Causal Effect of Selenium Levels on Osteoporosis: A Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
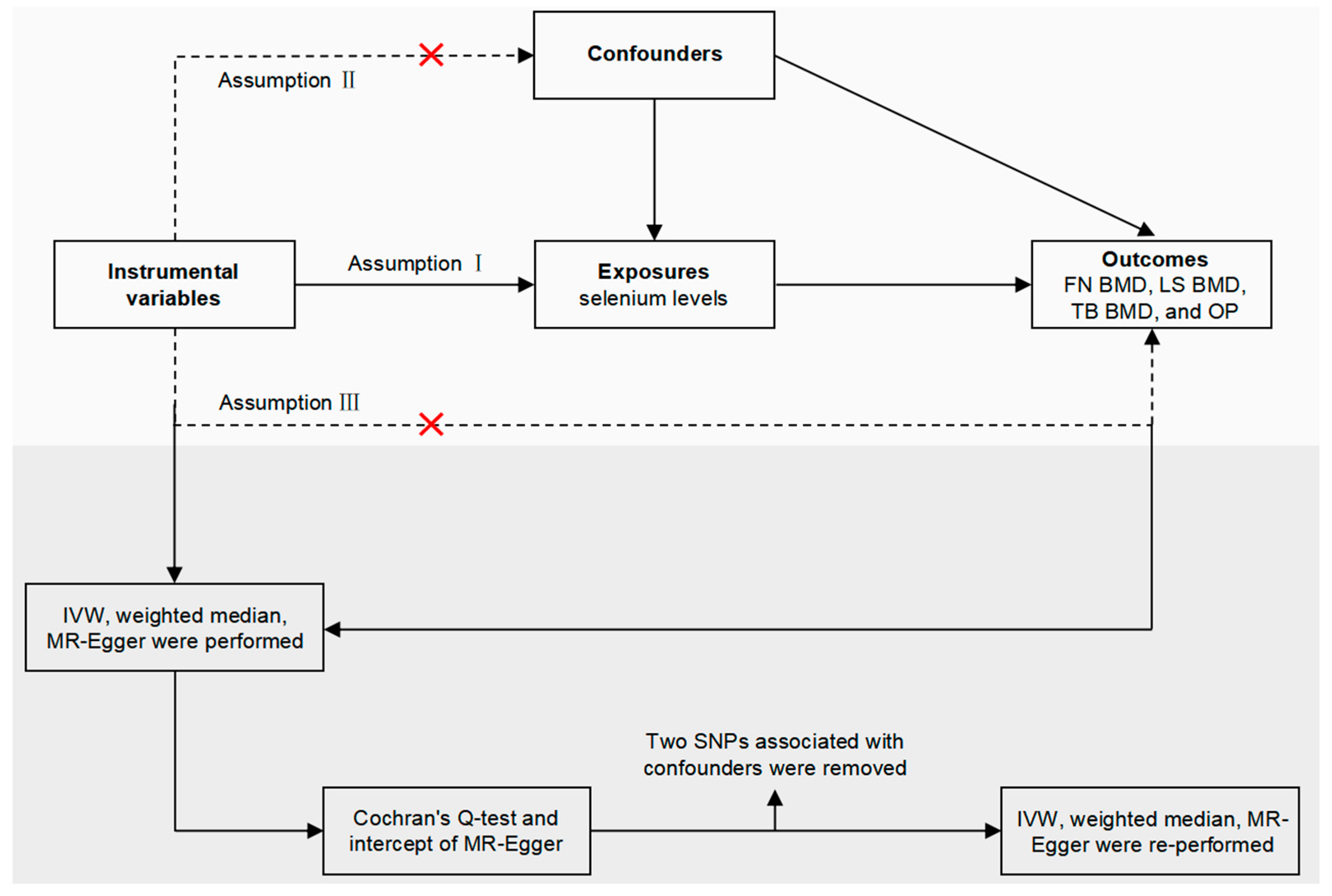
2.1. Study Overview
2.2. Summary Data for Se Levels
2.3. Instrumental Variable Selection
2.4. GWAS Summary Data for OP
2.5. Mendelian Randomization Analyses
