1. Introduction
Obesity is a chronic disease present worldwide, known to increase the risk factors for many pathological conditions, such as cardiovascular diseases, hypertension, infertility, diabetes mellitus and dyslipidemia [
1,
2]. The World Health Organization (WHO) has defined obesity as an abnormal or excessive accumulation of fat that represents a health risk, as indicated by a body mass index (BMI) ≥ 30 kg/m
2, while a body mass index (BMI) over 25 kg/m
2 is considered overweight [
3]. Previous studies have shown that a weight loss of 5–10% at baseline in subjects with obesity associated with other comorbidities can significantly improve health and counteract mortality [
4]. The etiology of obesity is multifactorial, involving, indeed, genetic and hormonal factors as well as social and environmental factors, mainly represented by a sedentary lifestyle and unhealthy eating habits [
4,
5,
6].
Gut hormones play an important role in the regulation of energy balance; they, indeed, modulate feeding behavior as well as energy expenditure and nutrient partitioning [
7]. Recently, it has been shown that gut hormone secretion can be regulated by G-protein-coupled taste receptor (TAS2R) family 2, a family of taste receptors activated by bitter molecules. Evolutionally, TAS2R has played important roles allowing organisms to detect potentially harmful substances and to avoid their ingestion. Being allocated in the gastrointestinal tract, it plays important roles in intestinal chemoreception [
8], coupling bitter molecules binding to gut hormone secretion.
The expression of TAS2Rs varies among people and there exist interindividual variations associated with gut hormone secretion upon taste receptor stimulation [
9,
10]. Previous in vivo studies indicate that the stimulation of TAS2R family members expressed by enteroendocrine cells (ECCs) is effective in inducing the release of gastrointestinal peptides, such as CCK and GLP-1, which are involved in the control of the sense of hunger and satiety, and sequentially of food intake.
The CCK hormone, released by the duodenal I cell after the consumption of a meal rich in lipids, is the most studied peptide up to date that is able to inhibit the sense of hunger and mediated by CCK1 receptors on vagal afferents [
11,
12].
Food intake and/or the ingestion of bitter substances can result in an increase in the release of CCK and a reduction in hormones, such as ghrelin and motilin, causing a decrease in gastrointestinal motility, in the hunger sense and, finally, in food intake [
13]. The effects on the secretion of gastrointestinal (GI) hormones and consequently on the slowing of gastric emptying play an important role in regulating energy intake, representing a new approach to the management or prevention of obesity and its comorbidity [
14,
15,
16].
To date, the common interest is to find a treatment that can counteract or prevent obesity, promoting and protecting individual health. A large amount of data indicate that a healthy diet and an active lifestyle play a key role in the prevention and treatment of obesity [
17,
18]. In particular, several dietary supplements, commonly called nutraceuticals, have been shown to have health benefits [
19,
20]. Furthermore, nutraceutical use associated with an appropriate diet and daily physical activity can represent an effective treatment for metabolic syndrome [
21,
22]. The aim of the present study was to assess the effects of a nutraceutical supplementation with
Cinchona succirubra (cinchona), a bitter taste agonist, on a population of overweight/obese adults. Cinchona is an arboreal plant belonging to the Rubiaceae family, from South America; in the past, this natural compound was widely used as a pharmacological treatment for its anti-malarial, analgesic and anti-flu properties [
23,
24,
25,
26,
27]. Previous studies demonstrate the beneficial effects of dietary cinchonine in preventing obesity [
25]; in particular, our purpose was to evaluate nutraceutical effects on satiety, weight loss, changes in body composition and nutritional status in a group of overweight/obese adults.
2. Materials and Methods
2.1. Study Design
The study protocol was approved by the Ethical Committee of the Federico II University Medical School of Naples—A.O.R.N. Cardarelli (EC approval code: 204/2023); all adults gave written informed consent. The clinical trial was registered at
www.isrctn.com, accessed on 25 July 2023 (ISRCTN 13055163 number).
Overweight/obese adults attending Outpatients Clinic of the Departmental program “Diet therapy in transplantation and chronic renal failure”, School of Medicine, “Federico II” University of Naples, were recruited. A low-calorie diet was recommended for all overweight/obese adults.
2.2. Inclusion and Exclusion Criteria
The adults eligible for the study were over 18 years of age with overweight/obesity, attending Outpatients Clinic of the Departmental program “Diet therapy in transplantation and chronic renal failure”, School of Medicine, “Federico II” University of Naples. Overweight/obesity was defined by a BMI > 25 and <45 kg/m2.
Adults were ineligible if the following were observed:
They participated in other clinical trials;
They declined to consent;
They showed a weight change of more than 3 kg in the last 2 months;
They were treated with weight loss medications and/or had a history of bariatric surgery;
They were treated with hormonal therapies (estrogens, thyroxine or progesterone);
They currently have cancer or received a cancer diagnosis within the last 5 years;
They suffered with acute or chronic metabolic and inflammatory diseases (Crohn’s disease, rheumatoid arthritis, etc.);
They suffered from type 1 or type 2 diabetes or had been treated with insulin and oral hypoglycemic drugs (changes in the regulation of intermediary metabolism).
Patients with type 2 diabetes, treated with diet alone, could be included in the study.
