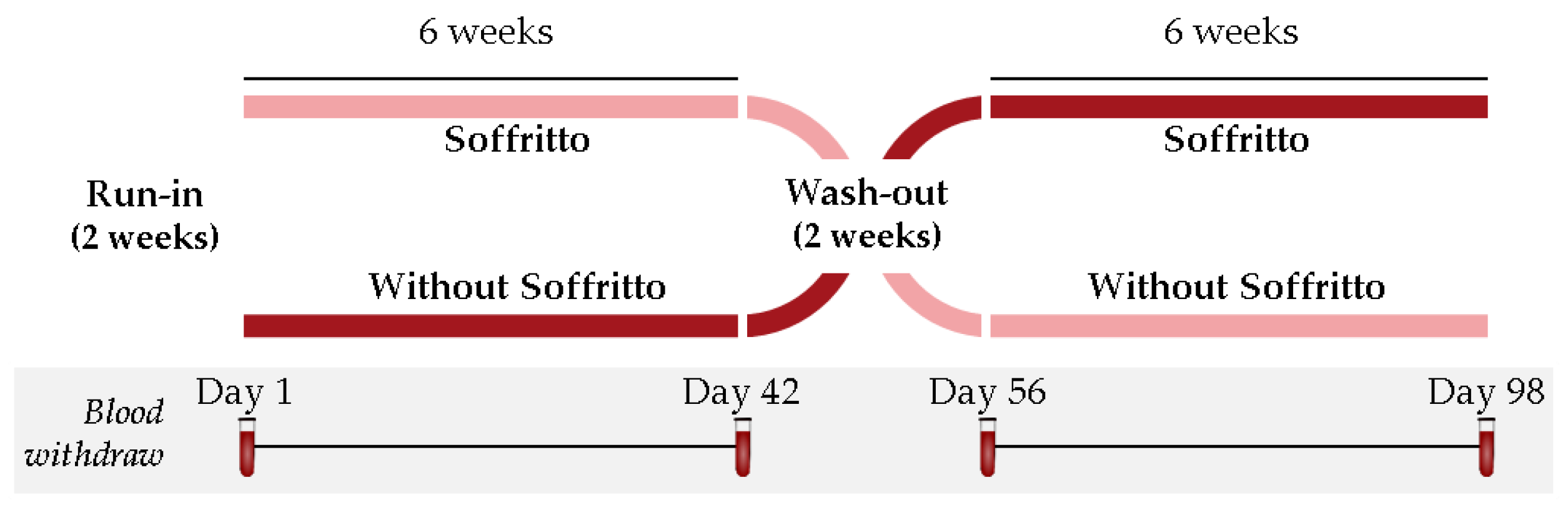
2.4. Study Design and Dietary Monitoring
2.5. Outcomes
2.5.1. Platelet Aggregation
2.5.2. Flow Cytometric Analysis of Circulating Extracellular Vesicles
2.5.3. Anthropometric Data, Blood Pressure, Lipid Profile, and Other Biochemical Measurements
2.5.4. Vascular Endothelial Function and Hemogram Profile
2.6. Statistical Analysis
3. Results
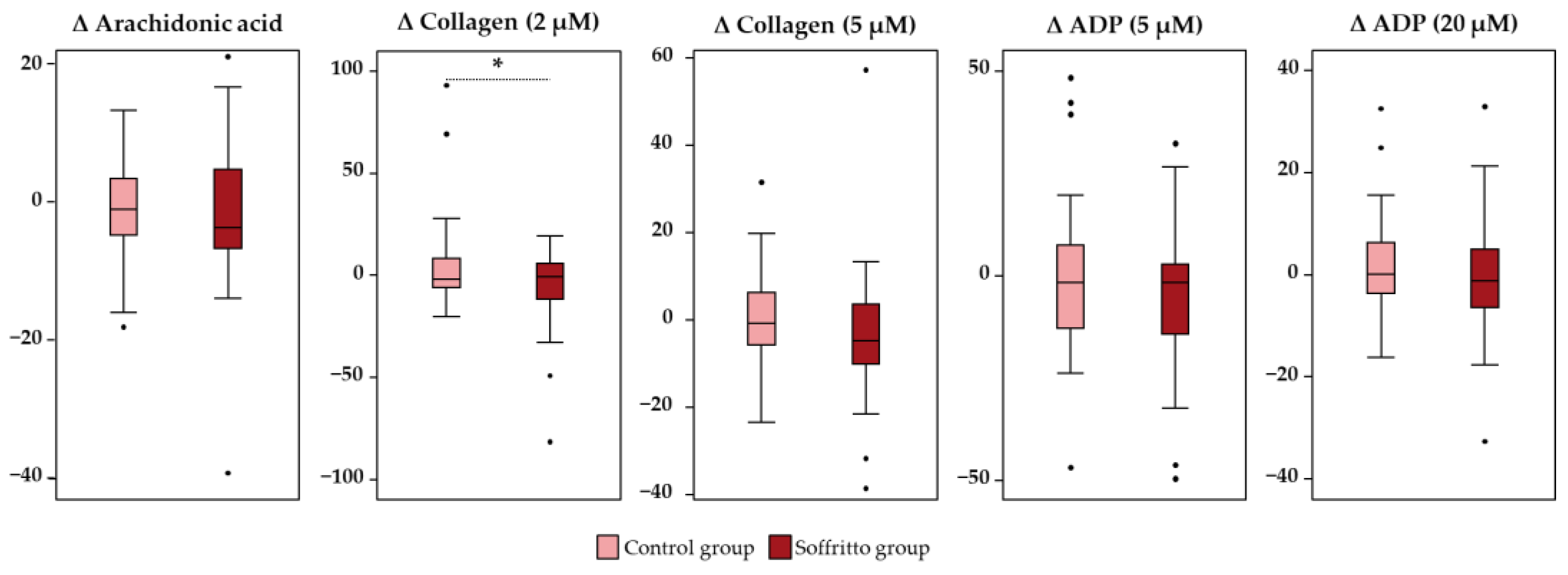
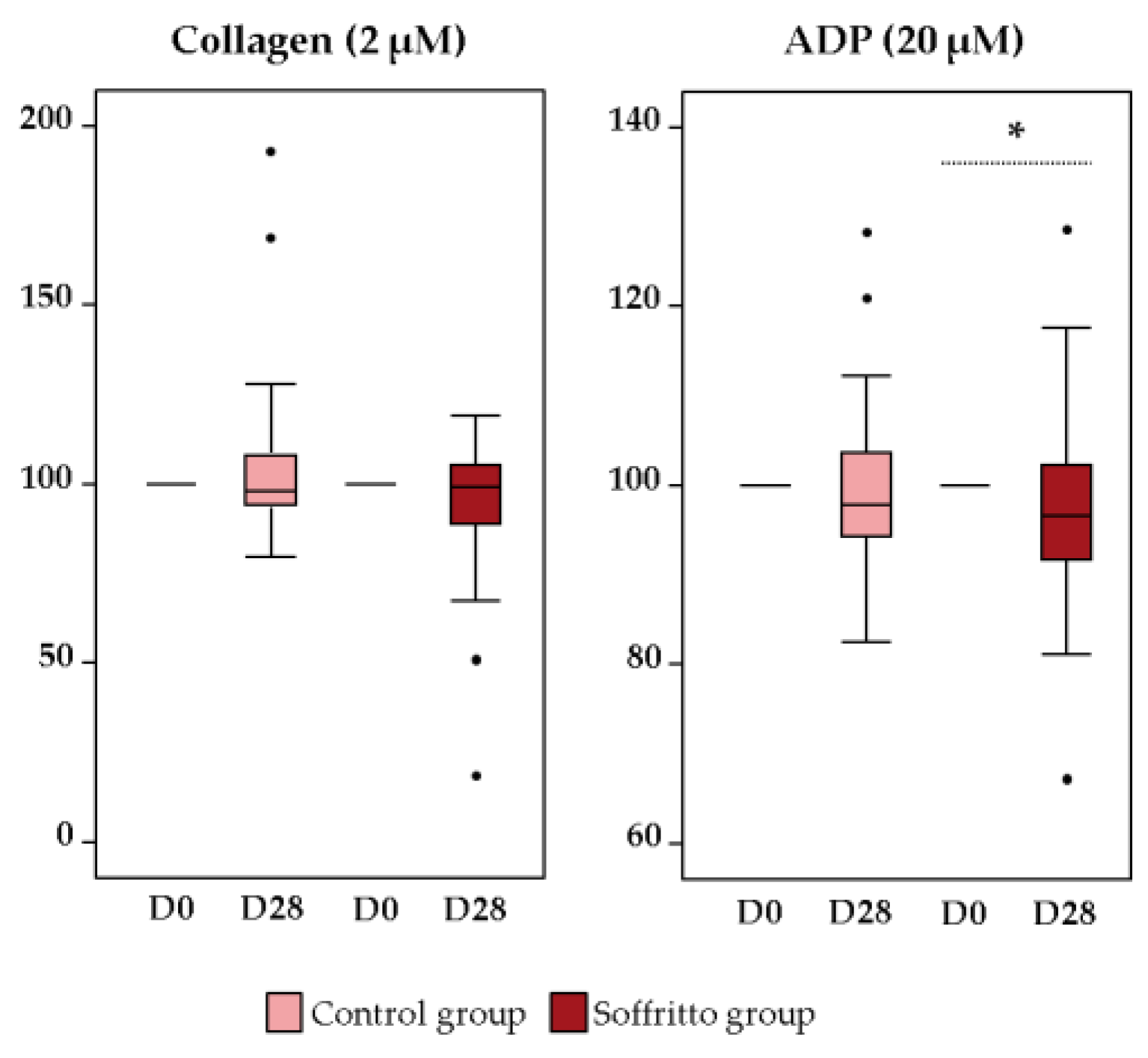
3.1. Platelet Function
3.2. Endothelial Function
3.3. Anthropometric and Biochemical Variables
3.4. Lipid Profile
3.5. Blood Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Obesity and Overweight. Available online: https://www.fao.org/about/meetings/icn2/preparations/document-detail/en/c/253841/ (accessed on 24 April 2023).
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato Juice Consumption Reduces Systemic Inflammation in Overweight and Obese Females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Pourahmadi, Z.; Mahboob, S.; Saedisomeolia, A.; Reykandeh, M.T. The Effect of Tomato Juice Consumption on Antioxidant Status in Overweight and Obese Females. Women Health 2015, 55, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef]
- López-Yerena, A.; Domínguez-López, I.; Abuhabib, M.M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Pérez, M. Tomato Wastes and By-Products: Upcoming Sources of Polyphenols and Carotenoids for Food, Nutraceutical, and Pharma Applications. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant Nutritional Quality of Tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; De Vos, R.C.H.; Hall, R.D. The Effect of Industrial Food Processing on Potentially Health-Beneficial Tomato Antioxidants. Crit. Rev. Food Sci. Nutr. 2010, 50, 919–930. [Google Scholar] [CrossRef]
- Ghavipour, M.; Sotoudeh, G.; Ghorbani, M. Tomato Juice Consumption Improves Blood Antioxidative Biomarkers in Overweight and Obese Females. Clin. Nutr. 2015, 34, 805–809. [Google Scholar] [CrossRef]