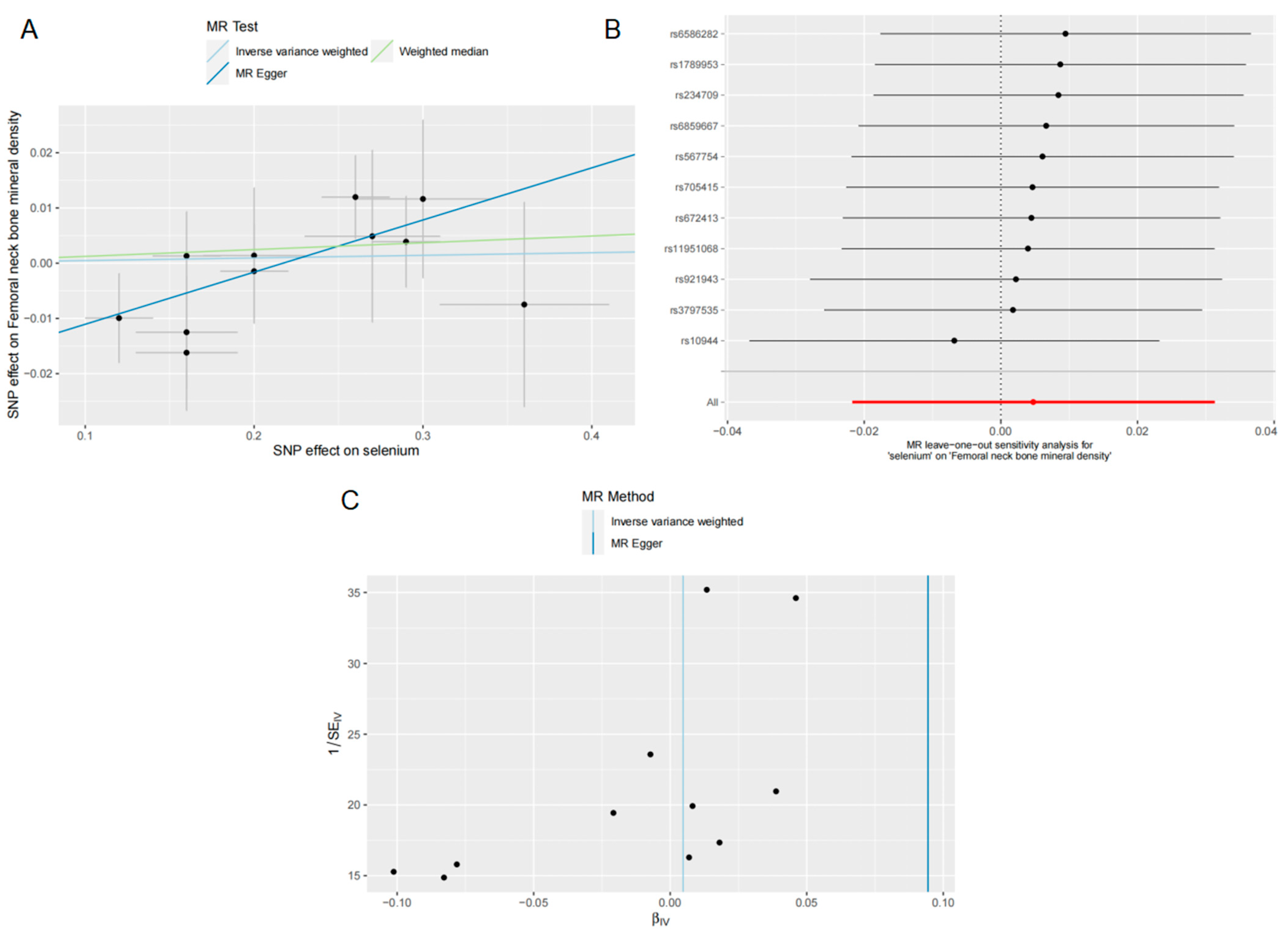
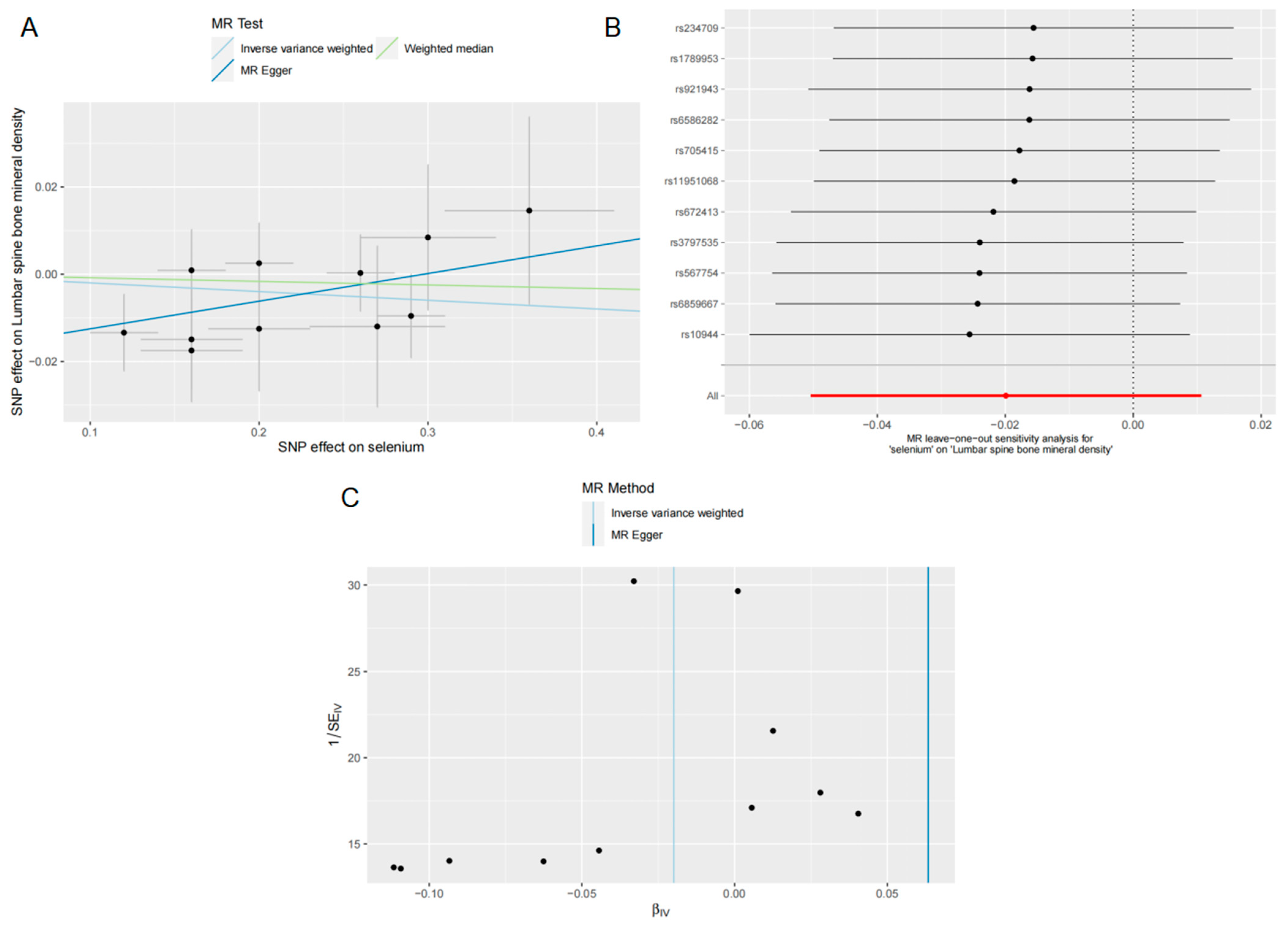
2.6. Sensitivity Analysis
3. Results
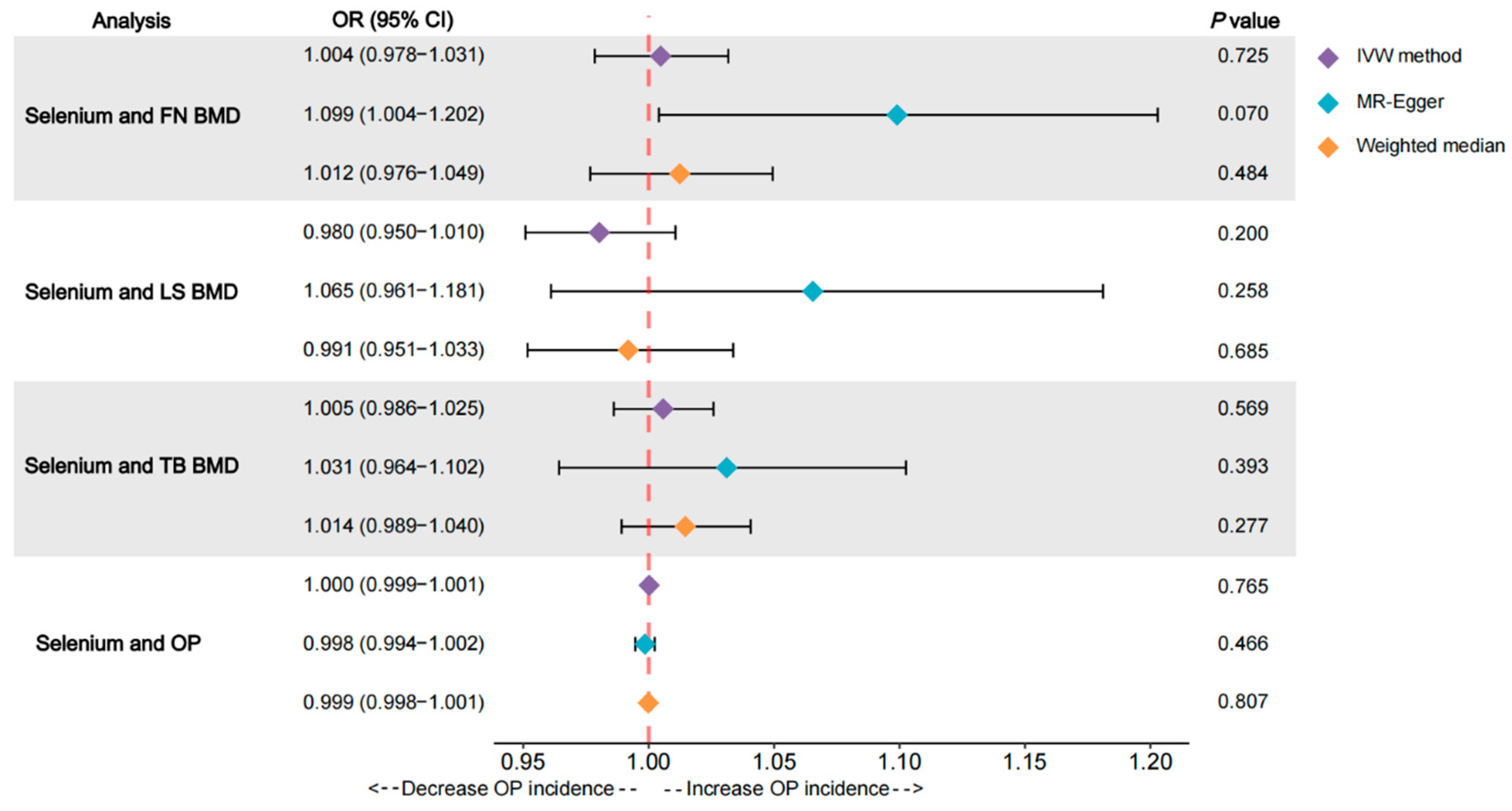
3.1. Effects of Se on FN BMD
3.2. Effects of Se on LS BMD
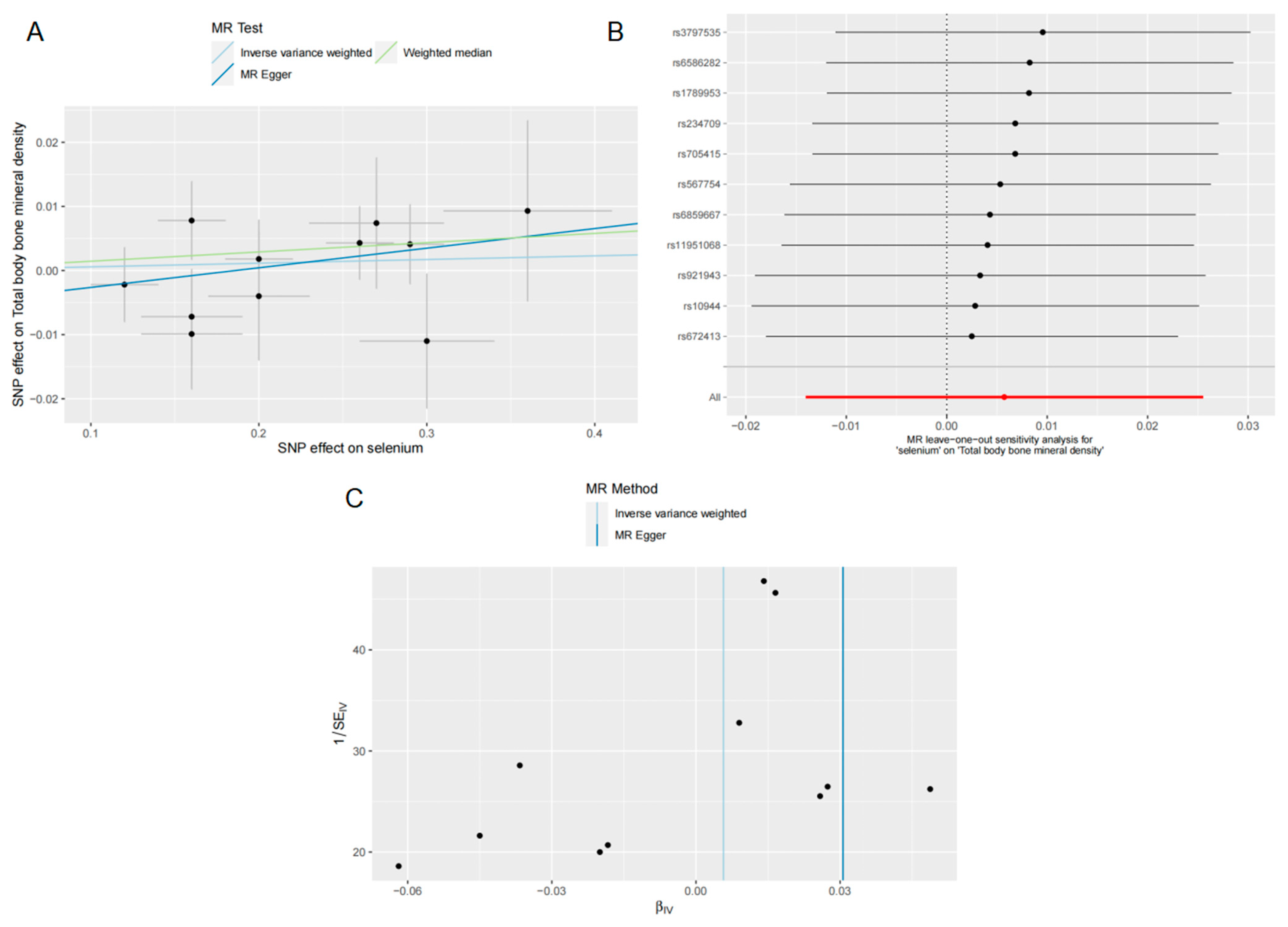
3.3. Effects of Se on TB BMD
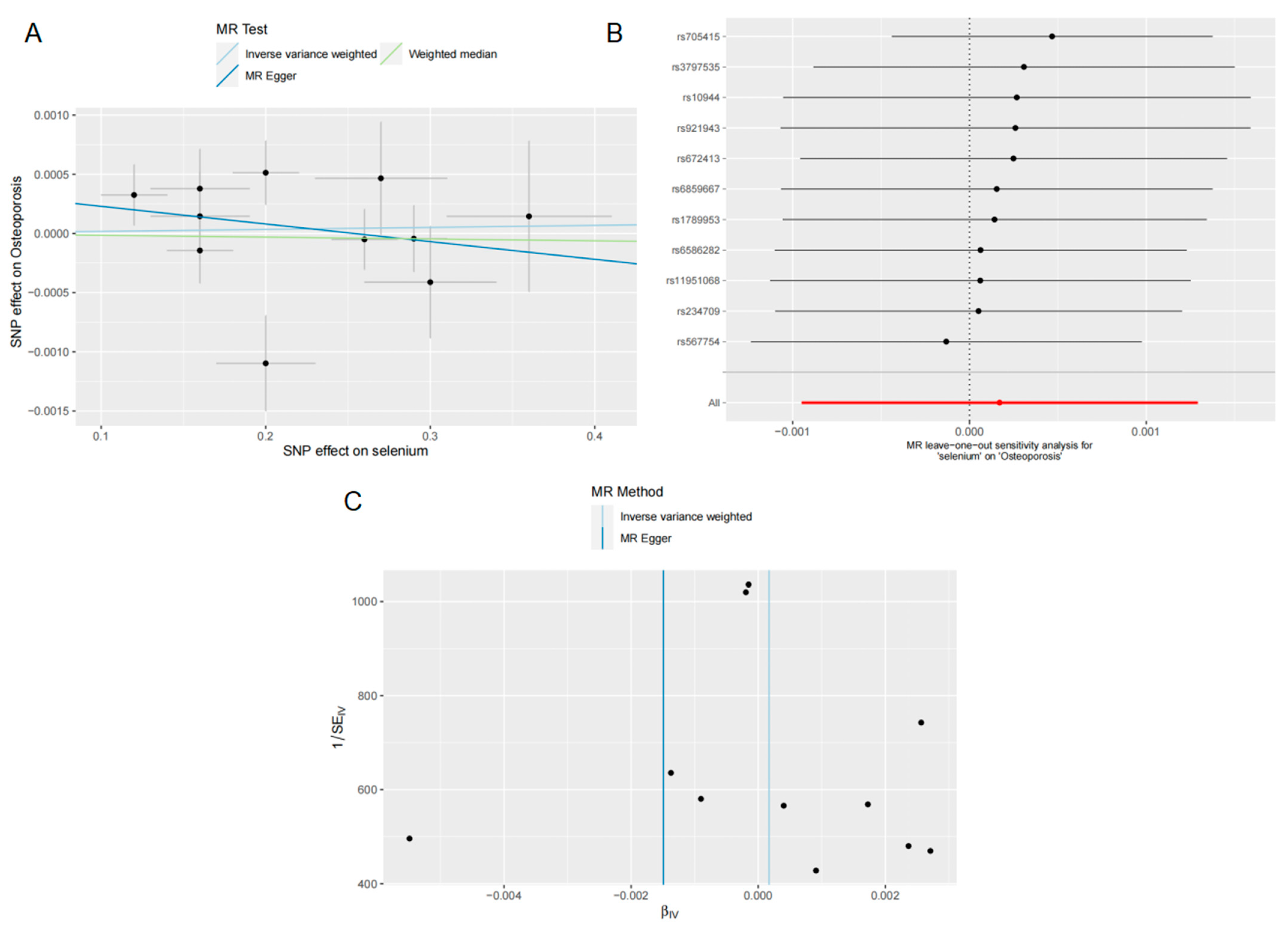
3.4. Effects of Se on OP
3.5. Second MR Analysis of Se Levels with FN BMD, LS BMD, TB BMD and OP
3.6. Sensitivity Test of the Second MR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, G.; Levin, B. An Evolution-Based Model of Causation for Aging-Related Diseases and Intrinsic Mortality: Explanatory Properties and Implications for Healthy Aging. Front. Public Health 2022, 10, 774668. [Google Scholar] [CrossRef]
- Schrempft, S.; Belsky, D.W.; Draganski, B.; Kliegel, M.; Vollenweider, P.; Marques-Vidal, P.; Preisig, M.; Stringhini, S. Associations between Life-Course Socioeconomic Conditions and the Pace of Aging. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2022, 77, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C.; Isfahani, N.S.; Fan, B.; Shepherd, J.A. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos. Int. 2017, 28, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: Highlights of the conference. South. Med. J. 2001, 94, 569–573.