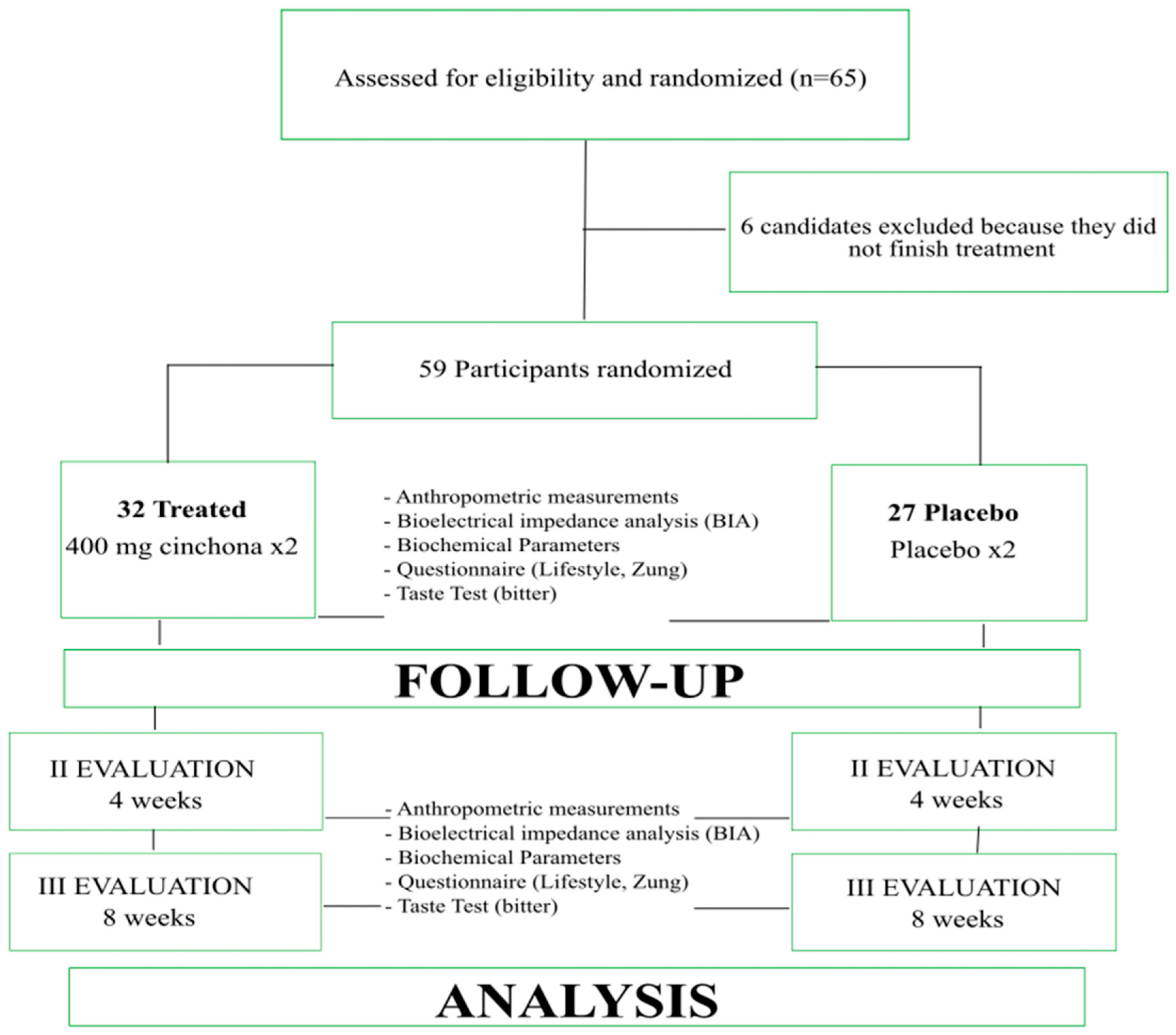
After baseline evaluations, subjects were randomized into 2 groups: the first group (32 adults) was treated with a hypocaloric diet for 2 months plus the supplementation of cinchona (T group); the second one (27 adults) was treated with a hypocaloric diet for 2 months plus a placebo supplementation (P-group). Adults affected by diseases, such as those reported above, cancer, acute and chronic metabolic and inflammatory diseases, type 1 and type 2 diabetes treated with insulin and/or hypoglycemic drugs or treated with drugs to lose weight or treated with hormone therapy were excluded.
2.3. Dietary Treatment
A personalized diet was recommended for each patient of both groups, om accordance with LARN (Livelli di Assunzione Raccomandata di Nutrienti) guidelines [
28,
29]. A hypocaloric diet (calorie restriction was 40% of the total energy needs), with 55–60% of the total caloric intake comprising carbohydrates, 10–15% of that comprising proteins and 20–25% of that being composed of fatty acids (<7% from saturated fat), was recommended for all obese adults.
2.4. Supplementation
The consumption of cinchona is not associated with health risks [
30,
31,
32].
The Cinchona succirubra bark powder used for the nutraceutical formulation was provided by NGN Healthcare New Generation Nutraceuticals srl. To allow the nutraceuticals to exclusively address duodenal EECs, the nutraceuticals were encapsulated in gastro-resistant capsules containing 400 mg of Cinchona succirubra bark powder microencapsulated with maltodextrins (two cinchona capsules per day, one hour before main meals). The placebo capsules used were produced using only maltodextrins and administered in accordance with the same methods. The subjects belonging to the T-group were treated with 800 mg of cinchona per day (two cinchona capsules per day, one hour before main meals). Participants who did not take pills for two or more days were excluded from the study.
2.5. Study Protocol
The subjects were evaluated at the moment of recruitment (time T0), after 30 (time T1) and 60 (time T2) days of treatment, using standardized protocols.