- Fenni, S.; Hammou, H.; Astier, J.; Bonnet, L.; Karkeni, E.; Couturier, C.; Tourniaire, F.; Landrier, J. Lycopene and Tomato Powder Supplementation Similarly Inhibit High-fat Diet Induced Obesity, Inflammatory Response, and Associated Metabolic Disorders. Mol. Nutr. Food Res. 2017, 61, 1601083. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Jiménez-Altayó, F.; Alsina, L.; Onetti, Y.; Rinaldi de Alvarenga, J.F.; Claro, C.; Ogalla, E.; Casals, N.; Lamuela-Raventos, R.M. Mediterranean Tomato-based Sofrito Protects against Vascular Alterations in Obese Zucker Rats by Preserving NO Bioavailability. Mol. Nutr. Food Res. 2017, 61, 1601010. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a Tomato-Rich Diet on Markers of Cardiovascular Disease Risk in Moderately Overweight, Disease-Free, Middle-Aged Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, F.J.; Jorge-Vidal, V.; Ros, G.; Periago, M.J. Effect of Consumption of Tomato Juice Enriched with N-3 Polyunsaturated Fatty Acids on the Lipid Profile, Antioxidant Biomarker Status, and Cardiovascular Disease Risk in Healthy Women. Eur. J. Nutr. 2012, 51, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Tominaga, N.; Ishikawa-Takano, Y.; Maeda-Yamamoto, M.; Nishihira, J. Effect of 12-Week Daily Intake of the High-Lycopene Tomato (Solanum lycopersicum), a Variety Named “PR-7”, on Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2019, 11, 1177. [Google Scholar] [CrossRef]
- Tian, Z.; Fan, D.; Li, K.; Zhao, D.; Liang, Y.; Ji, Q.; Gao, X.; Ma, X.; Zhao, Y.; Mao, Y. Four-Week Supplementation of Water-Soluble Tomato Extract Attenuates Platelet Function in Chinese Healthy Middle-Aged and Older Individuals: A Randomized, Double-Blinded, and Crossover Clinical Trial. Front. Nutr. 2022, 9, 891241. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, K.A.; Crosbie, L.; Gordon, M.J. Effects of Tomato Extract on Human Platelet Aggregation In Vitro. Platelets 2001, 12, 218–227. [Google Scholar] [PubMed]
- O’Kennedy, N.; Crosbie, L.; Whelan, S.; Luther, V.; Horgan, G.; Broom, J.I.; Webb, D.J.; Duttaroy, A.K. Effects of Tomato Extract on Platelet Function: A Double-Blinded Crossover Study in Healthy Humans. Am. J. Clin. Nutr. 2006, 84, 561–569. [Google Scholar] [CrossRef]
- Gärtner, C.; Stahl, W.; Sies, H. Lycopene Is More Bioavailable from Tomato Paste than from Fresh Tomatoes. Am. J. Clin. Nutr. 1997, 66, 116–122. [Google Scholar] [CrossRef]
- Rinaldi de Alvarenga, J.F.; Quifer-Rada, P.; Francetto Juliano, F.; Hurtado-Barroso, S.; Illan, M.; Torrado-Prat, X.; Lamuela-Raventós, R.M. Using Extra Virgin Olive Oil to Cook Vegetables Enhances Polyphenol and Carotenoid Extractability: A Study Applying the Sofrito Technique. Molecules 2019, 24, 1555. [Google Scholar] [CrossRef]