- Osnes, E.K.; Lofthus, C.M.; Meyer, H.E.; Falch, J.A.; Nordsletten, L.; Cappelen, I.; Kristiansen, I.S. Consequences of hip fracture on activities of daily life and residential needs. Osteoporos. Int. 2004, 15, 567–574. [Google Scholar] [CrossRef]
- Liu, J.; Curtis, E.M.; Cooper, C.; Harvey, N.C. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Investig. 2019, 42, 1149–1164. [Google Scholar] [CrossRef]
- Leder, B.Z.; Mitlak, B.; Hu, M.-Y.; Hattersley, G.; Bockman, R.S. Effect of Abaloparatide vs Alendronate on Fracture Risk Reduction in Postmenopausal Women with Osteoporosis. J. Clin. Endocrinol. Metab. 2020, 105, 938–943. [Google Scholar] [CrossRef]
- Black, D.M.; Geiger, E.J.; Eastell, R.; Vittinghoff, E.; Li, B.H.; Ryan, D.S.; Dell, R.M.; Adams, A.L. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. N. Engl. J. Med. 2020, 383, 743–753. [Google Scholar] [CrossRef]
- Xu, X.-H.; Dong, S.-S.; Guo, Y.; Yang, T.-L.; Lei, S.-F.; Papasian, C.J.; Zhao, M.; Deng, H.-W. Molecular Genetic Studies of Gene Identification for Osteoporosis: The 2009 Update. Endocr. Rev. 2010, 31, 447–505. [Google Scholar] [CrossRef]
- Wang, L.; Ran, L.; Zha, X.; Zhao, K.; Yang, Y.; Shuang, Q.; Liu, Y.; Hind, K.; Cheng, X.; Blake, G.M. Adjustment of DXA BMD measurements for anthropometric factors and its impact on the diagnosis of osteoporosis. Arch. Osteoporos. 2020, 15, s11657-020. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Ralston, S.H.; Uitterlinden, A.G. Genetics of Osteoporosis. Endocr. Rev. 2010, 31, 629–662. [Google Scholar] [CrossRef]
- Brommage, R.; Ohlsson, C. Translational studies provide insights for the etiology and treatment of cortical bone osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L.; Fleming, K.A.; Kuti, M.A.; Looi, L.M.; Lago, N.; Ru, K. Pathology and laboratory medicine in low-income and middle-income countries 1: Access to pathology and laboratory medicine services: A crucial gap. Lancet 2018, 391, 1927–1938. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Winfrey, V.P.; Kurokawa, S.; Mitchell, S.L.; Zhang, W. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014, 28, 3579–3588. [Google Scholar] [CrossRef]
- Bjorklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Nicholson, J.L.; Toh, P.; Alfulaij, N.; Berry, M.J.; Torres, D.J. New insights on selenoproteins and neuronal function. Free Radic. Biol. Med. 2022, 190, 55–61. [Google Scholar] [CrossRef]
- Arbogast, S.; Ferreiro, A. Selenoproteins and Protection against Oxidative Stress: Selenoprotein N as a Novel Player at the Crossroads of Redox Signaling and Calcium Homeostasis. Antioxid. Redox Signal. 2010, 12, 893–904. [Google Scholar] [CrossRef]
- Gao, X.; Ye, C.; Ma, H.; Zhang, Z.; Wang, J.; Zhang, Z.-H.; Zhao, X.; Ho, C.-T. Research Advances in Preparation, Stability, Application, and Possible Risks of Nanoselenium: Focus on Food and Food-Related Fields. J. Agric. Food Chem. 2023, 71, 8731–8745. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Phillips, J.A.; Mauer, C.; Edwards, M.; Balldin, V.H.; Hall, J.R.; Barber, R.; Conger, T.L.; Ho, E.J.; O’Bryant, S.E. The impact of GPX1 on the association of groundwater selenium and depression: A project FRONTIER study. BMC Psychiatry 2013, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Navas-Acien, A.; Pastor-Barriuso, R.; Guallar, E. Selenium and coronary heart disease: A meta-analysis. Am. J. Clin. Nutr. 2006, 84, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Stranges, S.; Chan, L.H.M. Circulating Selenium Concentration Is Inversely Associated with the Prevalence of Stroke: Results from the Canadian Health Measures Survey and the National Health and Nutrition Examination Survey. J. Am. Heart Assoc. 2019, 8, 12290. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes—A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef]
- Grili, P.P.d.F.; Vidigal, C.V.; da Cruz, G.F.; Albergaria, B.H.; Marques-Rocha, J.L.; Pereira, T.S.S.; Guandalini, V.R. Dietary consumption of selenium inversely associated with osteoporosis in postmenopausal women. Front. Nutr. 2022, 9, 997414. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, G.; Wang, D. Association of selenium intake with bone mineral density and osteoporosis: The national health and nutrition examination survey. Front. Endocrinol. 2023, 14, 1251838. [Google Scholar] [CrossRef]
- Zhang, J.J.; Munger, R.G.; West, N.A.; Cutler, D.R.; Wengreen, H.J.; Corcoran, C.D. Antioxidant intake and risk of osteoporotic hip fracture in Utah: An effect modified by smoking status. Am. J. Epidemiol. 2006, 163, 9–17. [Google Scholar] [CrossRef]