Nutritional status was assessed via anthropometric measurements: weight (Seca GmbH & Co. KG, Hamburg, Germany), height (measured using a wall-mounted stadiometer to the nearest 0.1 cm), body mass index (BMI), waist circumference (WC) and hip circumference (HC) [
33]. In particular, total body water (TBW), fat mass (FM) and fat-free mass (FFM) percentages were detected to evaluate the body composition, via bioelectrical impedance analysis (BIA) undertaken using a tetrapolar BIA (RJL 101; Akern SRL, Florence, Italy). BIA was performed with a single-frequency measurement (50 kHz) [
34]. The Zung test for assessing well-being was performed at baseline and at the end of the study. The Zung test represents a 20-item Likert scale, with scores ranging from 20, ‘‘no depression’’, to 80, ‘‘major depression’’, which are converted into index scores by dividing the sum of the raw scores by 80 and multiplying the result by 100 [
35].
Blood glucose (Gly), total cholesterol (TC), HDL cholesterol (HDL-c), LDL cholesterol (LDL-c), triglycerides (TG), uric acid, alanine amino transferase (ALT) and aspartate aminotransferase (AST) were measured and monitored during the treatment. All subjects, at T0 and at the end of the treatment, were tested for bitter taste to classify the subjects as tasters or non-tasters [
36] (
Figure 1).
2.6. Cinchona Alkaloid Status Assessment
To determine cinchona tasting status, all subjects consecutively placed five paper strips on their tongue; the first one was a control strip, whereas the others were impregnated with 0.4, 0.9, 2.4 and 6.0 mg of quinine extract, respectively. “Tasters” were defined as subjects who perceived bitter taste from any of the quinine impregnated blotting paper strips.
2.7. Dot Blot Protocol for CCK and Ghrelin Analyses
Serum CCK and ghrelin levels were measured via Dot-blot. Briefly, five microliters of serum samples was spotted on a nitrocellulose membrane to then be processed via Western blotting. CCK and ghrelin levels were immunodetected using anti-ghrelin (Santa Cruz, CA, USA, dilution 1:250) and anti-cholecystokinin (Santa Cruz, CA, USA, dilution 1:250) anti-bodies.
2.8. Compliance
Compliance with the dietary intervention was assessed by monitoring the dietary intake at baseline and every month until the end of the study using food frequency questionnaires [
37].
The assessment of compliance with physical activity was verified by asking subjects to complete a physical activity questionnaire [
38]. The questionnaires confirmed that adults belonging to both groups carried out regular physical activity for the entire duration of the treatment.
Satiety was assessed by means of visual analog scales (VAS), a previously validated questionnaire [
39].
Compliance with the nutraceutical supplementations was evaluated using a daily questionnaire asking each volunteer about the time of the consumption of the supplement as well as evaluating the presence of adverse events.