- Rinaldi de Alvarenga, J.F.; Quifer-Rada, P.; Westrin, V.; Hurtado-Barroso, S.; Torrado-Prat, X.; Lamuela-Raventós, R.M. Mediterranean Sofrito Home-cooking Technique Enhances Polyphenol Content in Tomato Sauce. J. Sci. Food Agric. 2019, 99, 6535–6545. [Google Scholar] [CrossRef]
- Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; De La Serrana, H.L. Phenols and the Antioxidant Capacity of Mediterranean Vegetables Prepared with Extra Virgin Olive Oil Using Different Domestic Cooking Techniques. Food Chem. 2015, 188, 430–438. [Google Scholar]
- Ross, A.B.; Vuong, L.T.; Ruckle, J.; Synal, H.A.; Schulze-Koenig, T.; Wertz, K.; Ruembeli, R.; Liberman, R.G.; Skipper, P.L.; Tannenbaum, S.R. Lycopene Bioavailability and Metabolism in Humans: An Accelerator Mass Spectrometry Study. Am. J. Clin. Nutr. 2011, 93, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Galvez, A.E.; Badimon, L.; Badimon, J.-J.; Fuster, V. Electrical Aggregometry in Whole Blood from Human, Pig and Rabbit. Thromb. Haemost. 1986, 56, 128–132. [Google Scholar] [CrossRef]
- Suades, R.; Padró, T.; Alonso, R.; Mata, P.; Badimon, L. High Levels of TSP1+/CD142+ Platelet-Derived Microparticles Characterise Young Patients with High Cardiovascular Risk and Subclinical Atherosclerosis. Thromb. Haemost. 2015, 114, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, R.; Berckmans, R.J.; McGregor, S.; Böing, A.N.; Th, M.; Romijn, F.P.H.; Westendorp, R.G.J.; Hack, C.E.; Sturk, A. Cellular Origin and Procoagulant Properties of Microparticles in Meningococcal Sepsis. Blood J. Am. Soc. Hematol. 2000, 95, 930–935. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro III, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Klimis-Zacas, D.; Del Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a Wild Blueberry (Vaccinium angustifolium) Drink Intervention on Markers of Oxidative Stress, Inflammation and Endothelial Function in Humans with Cardiovascular Risk Factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Erqou, S.; Kip, K.E.; Mulukutla, S.R.; Aiyer, A.N.; Reis, S.E. Endothelial Dysfunction and Racial Disparities in Mortality and Adverse Cardiovascular Disease Outcomes. Clin. Cardiol. 2016, 39, 338–344. [Google Scholar] [CrossRef]