- Arikan, D.C.; Coskun, A.; Ozer, A.; Kilinc, M.; Atalay, F.; Arikan, T. Plasma Selenium, Zinc, Copper and Lipid Levels in Postmenopausal Turkish Women and Their Relation with Osteoporosis. Biol. Trace Elem. Res. 2011, 144, 407–417. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Cvijetic, S.; Baric, I.C.; Cecic, I.; Saric, M.; Crncevic-Orlic, Z.; Blanusa, M.; Korsic, M. Nutrition and lifestyle in relation to bone health and body weight in Croatian postmenopausal women. Int. J. Food Sci. Nutr. 2009, 60, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, E.; Turan, M.; Aydin, A.; Akay, C.; Kutlu, M. Magnesium, zinc, copper, manganese, and selenium levels in postmenopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann. Acad. Med. Singap. 2008, 37, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafo, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Smith, G.D. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Ala-Korpela, M.; Smith, G.D. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat. Rev. Cardiol. 2017, 14, 78. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA-J. Am. Med. Assoc. 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Cheng, L.; Zhuang, H.; Yang, S.; Jiang, H.; Wang, S.; Zhang, J. Exposing the Causal Effect of C-Reactive Protein on the Risk of Type 2 Diabetes Mellitus: A Mendelian Randomization Study. Front. Genet. 2018, 9, 657. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, J.; Cheng, L.; Liu, S.-L. A Positive Causal Influence of IL-18 Levels on the Risk of T2DM: A Mendelian Randomization Study. Front. Genet. 2019, 10, 295. [Google Scholar] [CrossRef]
- Chen, X.; Kong, J.; Pan, J.; Huang, K.; Zhou, W.; Diao, X.; Cai, J.; Zheng, J.; Yang, X.; Xie, W.; et al. Kidney damage causally affects the brain cortical structure: A Mendelian randomization study. Ebiomedicine 2021, 72, 103592. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Fornage, M.; Foy, M.; Xun, P.; Gladyshev, V.N.; Morris, S.; Chasman, D.I.; Hu, F.B.; Rimm, E.B.; Kraft, P.; et al. Genome-wide association study of selenium concentrations. Hum. Mol. Genet. 2015, 24, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.M.; Zhu, G.; Dy, V.; Heath, A.C.; Madden, P.A.F.; Kemp, J.P.; McMahon, G.; St Pourcain, B.; Timpson, N.J.; Golding, J.; et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum. Mol. Genet. 2013, 22, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, L.; Ma, F.; Xue, S.; Sun, T.; Xu, Z. Effects of Selenium on Chronic Kidney Disease: A Mendelian Randomization Study. Nutrients 2022, 14, 4458. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat. Med. 2011, 30, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-F.; Forgetta, V.; Hsu, Y.-H.; Estrada, K.; Rosello-Diez, A.; Leo, P.J.; Dahia, C.L.; Park-Min, K.H.; Tobias, J.H.; Kooperberg, C.; et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature 2015, 526, 112. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gomez, C.; Kemp, J.P.; Trajanoska, K.; Luan, J.a.; Chesi, A.; Ahluwalia, T.S.; Mook-Kanamori, D.O.; Ham, A.; Hartwig, F.P.; Evans, D.S.; et al. Life-Course Genome-wide Association Study Meta-analysis of Total Body BMD and Assessment of Age-Specific Effects. Am. J. Hum. Genet. 2018, 102, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Donertas, H.M.; Fabian, D.K.; Fuentealba, M.; Partridge, L.; Thornton, J.M. Common genetic associations between age-related diseases. Nat. Aging 2021, 1, 400. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Smith, G.D.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Grover, S.; Del Greco, M.F.; Stein, C.M.; Ziegler, A. Mendelian Randomization. Methods Mol. Biol. 2017, 1666, 581–628. [Google Scholar] [CrossRef]
- Chen, X.; Kong, J.; Diao, X.; Cai, J.; Zheng, J.; Xie, W.; Qin, H.; Huang, J.; Lin, T. Depression and prostate cancer risk: A Mendelian randomization study. Cancer Med. 2020, 9, 9160–9167. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Gronau, Q.F.; Wagenmakers, E.-J. Limitations of Bayesian Leave-One-Out Cross-Validation for Model Selection. Comput. Brain Behav. 2019, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Widmann, T.; Herrmann, W. Homocysteine—A newly recognised risk factor for osteoporosis. Clin. Chem. Lab. Med. 2005, 43, 1111–1117. [Google Scholar] [CrossRef]