In addition, the number of capsules at the end of the study was recorded.
2.9. Sample Size and Statistical Power
Sample size calculation was performed using MedCalc. The outcome for the calculation of sample size was the reduction in body weight in the T-group. A difference of 25% between the T-group and P-group was estimated. Power and significance levels were set at 0.80 and 0.05, respectively. Using these parameters, the estimated sample size was 30 participants per group.
2.10. Statistical Analysis
All data were expressed as the mean ± standard error of the mean (SEM). Data were analyzed with the IBM SPSS 20.0 program (Statistical Package for Social Science, SPSS, Chicago, IL, USA). A paired-sample
t-test, independent-sample
t-test and chi-squared test were performed. Hormone levels were compared among groups using ordinary two-way ANOVA, correcting for multiple comparisons, and controlling the false discovery rate using the original FDR method of Benjamini and Hochberg [
40,
41]. Statistical significance was set at
p < 0.05.
3. Results
3.1. Nutritional Status Evaluation
All adults were accurately evaluated at baseline and were reconsidered after 30 and 60 days. No significant differences in all parameters were observed at the baseline among the two groups with the exception of hip circumference (P-group vs. T-group:
p = 0.019) and the percentage of total body water (P-group vs. T-group:
p = 0.49) (
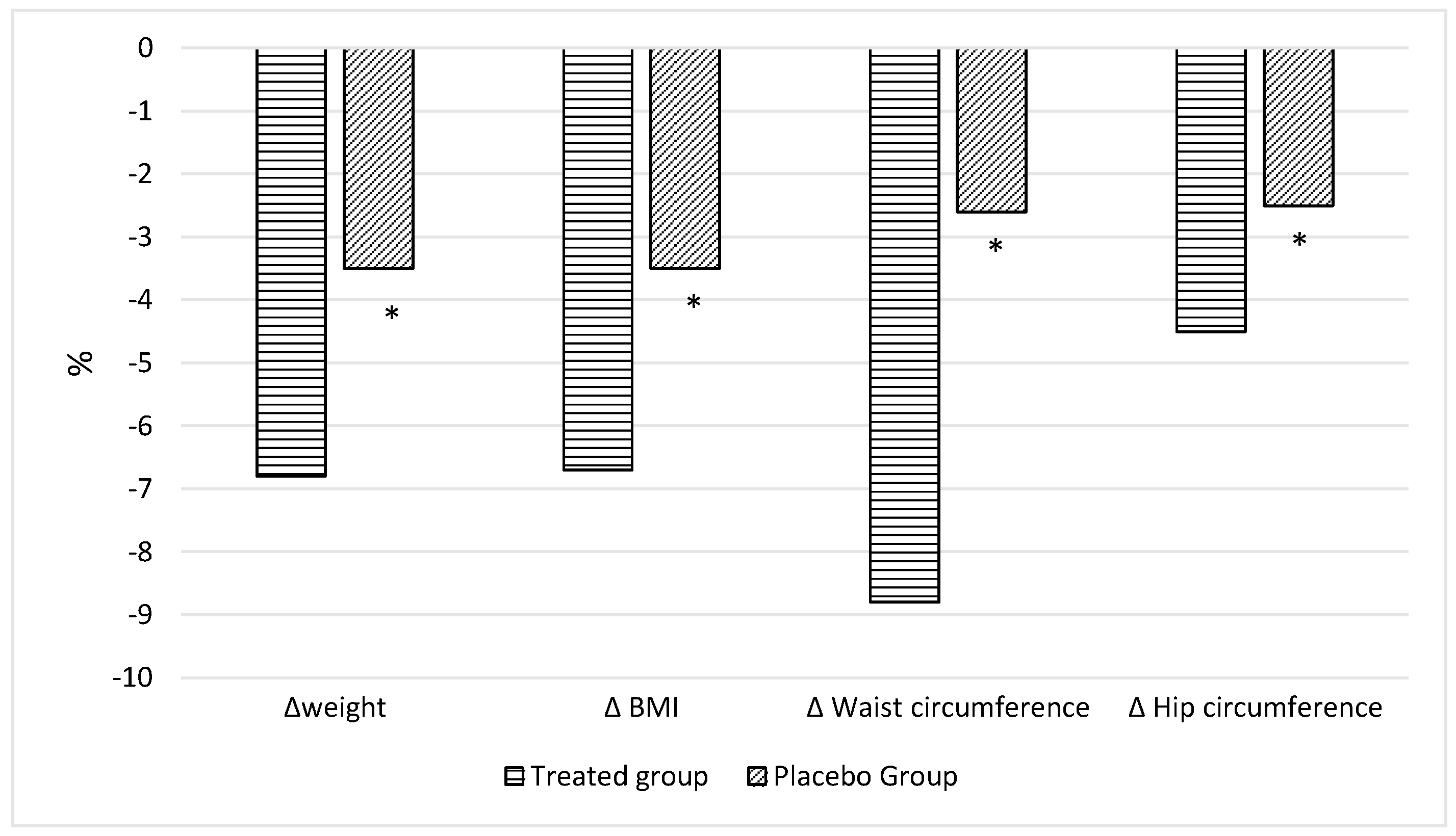
Table 1). On the basis of the dietary questionnaire, in the whole cohort, all adults had high adherence to the Med-Diet. At the end of the observations, the adults belonging to T-group presented significant changes in nutritional status and body composition compared to those at T0 (
Table 2) and a higher improvement compared to those in the P-groups (
Table 2), in particular in body weight (
p < 0.0001
Table 2,
Figure 2) as well as in BMI (
p < 0.0001,
Table 2,
Figure 2), waist circumference (
p = 0.047,
Table 2,
Figure 2), hip circumference (
p = 0.016,
Table 2,
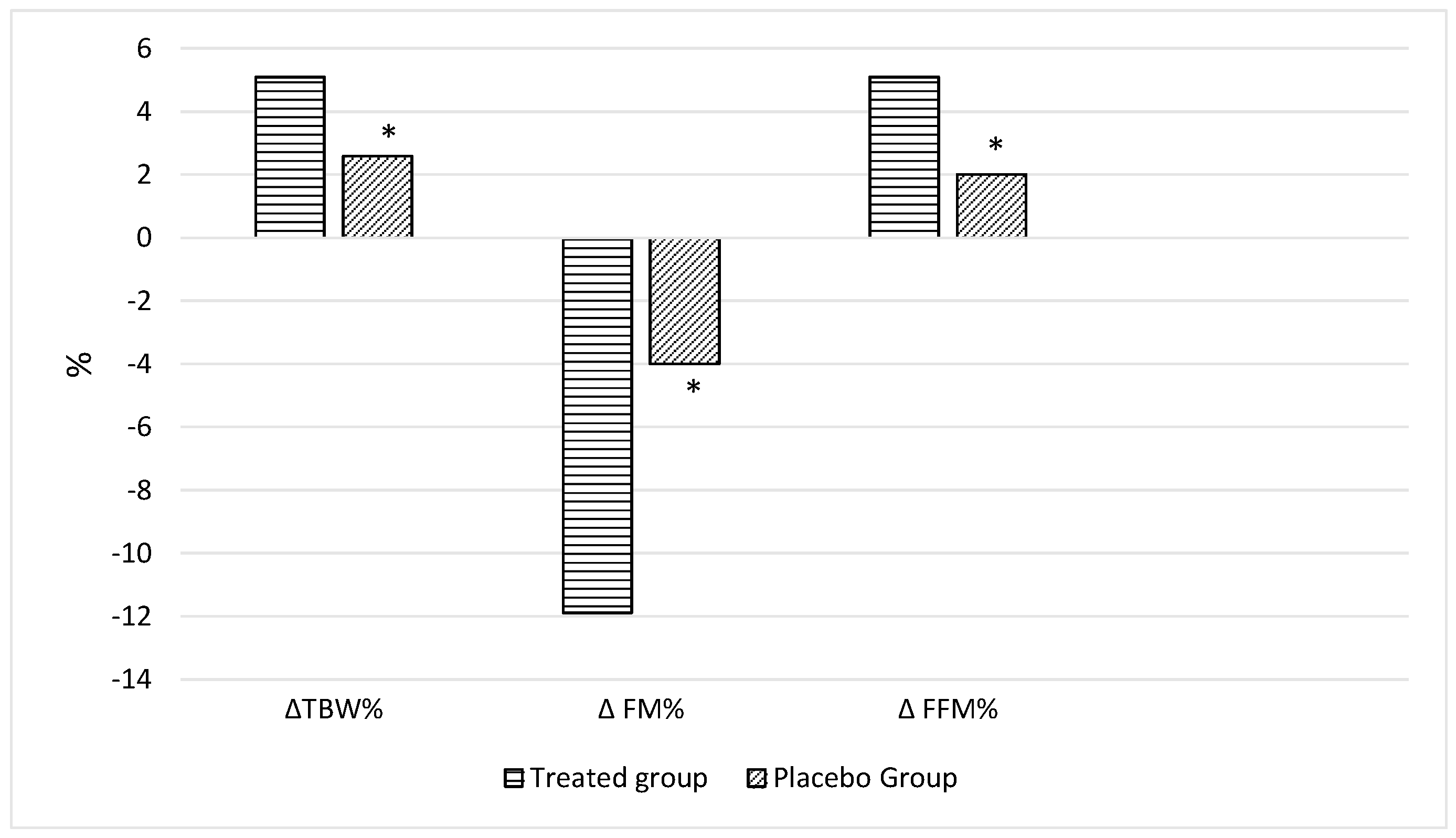
Figure 2), FM% (
p = 0.013,
Table 2,
Figure 3), FFM% (
p = 0.006,
Table 2,
Figure 3) and TBW% (
p = 0.004,
Table 2,
Figure 3). Zung scores for adults belonging to both groups were less than 45 and therefore did not indicate a depression, at the end of the treatment. However, a significant reduction was observed in both the treated (
p < 0.0001) and the placebo group (
p = 0.008) (
Table 2). Biochemical parameters were monitored throughout the study; parameters, such as HDL-c, and both transaminases remained stable throughout the study in both groups. Moreover, adults perceived the bitter taste of quinine-impregnated blotting paper and were thus all classified as “Tasters in both groups”.