- Valderas-Martinez, P.; Chiva-Blanch, G.; Casas, R.; Arranz, S.; Martínez-Huélamo, M.; Urpi-Sarda, M.; Torrado, X.; Corella, D.; Lamuela-Raventós, R.M.; Estruch, R. Tomato Sauce Enriched with Olive Oil Exerts Greater Effects on Cardiovascular Disease Risk Factors than Raw Tomato and Tomato Sauce: A Randomized Trial. Nutrients 2016, 8, 170. [Google Scholar] [CrossRef]
- Patrono, C.; Andreotti, F.; Arnesen, H.; Badimon, L.; Baigent, C.; Collet, J.-P.; De Caterina, R.; Gulba, D.; Huber, K.; Husted, S. Antiplatelet Agents for the Treatment and Prevention of Atherothrombosis. Eur. Heart J. 2011, 32, 2922–2932. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The Key Contribution of Platelet and Vascular Arachidonic Acid Metabolism to the Pathophysiology of Atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M. Light Transmission Aggregometry and ATP Release for the Diagnostic Assessment of Platelet Function. Semin. Thromb. Hemost. 2009, 35, 158–167. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, N.; Crosbie, L.; van Lieshout, M.; Broom, J.I.; Webb, D.J.; Duttaroy, A.K. Effects of Antiplatelet Components of Tomato Extract on Platelet Function In Vitro and Ex Vivo: A Time-Course Cannulation Study in Healthy Humans. Am. J. Clin. Nutr. 2006, 84, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, G.; Wang, Y.; Tzu, N.-H.; Fong, T.-H.; Shen, M.-Y.; Lin, K.-H.; Chou, D.-S.; Sheu, J.-R. Inhibitory Effects of Lycopene on In Vitro Platelet Activation and In Vivo Prevention of Thrombus Formation. J. Lab. Clin. Med. 2005, 146, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Forero-Doria, O.; Carrasco, G.; Maricán, A.; Santos, L.S.; Alarcón, M.; Palomo, I. Effect of Tomato Industrial Processing on Phenolic Profile and Antiplatelet Activity. Molecules 2013, 18, 11526–11536. [Google Scholar] [CrossRef]
- Cámara, M.; Fernández-Ruiz, V.; Sánchez-Mata, M.-C.; Domínguez Díaz, L.; Kardinaal, A.; van Lieshout, M. Evidence of Antiplatelet Aggregation Effects from the Consumption of Tomato Products, according to EFSA Health Claim Requirements. Crit. Rev. Food Sci. Nutr. 2020, 60, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial Dysfunction: A Marker of Atherosclerotic Risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Palomo, I.; Fuentes, E.; Padro, T.; Badimon, L. Platelets and Atherogenesis: Platelet Anti-Aggregation Activity and Endothelial Protection from Tomatoes (Solanum lycopersicum L.). Exp. Ther. Med. 2012, 3, 577–584. [Google Scholar] [CrossRef]