- Walsh, J.S.; Jacques, R.M.; Schomburg, L.; Hill, T.R.; Mathers, J.C.; Williams, G.R.; Eastell, R. Effect of selenium supplementation on musculoskeletal health in older women: A randomised, double-blind, placebo-controlled trial. Lancet Healthy Longev. 2021, 2, E212–E221. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, N.; He, H.; Yang, Z.; Wu, J.; Yang, T.; Wang, Y. The association between selenium and bone health: A meta-analysis. Bone Jt. Res. 2023, 12, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-C.; Kwon, Y.; Lee, Y.; Kim, D.K.; Jang, Y.; Lee, S. Low selenium levels are associated with decreased bone mineral densities. J. Trace Elem. Med. Biol. 2020, 61, 126534. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Liu, R. Association between dietary selenium intake and bone mineral density in the US general population. Ann. Transl. Med. 2022, 10, 3441. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Looker, A.C.; Lu, Z.H.; Fan, R.Z.; Eicher-Miller, H.A.; Fakhouri, T.H.; Gahche, J.J.; Weaver, C.M.; Mills, J.L. B-vitamin status and bone mineral density and risk of lumbar osteoporosis in older females in the United States. Am. J. Clin. Nutr. 2015, 102, 687–694. [Google Scholar] [CrossRef]
- Bucciarelli, P.; Martini, G.; Martinelli, I.; Ceccarelli, E.; Gennari, L.; Bader, R.; Valenti, R.; Franci, B.; Nuti, R.; Mannucci, P.M. The relationship between plasma homocysteine levels and bone mineral density in post-menopausal women. Eur. J. Intern. Med. 2010, 21, 301–305. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Qian, Y.; Ye, D.; Huang, H.; Wu, D.J.H.; Zhuang, Y.; Jiang, X.; Mao, Y. Coffee Consumption and Risk of Stroke: A Mendelian Randomization Study. Ann. Neurol. 2020, 87, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.C.; Choi, H.K.; Do, R.; Merriman, T.R. Insight into rheumatological cause and effect through the use of Mendelian randomization. Nat. Rev. Rheumatol. 2016, 12, 486–496. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Tchetgen, E.J.T.; Cornelis, M.; Kraft, P. Methodological Challenges in Mendelian Randomization. Epidemiology 2014, 25, 427–435. [Google Scholar] [CrossRef]
| SNP | Chromosome | Effect Allele | Other Allele | Frequency | Beta | Se | P |
|---|---|---|---|---|---|---|---|
| rs672413 | 5 | A | G | 0.32 | 0.164418 | 0.021835 | 5.21 × 10−14 |
| rs705415 | 5 | T | C | 0.14 | −0.20006 | 0.032113 | 4.64 × 10−10 |
| rs3797535 | 5 | T | C | 0.08 | 0.298102 | 0.037544 | 2.05 × 10−15 |
| rs11951068 | 5 | A | G | 0.07 | 0.268264 | 0.03992 | 1.86 × 10−11 |
| rs921943 | 5 | T | C | 0.29 | 0.294952 | 0.022447 | 1.90 × 10−39 |
| rs10944 | 5 | T | G | 0.49 | 0.257746 | 0.020375 | 1.13 × 10−36 |
| rs567754 | 5 | T | C | 0.34 | −0.19588 | 0.021502 | 8.38 × 10−20 |
| rs6859667 | 5 | T | C | 0.96 | −0.35969 | 0.051978 | 4.40 × 10−12 |
| rs6586282 | 21 | T | C | 0.17 | −0.15971 | 0.027116 | 3.96 × 10−9 |
| rs1789953 | 21 | T | C | 0.14 | 0.162035 | 0.029354 | 3.40 × 10−8 |
| rs234709 | 21 | T | C | 0.45 | −0.11957 | 0.020474 | 5.23 × 10−9 |
| GWAS ID | Trait | Sample Size | Number of SNPs | Population | PMID |
|---|---|---|---|---|---|
| ieu-a-980 | FN BMD | 32,735 | 10,586,900 | European | 26367794 |
| ieu-a-982 | LS BMD | 28,498 | 10,582,867 | European | 26367794 |
| ebi-a-GCST005348 | TB BMD | 56,284 | 16,162,733 | European | 29304378 |
| ebi-a-GCST90038656 | OP | 484,598 | 9,587,836 | European | 33959723 |
| Exposure | Outcome | Heterogeneity Test MR–Egger | Heterogeneity Test IVW | Pleiotropy Test MR–Egger | |||
|---|---|---|---|---|---|---|---|
| Q | P | Q | P | Intercept | P | ||
| Se levels | FN BMD | 4.919 | 0.841 | 9.058 | 0.526 | −0.020 | 0.072 |
| Se levels | LS BMD | 4.827 | 0.849 | 7.574 | 0.670 | −0.018 | 0.131 |
| Se levels | TB BMD | 6.460 | 0.693 | 7.040 | 0.721 | −0.005 | 0.465 |
| Se levels | OP | 14.772 | 0.097 | 16.063 | 0.097 | 0.000 | 0.398 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, H.; Ullah, A.; Yao, S.; Lyu, Q.; Kou, G. Causal Effect of Selenium Levels on Osteoporosis: A Mendelian Randomization Study. Nutrients 2023, 15, 5065. https://doi.org/10.3390/nu15245065
Li J, Li H, Ullah A, Yao S, Lyu Q, Kou G. Causal Effect of Selenium Levels on Osteoporosis: A Mendelian Randomization Study. Nutrients. 2023; 15(24):5065. https://doi.org/10.3390/nu15245065
Chicago/Turabian StyleLi, Jinjie, Hong Li, Amin Ullah, Shuyuan Yao, Quanjun Lyu, and Guangning Kou. 2023. "Causal Effect of Selenium Levels on Osteoporosis: A Mendelian Randomization Study" Nutrients 15, no. 24: 5065. https://doi.org/10.3390/nu15245065
APA StyleLi, J., Li, H., Ullah, A., Yao, S., Lyu, Q., & Kou, G. (2023). Causal Effect of Selenium Levels on Osteoporosis: A Mendelian Randomization Study. Nutrients, 15(24), 5065. https://doi.org/10.3390/nu15245065