Furthermore, satiety questionnaires administered in both groups revealed that the T-group showed greater satiety throughout the duration of treatment compared with that in the P-group confirming the data obtained via hormonal measurements.
VAS analysis revealed that, compared to those in the P-group, adults belonging to the T-group felt more satiated before (3.5 + −0.9 and 4.2 + −0.6 for the P- and T-group, respectively; p value = 0.0059) and during the meals (3.3 + −0.7 and 4.3 + −0.7 for the P- and T-group, respectively; p value < 0.0001), suggesting that cinchona prolonged satiety throughout the day.
3.2. Treated Group (T-Group)
Nutraceutical supplementation improved the nutritional status of adults belonging to the T-group, inducing a significant reduction in Body weight (90.4 ± 1.7 vs. 96.9 ± 1.6 kg, p < 0.0001; T2 vs. T0) as well as in BMI (32.4 ± 1.1 vs. 34.6 ± 1.0 kg/m2, p < 0.0001; T2 vs. T0), waist circumference (96.1 ± 1.0 vs. 102.7 ± 1.1 cm, p < 0.0001; T2 vs. T0) and hip circumference (115.2 ± 1.1 vs. 120.7 ± 1.1 cm, p < 0.0001; T2 vs. T0) when compared with those at baseline. Finally, bioimpedance analysis showed a significant reduction in FM% (31.1 ± 2.1% vs. 34.6 ± 1.7%; p < 0.0001, T2 vs. T0) in the T-group after 2 months of nutraceutical supplementation. There was, moreover, a significant increase in FFM% (68.9 ± 1.4% vs. 65.4 ± 1.2%, p < 0.0001; T2 vs. T0) and TBW% (50.6 ± 1.2% vs. 48.0 ± 1.1%, p < 0.0001; T2 vs. T0) when compared with those at baseline.
It is interesting to observe the bioumoral parameter improvements; in particular, significant reductions in glucose (87.4 ± 1.0 vs. 92.6 ± 1.4 mg/dL,
p = 0.025; T2 vs. T0), TC (173.4 ± 2.9 vs. 189.2 ± 3.0 mg/dL,
p = 0.04; T2 vs. T0), LDL-c (106.7 ± 3.5 vs. 118.9 ± 3.5 mg/dL,
p = 0.029; T2 vs. T0) and uric acid (3.3 ± 0.7 vs. 3.9 ± 0.6 mg/dL,
p = 0.07; T2 vs. T0) were detected when compared with those at baseline. Conversely, HDL-c as well as Try, both transaminases, PA, SM and SMI remained stable throughout the study (
Table 2).
3.3. Placebo Group (P-Group)
Adults belonging to the P-group showed an improvement in body weight (87.5 ± 2.0 vs. 90.7 ± 2.0 kg,
p < 0.0001; T2 vs. T0) as well as in BMI (31.8 ± 0.8 vs. 33.0 ± 0.8 kg/m
2,
p < 0.0001; T2 vs. T0). Furthermore, a significant reduction in waist circumference (98.9 ± 1.4 vs. 100.7 ± 1.5 cm,
p < 0.0001; T2 vs. T0) as well as in hip circumference (111.7 ± 0.7 vs. 113.7 ± 0.9 cm,
p = 0.001; T2 vs. T0) was detected after 60 days of treatment. Finally, bioimpedance analysis showed a significant reduction in FM% (28.4 ± 1.6 vs. 29.8 ± 1.6%;
p = 0.02, T2 vs. T0) in the P-group after 60 days of placebo supplementation and an increase in FFM% (71.5 ± 1.0 vs. 70.2 ± 1.0%,
p = 0.01; T2 vs. T0). Conversely, no significant differences were detected in TBW%, PA, SM and SMI compared with those at baseline. A significant improvement was detected in bioumoral parameters after 60 days; in particular, significant reductions in glucose (91.1 ± 1.3 vs. 96.0 ± 1.6 mg/dL,
p = 0.001; T2 vs. T0), TC (171.1 ± 2.8 vs. 185.9 ± 3.2 mg/dL,
p = 0.05 T2; vs. T0), LDL-c (106.7 ± 3.0 vs. 122.3 ± 3.5 mg/dL,
p = 0.035 T2; vs. T0), Try (84.6 ± 3.8 vs. 109.5 ± 6.8 mg/dL,
p = 0.023; T2 vs. T0) and uric acid (3.0 ± 0.6 vs. 4.1 ± 0.8 mg/dL,
p = 0.010; T2 vs. T0) were observed compared with those at baseline. Conversely, HDL-c and both transaminases remained stable throughout the study (
Table 2).