- Stangl, V.; Kuhn, C.; Hentschel, S.; Jochmann, N.; Jacob, C.; Böhm, V.; Fröhlich, K.; Müller, L.; Gericke, C.; Lorenz, M. Lack of Effects of Tomato Products on Endothelial Function in Human Subjects: Results of a Randomised, Placebo-Controlled Cross-over Study. Br. J. Nutr. 2011, 105, 263–267. [Google Scholar] [CrossRef]
- Xaplanteris, P.; Vlachopoulos, C.; Pietri, P.; Terentes-Printzios, D.; Kardara, D.; Alexopoulos, N.; Aznaouridis, K.; Miliou, A.; Stefanadis, C. Tomato Paste Supplementation Improves Endothelial Dynamics and Reduces Plasma Total Oxidative Status in Healthy Subjects. Nutr. Res. 2012, 32, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Park, H.W.; Lee, J.H.; Jang, Y.; Lee, J.H. Effects of Lycopene Supplementation on Oxidative Stress and Markers of Endothelial Function in Healthy Men. Atherosclerosis 2011, 215, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Cubedo, J.; Padró, T.; Casaní, L.; Mendieta, G.; González, A.; Badimon, L. Intake of Cooked Tomato Sauce Preserves Coronary Endothelial Function and Improves Apolipoprotein AI and Apolipoprotein J Protein Profile in High-Density Lipoproteins. Transl. Res. 2015, 166, 44–56. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Tulipani, S.; Estruch, R.; Escribano, E.; Illán, M.; Corella, D.; Lamuela-Raventós, R.M. The Tomato Sauce Making Process Affects the Bioaccessibility and Bioavailability of Tomato Phenolics: A Pharmacokinetic Study. Food Chem. 2015, 173, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Perez-Cornago, A.; Navas-Carretero, S.; Bondia-Pons, I.; Zulet, M.A.; Martinez, J.A. A Regular Lycopene Enriched Tomato Sauce Consumption Influences Antioxidant Status of Healthy Young-Subjects: A Crossover Study. J. Funct. Foods 2013, 5, 28–35. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Chávez-Manzanera, E.; Meza-Arana, C.E.; Brito-Córdova, G.; Mehta, R.; Pérez-Méndez, O.; Gómez-Pérez, F.J. Effect of Tomato Consumption on High-Density Lipoprotein Cholesterol Level: A Randomized, Single-Blinded, Controlled Clinical Trial. Diabetes Metab. Syndr. Obes. 2013, 6, 263–273. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Martínez-Huélamo, M.; Rinaldi de Alvarenga, J.F.; Quifer-Rada, P.; Vallverdú-Queralt, A.; Pérez-Fernández, S.; Lamuela-Raventós, R.M. Acute Effect of a Single Dose of Tomato Sofrito on Plasmatic Inflammatory Biomarkers in Healthy Men. Nutrients 2019, 11, 851. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and Lycopene Supplementation and Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [PubMed]
| Ingredients of the Soffritto (%) | |
| Tomato paste | 35 |
| Onion | 10 |
| Extra-virgin olive oil | 5 |
| Sugar | 3 |
| Salt | 2 |
| Mean Nutritional Values (100 g) | |
| Energetic value | 96 Kcal/400 KJ |
| Proteins | 1.8 g |
| Carbohydrates | 10.6 g |
| Fat | 5.1 g |
| Baseline Characteristics | ∆ | |||||
|---|---|---|---|---|---|---|
| Control | Soffritto | p-Value | Control | Soffritto | p-Value | |
| RHI | 2.01 ± 0.13 | 1.85 ± 0.06 | 0.106 | −0.02 ± 0.13 | 0.12 ± 0.09 | 0.364 |
| lnRHI | 0.64 ± 0.05 | 0.60 ± 0.03 | 0.320 | 0.00 ± 0.05 | 0.04 ± 0.04 | 0.526 |
| FRHI | 0.35 ± 0.07 | 0.30 ± 0.05 | 0.306 | −0.03 ± 0.06 | 0.01 ± 0.04 | 0.533 |
| AI@75 | −1.27 ± 1.85 | −0.39 ± 2.10 | 0.619 | 2.49 ± 1.46 | −0.21 ± 1.45 | 0.270 |
| Baseline Characteristics | ∆ | |||||
|---|---|---|---|---|---|---|
| Control | Soffritto | p-Value | Control | Soffritto | p-Value | |
| Anthropometric parameters | ||||||
| Weight (kg) | 90.00 ± 2.31 | 89.78 ± 2.12 | 0.110 | −0.38 ± 0.29 | −0.08 ± 0.24 | 0.401 |
| BMI (kg/m2) | 31.16 ± 0.56 | 31.05 ± 0.51 | 0.481 | −0.13 ± 0.13 | 0.03 ± 0.09 | 0.386 |
| WC (cm) | 100.53 ± 1.86 | 100.86 ± 1.78 | 1.000 | −0.78 ± 0.78 | −0.98 ± 0.80 | 0.324 |
| WtHR (cm/cm) | 0.59 ± 0.01 | 0.60 ± 0.01 | 1.000 | 0.00 ± 0.00 | −0.01 ± 0.00 | 0.221 |
| Hemodynamic control | ||||||
| SBP (mmHg) | 125.79 ± 2.20 | 124.35 ± 1.78 | 0.362 | −2.50 ± 1.80 | −0.23 ± 1.37 | 0.341 |
| DBP (mmHg) | 72.59 ± 1.95 | 70.78 ± 1.66 | 0.172 | −2.83 ± 1.28 | 0.68 ± 1.18 | 0.040 |
| Biochemical parameters | ||||||
| Total protein (g/L) | 68.82 ± 0.60 | 66.93 ± 1.68 | 0.219 | −1.21 ± 0.47 | 0.79 ± 1.90 | 0.301 |
| Glucose (mM) | 4.91 ± 0.14 | 4.83 ± 0.13 | 0.329 | −0.10 ± 0.06 | −0.09 ± 0.07 | 0.981 |