3.4. Serum CCK and Ghrelin Level
To confirm nutraceutical supplementation components addressing enteroendocrine cells (EECs), CCK and ghrelin serum levels were measured in treated and placebo groups, both at T0 and T2. Previously, we showed that bitter taste receptors are expressed in gastric EECs as well as in intestinal EECs. Gastric EECs secrete ghrelin while intestinal EECs secrete CCK [
42,
43]. Considering that nutraceutical supplementation was administered with gastro-resistant pills, we expected the stimulation of intestinal EECs but not of gastric EECs. As shown in
Table 3 at T0 and T2, placebo and treated adult serum samples were processed via Dot-blot to detect circulating CCK and ghrelin. It is worth noting that the hypocaloric diet decreased CCK levels at T2 in placebo adults (delta −40% ± 10,
p = 0.046); this reduction has been linked to increased hunger in adults undergoing hypocaloric diets. On the contrary, in adults receiving the nutraceutical supplementation, we did not measure a statistically significant reduction in CCK levels, indicating that nutraceuticals were effective in stimulating intestinal EECs to release CCK. Moreover, we measured ghrelin levels, which were unchanged in both placebo and treated group at the end of observations, indicating that there was no stimulation of gastric EECs.
4. Discussion
The results of the present study indicate that overweight/obese adults attending Outpatients Clinic of the Departmental program “Diet therapy in transplantation and chronic renal failure”, School of Medicine, “Federico II” University of Naples, treated with the supplementation of Cinchona succirubra, showed a significant improvement in nutritional status and body composition after 60 days of treatment compared with baseline. These adults were compared with a group of overweight/obese adults treated with a hypocaloric diet without supplementation. Interestingly, the cinchona-treated adults showed a reduction in body weight, BMI, waist circumference, hip circumference and FM% that was higher than that detected in the subjects receiving the placebo. Furthermore, a higher improvement in the percentage of FFM and TBW was observed in the T-group, when compared with baseline and adults belonging to the P-group. This study, indeed, demonstrates that the association of hypocaloric diet with cinchona nutraceutical supplementation is effective in inducing higher weight loss than that obtained with a hypocaloric diet.
The study justified the choice of Cinchona succirubra as a dietary supplement in the broader context of the use of natural substances or nutraceuticals for the treatment of obesity and weight control. The selection of cinchona was influenced by its role as a bitter taste agonist. This property was considered potentially beneficial for modulating food intake, therefore leading to improved weight management. In this context, previous preclinical studies strongly supported our selection.
Our results are in agreement with those of a previous study where Jung and coworkers investigated the effects of dietary cinchonine on the reduction in high-fat-diet- (HFD) induced adipogenesis and inflammation in a mouse model. In particular, the authors showed that HFD-fed mice treated with 0.05% dietary cinchonine for 10 weeks presented a reduction in body weight as well as an improvement in blood parameters, such as triglycerides, cholesterol and glycemia, accompanied by an attenuation in proinflammatory cytokine production. Therefore, the authors suggested that cinchona is able to prevent obesity due to its effects on adipogenesis and inflammation [
25].
Furthermore, adults belonging to the treated group showed a surprising reduction in the percentage of FM compared with that in the placebo group, confirming previous data presented by Cettour-Rose and coworkers, who studied the effect of 0.1% quinine on body weight and body composition in male mice fed a balanced diet. In particular, they showed that quinine, a cinchona alkaloid belonging to the aryl-amino alcohol group of drugs, is effective in the management and control of body weight and fat mass without affecting food intake. Therefore, the authors indicated that this could represent a novel tool to counteract obesity [
44]. Our present data support the previous suggestions, indicating that the stimulation of taste receptors by nutraceuticals based on cinchona affect the complex interplay of molecules involved in the regulation of food intake, body metabolism and total energy introduction and expenditure, facilitating real control of individual body weight.
It is interesting that adults belonging to the treated group revealed greater satiety throughout the duration of treatment, suggesting that nutraceutical treatment with cinchona is able to modulate gastrointestinal (GI) functions, including intestinal hormones, leading to an increased satiety and the consequent fine modulation of energy intake.
The results of this study indicate that the treatment with cinchona plus hypocaloric diet was accompanied by an interesting resetting of hormonal secretion by intestinal endocrine cells. The hypocaloric diet in the P-group, indeed, induced a decrease in CCK secretion and blood levels, as previously observed in the induction of weight loss via hypocaloric diet treatment. However, it is worth noting that the chinchona supplementation plus the hypocaloric diet did not modify the CCK secretion and blood levels, suggesting that cinchona was effective in facilitating CCK secretion, which was sequentially accompanied by higher satiety in the adults, as detected in our T-group. Therefore, CCK secretion and unmodified blood levels trigger the adaptation to weight loss induced by cinchona, as characterized by a decrease in hunger stimulation and better compliance to the hypocaloric diet. This likely suggests that this adaptation facilitates weight loss with a real improvement in all the morphometric and functional parameters under all time periods of treatment in our treated cases compared to those in the placebo group. On the other hand, in the P-group, the decrease in CCK secretion and blood levels indicate that the risk of hunger stimulation may play a key role in major alterations or failure in weight loss management.
In conclusion, weight loss management triggers the adaptation of circulating hormones, which is characterized by a decrease in CCK secretion with the consequent risk of failure of weight loss maintenance. Supplementation with cinchona in a hypocaloric diet facilitates higher circulating levels of CCK, which are effective in promoting patient satiety and reducing the risk of hunger stimulation and failure in weight loss attempts. These data call for further studies to clarify the complex interplay of different gut-derived hormones in the modulation of food and energy intake.
5. Limitation
Although this study presents promising results regarding the efficacy of Cinchona succirubra supplementation in conjunction with a hypocaloric diet for weight loss in overweight/obese patients, there are several limitations that must be acknowledged. First, the sample size and demographic diversity of the study were limited, calling for further research to confirm these findings across a broader population. Additionally, the study duration was relatively short (60 days), indicating the necessity for longer-term studies to evaluate the sustainability and prolonged effects of such treatment. While the study offers promising initial results for morphometric and functional parameters, an in-depth exploration of the long-term implications and potential side effects would be really useful. Furthermore, the biochemical mechanisms contributing to the observed weight loss and hormonal regulation also require further detailed investigation. Finally, while the study suggests an interesting resetting of hormonal secretion and appetite control, these results need to be verified with larger and diverse study populations over longer periods of time to validate the efficacy and safety of cinchona supplementation and the complex interplay of different gastro-intestinal hormones in weight management.
In this manuscript, we only measure fasting levels of CCK and ghrelin in adults receiving Cinchona succirubra. In the future, it will be interesting to measure postprandial levels of these appetite hormones upon Cinchona succirubra supplementation. Their levels will confirm the stimulatory mechanism of action of cinchona on intestinal bitter receptors and/or eventually highlight an unprecedented mechanism of action involving long-term transcriptional modulatory activity on enteroendocrine cells.
6. Conclusions
The results obtained from this study pave the way for a range of future research opportunities, which are crucial to deepening our understanding and applications in clinical settings. One immediate area to explore is the longitudinal evaluation of the effects of cinchona on weight and body metabolism. Studies extending over longer periods will be critical to assess the long-term safety and efficacy of this dietary supplement. The diversification of the study population in future trials is also essential. Including participants from various demographic backgrounds, different age groups and ethnicities will help determine the effectiveness of cinchona supplementation across a broader spectrum of the population. Furthermore, the mechanisms triggered by cinchona, affecting weight loss and metabolic parameters, need to be clarified. Such studies would provide valuable insights into its role in appetite regulation and energy balance, contributing to an understanding of its therapeutic potential. Significantly, comparative studies will also be interesting in terms of assessing the efficacy of cinchona, when combined with other plant matrices, as sources of alkaloids able to activate bitter taste receptors. Such combinations could potentially enhance the beneficial potential or mitigate any adverse effects, offering a new approach to weight management.
Author Contributions
M.C., B.D.C., A.C., B.G., G.C.T. and E.N. designed and supervised this study. M.C., B.D.C., M.D.L., E.S., M.S. and G.N. performed procedures in clinical research and analyzed the data. M.C., B.D.C., G.N., A.C., B.G. and E.N. supervised this paper. All authors contributed to this article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethical Committee of the Federico II University Medical School of Naples—A.O.R.N. Cardarelli (Project identification code 204/2023, approval date 5 June 2023), and all adults gave written informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are stored in a database at the Department of Clinical Medicine and Surgery, University of Naples “Federico II”, 80131 Naples, Italy. The data are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pi-Sunyer, X. The Medical Risks of Obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Chiurazzi, M.; Di Maro, M.; Cozzolino, M.; Colantuoni, A. Mitochondrial Dynamics and Microglia as New Targets in Metabolism Regulation. Int. J. Mol. Sci. 2020, 21, 3450. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Technical Report Series 894. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2000.
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Prim. 2017, 3, 17034. [Google Scholar] [CrossRef]
- Chiurazzi, M.; Cozzolino, M.; Orsini, R.C.; Di Maro, M.; Di Minno, M.N.D.; Colantuoni, A. Impact of Genetic Variations and Epigenetic Mechanisms on the Risk of Obesity. Int. J. Mol. Sci. 2020, 21, 9035. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, J.W.; Pawlik, T.; Brzozowski, T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharmacol. 2004, 55 Pt 2, 137–154. [Google Scholar]
- Rozengurt, E.; Sternini, C. Taste receptor signaling in the mammalian gut. Curr. Opin. Pharmacol. 2007, 7, 557–562. [Google Scholar] [CrossRef]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296, Erratum in: J. Cell Biol. 2010, 191, 429. [Google Scholar] [CrossRef]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef]
- Baldwin, B.; Parrott, R.; Ebenezer, I. Food for thought: A critique on the hypothesis that endogenous cholecystokinin acts as a physiological satiety factor. Prog. Neurobiol. 1998, 55, 477–507. [Google Scholar] [CrossRef]