| Creatinine (µM) | 76.23 ± 1.79 | 75.70 ± 1.67 | 0.460 | −0.39 ± 0.72 | 0.80 ± 0.92 | 0.256 |
| Urea (mM) | 5.48 ± 0.19 | 5.22 ± 0.17 | 0.151 | 0.19 ± 0.17 | 0.22 ± 0.19 | 0.914 |
| Uric acid (mM) | 351.00 ± 16.70 | 349.35 ± 16.16 | 0.603 | 14.95 ± 6.75 | 3.73 ± 9.65 | 0.284 |
| ALT (U/L) | 25.03 ± 3.03 | 22.90 ± 2.20 | 0.200 | −4.88 ± 3.22 | −0.45 ± 2.40 | 0.326 |
| AST (U/L) | 22.46 ± 2.64 | 22.05 ± 1.22 | 0.873 | −0.15 ± 1.76 | −0.15 ± 1.51 | 1.000 |
| GGT (U/L) | 31.87 ± 4.76 | 30.28 ± 4.41 | 0.242 | 1.78 ± 1.48 | 1.93 ± 1.53 | 0.920 |
| Lipid profile | ||||||
| TC (mg/dL) | 194.87 ± 4.76 | 193.84 ± 5.27 | 0.347 | −1.03 ± 3.02 | 1.54 ± 2.08 | 0.468 |
| HDLc (mg/dL) | 46.40 ± 1.79 | 45.80 ± 1.56 | 0.621 | −0.15 ± 0.82 | 0.27 ± 0.69 | 0.728 |
| LDLc (mg/dL) | 116.41 ± 4.26 | 118.91 ± 4.22 | 0.484 | 0.54 ± 3.00 | −0.97 ± 1.96 | 0.678 |
| Non-HDLc (mg/dL) | 148.47 ± 4.88 | 146.03 ± 5.30 | 0.369 | −0.88 ± 2.64 | 1.27 ± 2.05 | 0.494 |
| LDLc/HDLc | 2.60 ± 0.12 | 2.69 ± 0.13 | 0.239 | 0.03 ± 0.06 | −0.08 ± 0.05 | 0.170 |
| TAG (mg/dL) | 159.28 ± 21.22 | 131.26 ± 13.27 | 0.115 | −8.91 ± 11.50 | 7.76 ± 8.40 | 0.265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Yerena, A.; Padro, T.; de Santisteban Villaplana, V.; Muñoz-García, N.; Pérez, A.; Vilahur, G.; Badimon, L. Vascular and Platelet Effects of Tomato Soffritto Intake in Overweight and Obese Subjects. Nutrients 2023, 15, 5084. https://doi.org/10.3390/nu15245084
López-Yerena A, Padro T, de Santisteban Villaplana V, Muñoz-García N, Pérez A, Vilahur G, Badimon L. Vascular and Platelet Effects of Tomato Soffritto Intake in Overweight and Obese Subjects. Nutrients. 2023; 15(24):5084. https://doi.org/10.3390/nu15245084
Chicago/Turabian StyleLópez-Yerena, Anallely, Teresa Padro, Victoria de Santisteban Villaplana, Natàlia Muñoz-García, Antonio Pérez, Gemma Vilahur, and Lina Badimon. 2023. "Vascular and Platelet Effects of Tomato Soffritto Intake in Overweight and Obese Subjects" Nutrients 15, no. 24: 5084. https://doi.org/10.3390/nu15245084
APA StyleLópez-Yerena, A., Padro, T., de Santisteban Villaplana, V., Muñoz-García, N., Pérez, A., Vilahur, G., & Badimon, L. (2023). Vascular and Platelet Effects of Tomato Soffritto Intake in Overweight and Obese Subjects. Nutrients, 15(24), 5084. https://doi.org/10.3390/nu15245084