- Andreozzi, P.; Sarnelli, G.; Pesce, M.; Zito, F.P.; Alessandro, A.D.; Verlezza, V.; Palumbo, I.; Turco, F.; Esposito, K.; Cuomo, R. The Bitter Taste Receptor Agonist Quinine Reduces Calorie Intake and Increases the Postprandial Release of Cholecystokinin in Healthy Subjects. J. Neurogastroenterol. Motil. 2015, 21, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Verbeure, W.; Mori, H.; Schol, J.; Van den Houte, K.; Huang, I.; Balsiger, L.; Broeders, B.; Colomier, E.; Scarpellini, E.; et al. The gastrointestinal tract in hunger and satiety signalling. United Eur. Gastroenterol. J. 2021, 9, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Wu, S.V.; Reeve, J.R., Jr.; Rozengurt, E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: Role of L-type voltage-sensitive Ca2+ channels. Am. J. Physiol. Cell Physiol. 2006, 291, C726–C739. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Rotondo, A.; Thijs, T.; Andrews, C.N.; Janssen, P.; Tack, J.; Depoortere, I. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep. 2015, 5, 15985. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Egan, J.M.; Jang, H.-J. Denatonium induces secretion of glucagon-like peptide-1 through activation of bitter taste receptor pathways. Diabetologia 2014, 57, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Hess, S. The role of physical activity in the prevention and management of obesity. J. Am. Diet. Assoc. 1998, 98 (Suppl. S2), S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [CrossRef]
- Kasbia, G.S. Functional foods and nutraceuticals in the management of obesity. Nutr. Food Sci. 2005, 35, 344–352. [Google Scholar] [CrossRef]
- Poddar, K.; Kolge, S.; Bezman, L.; Mullin, G.E.; Cheskin, L.J. Nutraceutical supplements for weight loss: A systematic review. Nutr. Clin. Pract. 2011, 26, 539–552. [Google Scholar] [CrossRef]
- Patti, A.M.; Al-Rasadi, K.; Giglio, R.V.; Nikolic, D.; Mannina, C.; Castellino, G.; Chianetta, R.; Banach, M.; Cicero, A.F.; Lippi, G.; et al. Natural approaches in metabolic syndrome management. Arch. Med. Sci. 2018, 14, 422–441. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Pavanello, C.; Calabresi, L.; Ruscica, M. Nutraceutical approaches to metabolic syndrome. Ann. Med. 2017, 49, 678–697. [Google Scholar] [CrossRef] [PubMed]
- Achan, J.E.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Gachelin, G.; Garner, P.; Ferroni, E.; Tröhler, U.; Chalmers, I. Evaluating Cinchona bark and quinine for treating and preventing malaria. J. R. Soc. Med. 2017, 110, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.A.; Choi, M.; Kim, S.; Yu, R.; Park, T. Cinchonine Prevents High-Fat-Diet-Induced Obesity through Downregulation of Adipogenesis and Adipose Inflammation. PPAR Res. 2012, 2012, 541204. [Google Scholar] [CrossRef]
- Mitsui, N.; Noro, T.; Kuroyanagi, M.; Miyase, T.; Umehara, K.; Ueno, A. Monoamine oxidase inhibitors from Cinchonae Cortex. Chem. Pharm. Bull. 1989, 37, 363–366. [Google Scholar] [CrossRef]
- Gurung, P.; De, P. Spectrum of biological properties of cinchon alkaloids: A brief review. J. Pharmacogn. Phytochem. 2017, 6, 162–166. [Google Scholar]
- Muscariello, E.; Nasti, G.; Siervo, M.; Di Maro, M.; Lapi, D.; D’Addio, G.; Colantuoni, A. Dietary protein intake in sarcopenic obese older women. Clin. Interv. Aging 2016, 11, 133–140. [Google Scholar] [CrossRef]
- Vitiello, V.; Germani, A.; Capuzzo Dolcetta, E.; Donini, L.M.; Del Balzo, V. The New Modern Mediterranean Diet Italian Pyramid. Ann. Ig. 2016, 28, 179–186. [Google Scholar] [CrossRef]
- Goldenberg, A.M.; Wexler, L.F. Quinine overdose: Review of toxicity and treatment. Clin. Cardiol. 1988, 11, 716–718. [Google Scholar] [CrossRef]
- Bateman, D.N.; Dyson, E.H. Quinine toxicity. Advers. Drug React. Acute Poisoning Rev. 1986, 5, 215–233. [Google Scholar]
- Cheng, G.-G.; Cai, X.-H.; Zhang, B.-H.; Li, Y.; Gu, J.; Bao, M.-F.; Liu, Y.-P.; Luo, X.-D. Cinchona Alkaloids from Cinchona succirubra and Cinchona ledgeriana. Planta Medica 2014, 80, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Stephan, B.; Nasti, G.; Colantuoni, A. Ageing, adiposity indexes and low muscle mass in a clinical sample of overweight and obese women. Obes. Res. Clin. Pract. 2012, 6, e1–e90. [Google Scholar] [CrossRef]
- Piccoli, A. Patterns of bioelectrical impedance vector analysis: Learning from electrocardiography and forgetting electric circuit models. Nutrition 2002, 18, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W. A rating instrument for anxiety disorders. Psychosomatics 1971, 12, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.K.; Drayna, D. Genetics of individual differences in bitter taste perception: Lessons from the PTC gene. Clin. Genet. 2005, 67, 275–280, Erratum in: Clin. Genet. 2005, 67, 534. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian EPIC cohorts: Presentation of data and methodological issues. Tumori J. 2003, 89, 594–607. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Janssen, S.; Laermans, J.; Verhulst, P.-J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef]
- Deloose, E.; Vos, R.; Corsetti, M.; Depoortere, I.; Tack, J. Endogenous motilin, but not ghrelin plasma levels fluctuate in accordance with gastric phase III activity of the migrating motor complex in man. Neurogastroenterol. Motil. 2014, 27, 63–71. [Google Scholar] [CrossRef]
- Latorre, R.; Huynh, J.; Mazzoni, M.; Gupta, A.; Bonora, E.; Clavenzani, P.; Chang, L.; Mayer, E.A.; De Giorgio, R.; Sternini, C. Expression of the Bitter Taste Receptor, T2R38, in Enteroendocrine Cells of the Colonic Mucosa of Overweight/Obese vs. Lean Subjects. PLoS ONE 2016, 11, e0147468. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Takahashi, C.; Taniguchi, Y.; Narukawa, M.; Misaka, T.; Ano, Y. Bitter taste receptor activation by hop-derived bitter components induces gastrointestinal hormone production in enteroendocrine cells. Biochem. Biophys. Res. Commun. 2020, 533, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Cettour-Rose, P.; Bezençon, C.; Darimont, C.; le Coutre, J.; Damak, S. Quinine controls body weight gain without affecting food intake in male C57BL6 mice. BMC Physiol. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).