High-Dose Vitamin D Supplementation Significantly Affects the Placental Transcriptome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Study Population
2.2. Intervention
2.3. Collection of Placental Tissue
2.4. Random Sample Collection
2.5. RNA Purification
2.6. Next-Generation RNA Sequencing
2.7. Data Preparation and Statistics
2.8. Ethical Approval
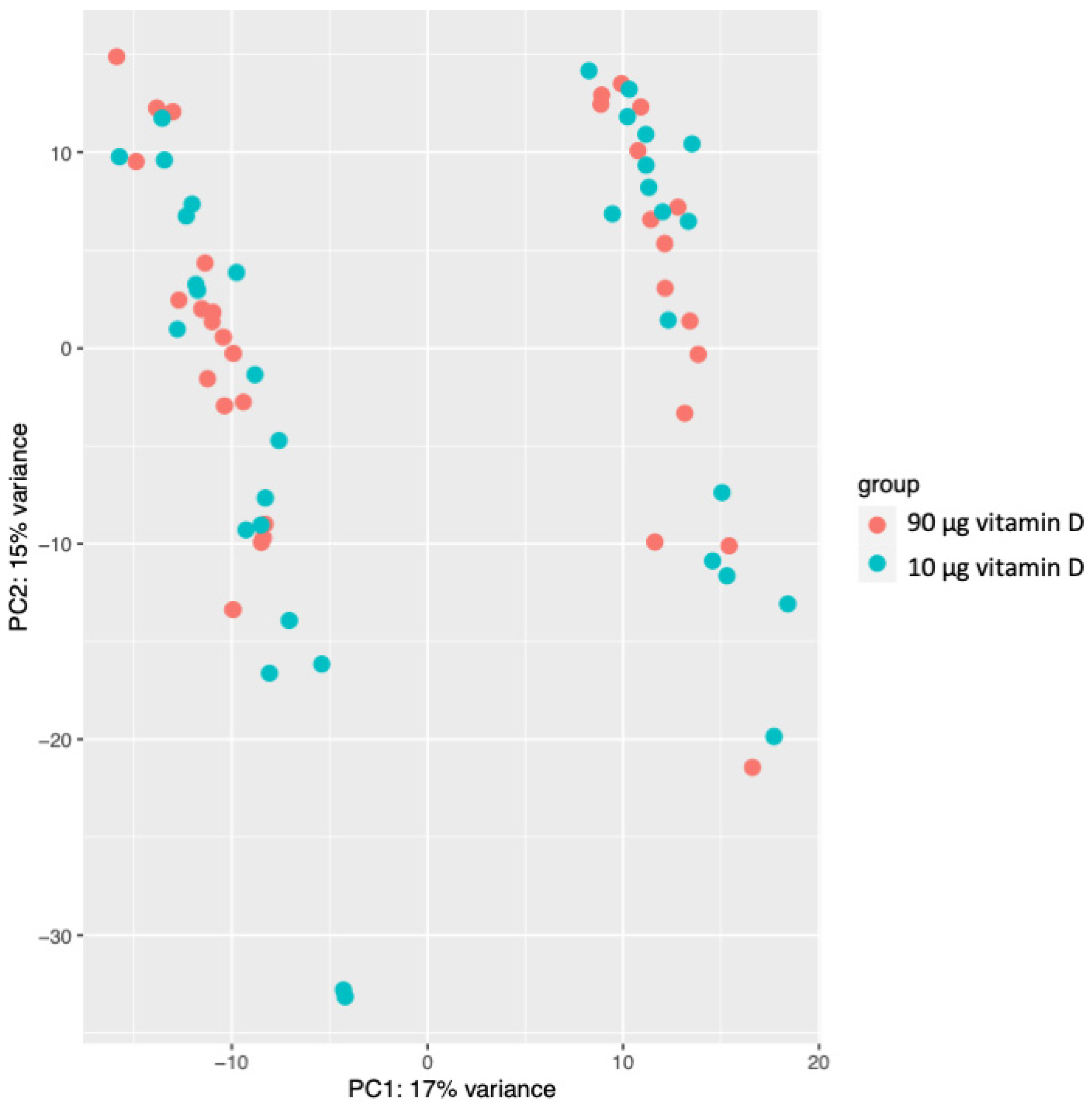
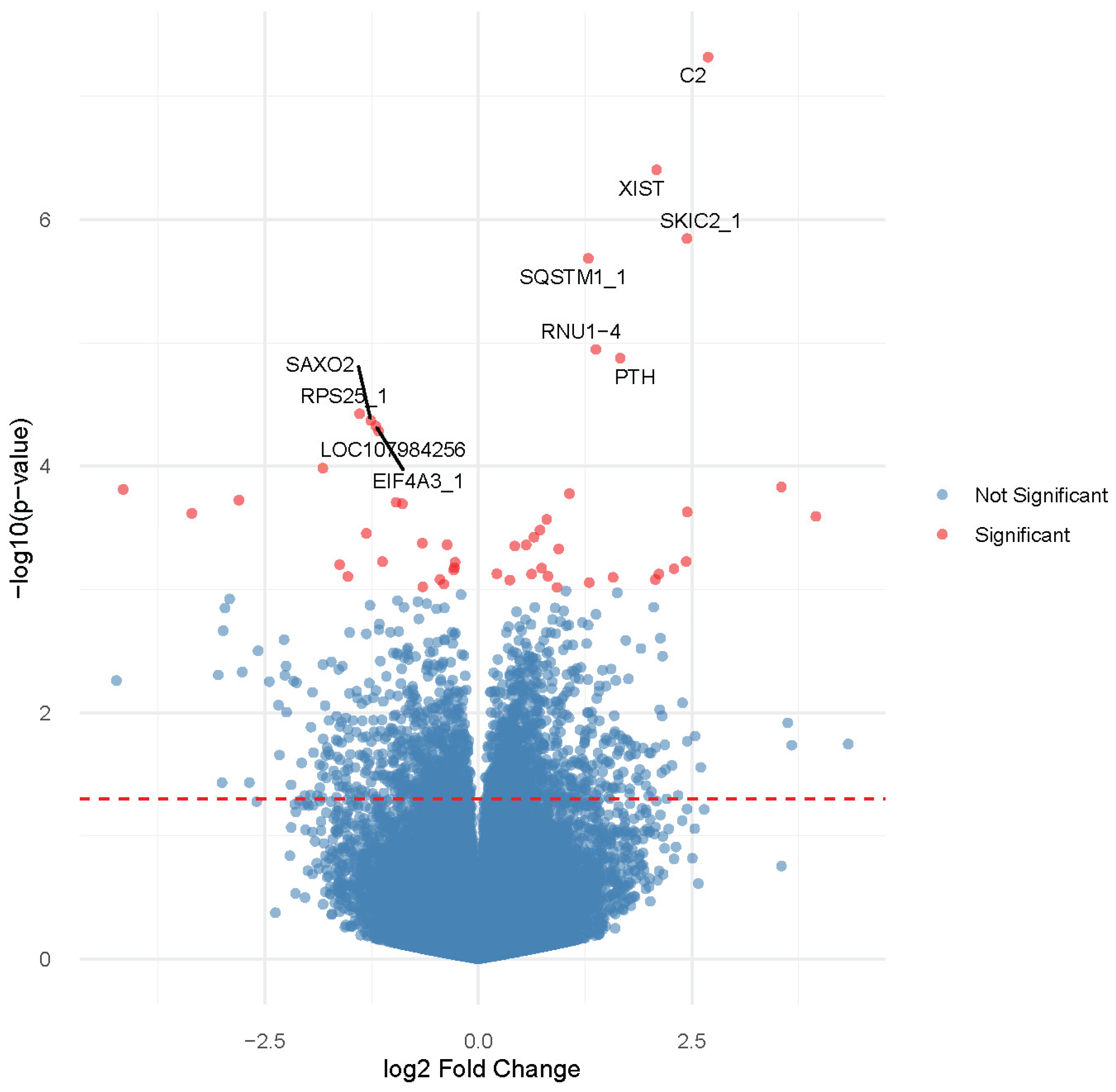
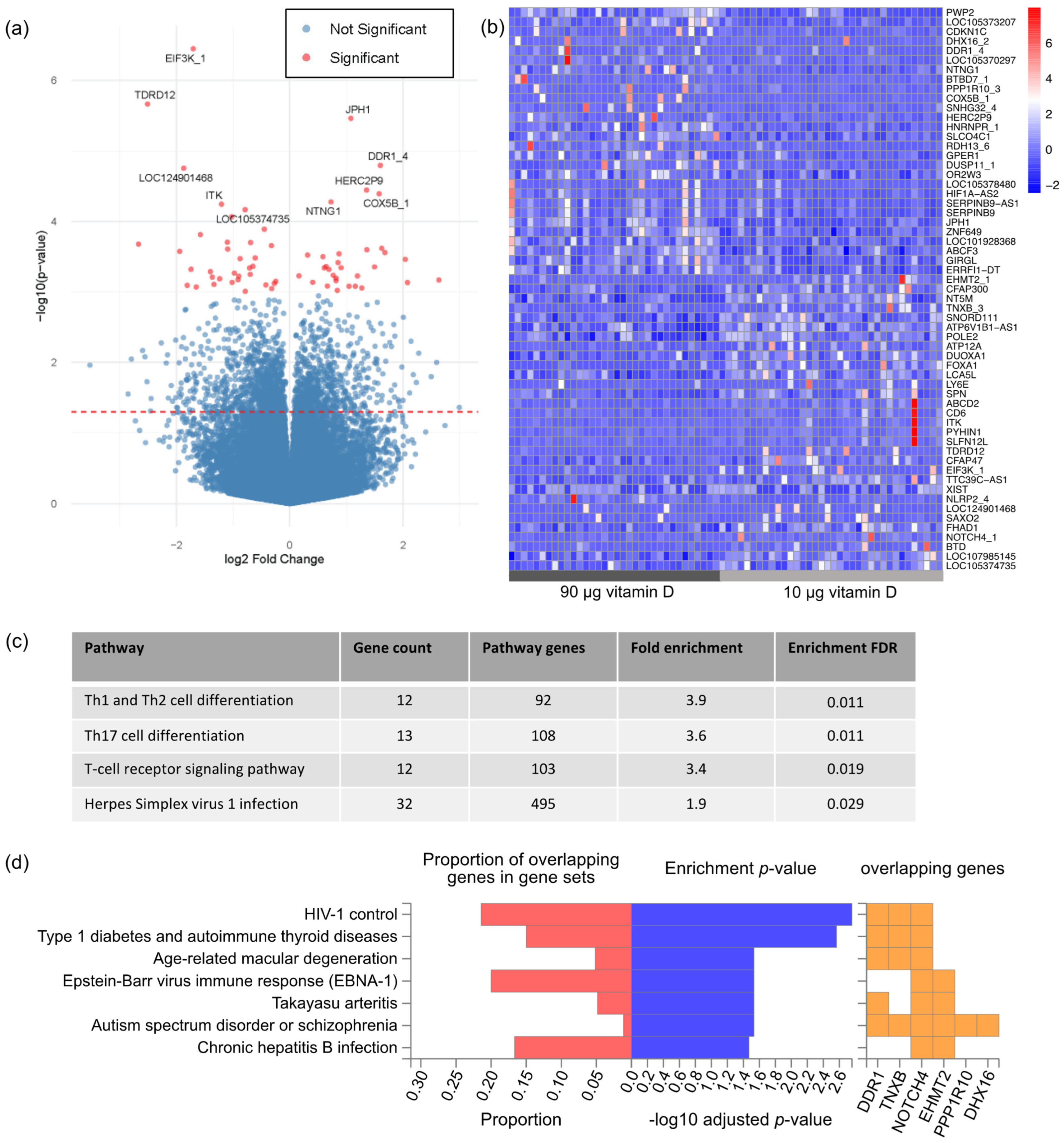
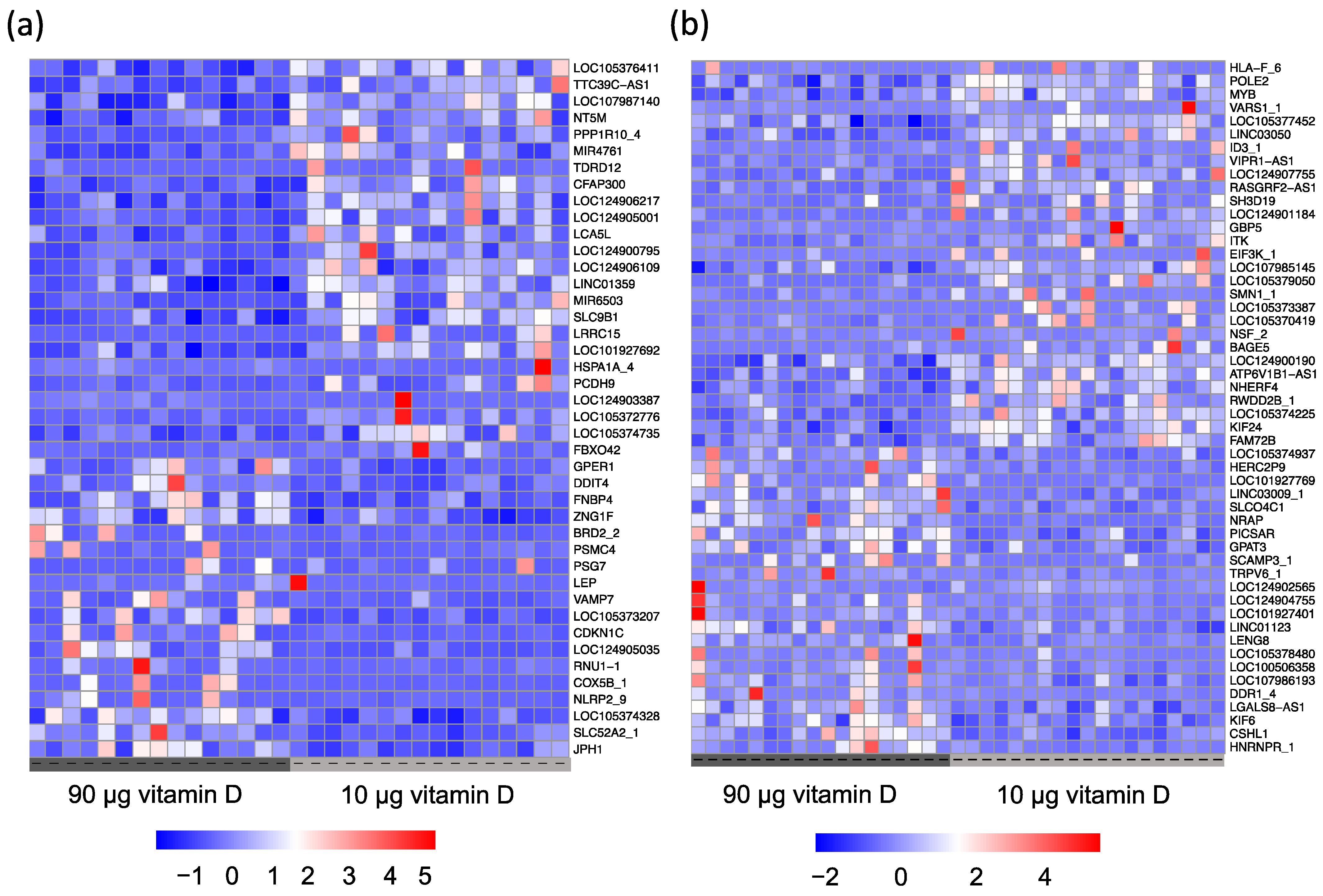
3. Results
3.1. Sample Characteristics
3.2. Gene Expression Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, C.L.; Hollis, B.W.; Kotsa, K.; Fakhoury, H.; Karras, S.N. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev. Endocr. Metab. Disord. 2017, 18, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Ponsonby, A.L.; Lucas, R.M.; Lewis, S.; Halliday, J. Vitamin D status during pregnancy and aspects of offspring health. Nutrients 2010, 2, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Ideraabdullah, F.Y.; Belenchia, A.M.; Rosenfeld, C.S.; Kullman, S.W.; Knuth, M.; Mahapatra, D.; Bereman, M.; Levin, E.D.; Peterson, C.A. Maternal vitamin D deficiency and developmental origins of health and disease (DOHaD). J. Endocrinol. 2019, 241, R65–R80. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, F.; Cardoso, I.; Keller, A.; Stougaard, M.; Frederiksen, P.; Cohen, A.S.; Maslova, E.; Jacobsen, R.; Backer, V.; Heitmann, B.L. Neonatal Vitamin D Status and Risk of Asthma in Childhood: Results from the D-Tect Study. Nutrients 2020, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Raia-Barjat, T.; Sarkis, C.; Rancon, F.; Thibaudin, L.; Gris, J.-C.; Alfaidy, N.; Chauleur, C. Vitamin D deficiency during late pregnancy mediates placenta-associated complications. Sci. Rep. 2021, 11, 20708. [Google Scholar] [CrossRef] [PubMed]
- Hutabarat, M.; Wibowo, N.; Obermayer-Pietsch, B.; Huppertz, B. Impact of vitamin D and vitamin D receptor on the trophoblast survival capacity in preeclampsia. PLoS ONE 2018, 13, e0206725. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K.M.; Kiely, M. Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy. Nutrients 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-Q.; Qi, H.-P.; Luo, Z.-C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef]
- Kiely, M.E.; Zhang, J.Y.; Kinsella, M.; Khashan, A.S.; Kenny, L.C. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am. J. Clin. Nutr. 2016, 104, 354–361. [Google Scholar] [CrossRef]
- Silva-Zolezzi, I.; Samuel, T.M.; Spieldenner, J. Maternal nutrition: Opportunities in the prevention of gestational diabetes. Nutr. Rev. 2017, 75, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Hossein-Nezhad, A.; Karimi, F.; Shafaei, A.R.; Larijani, B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab. Res. Rev. 2008, 24, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Tao, R.X.; Hu, H.L.; Zhang, Y.; Jiang, X.M.; Zhang, M.X.; Jin, D.; Yao, M.N.; Tao, F.B.; Zhu, P. The association of vitamin D status and supplementation during pregnancy with gestational diabetes mellitus: A Chinese prospective birth cohort study. Am. J. Clin. Nutr. 2020, 111, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Rifas-Shiman, S.L.; Kleinman, K.; Litonjua, A.A.; Huh, S.Y.; Rich-Edwards, J.W.; Camargo, C.A., Jr.; Gillman, M.W. Vitamin D deficiency in pregnancy and gestational diabetes mellitus. Am. J. Obs. Gynecol. 2012, 207, 182.E1–182.E8. [Google Scholar] [CrossRef] [PubMed]
- Saraf, R.; Morton, S.M.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child. Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Barker, D.J.; Thornburg, K.L. Placental programming of chronic diseases, cancer and lifespan: A review. Placenta 2013, 34, 841–845. [Google Scholar] [CrossRef]
- Guttmacher, A.E. The Human Placenta Project: Placental structure, development, and function in real time. Placenta 2014, 35, 303–304. [Google Scholar] [CrossRef]
- Barker, D.J.; Larsen, G.; Osmond, C.; Thornburg, K.L.; Kajantie, E.; Eriksson, J.G. The placental origins of sudden cardiac death. Int. J. Epidemiol. 2012, 41, 1394–1399. [Google Scholar] [CrossRef]
- Evans, K.N.; Bulmer, J.N.; Kilby, M.D.; Hewison, M. Vitamin D and placental-decidual function. J. Soc. Gynecol. Investig. 2004, 11, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Wood, M.R.; Malysheva, O.V.; Jones, S.; Mehta, S.; Brannon, P.M.; Caudill, M.A. Placental vitamin D metabolism and its associations with circulating vitamin D metabolites in pregnant women. Am. J. Clin. Nutr. 2017, 106, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Vitamin D and pregnancy: Skeletal effects, nonskeletal effects, and birth outcomes. Calcif. Tissue Int. 2013, 92, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef]
- Ali, A.M.; Alobaid, A.; Malhis, T.N.; Khattab, A.F. Effect of vitamin D3 supplementation in pregnancy on risk of pre-eclampsia -Randomized controlled trial. Clin. Nutr. 2019, 38, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Kanani, F.H.; Ramzan, S.; Kausar, R.; Ayaz, S.; Khanani, R.; Pal, L. Obstetric and Neonatal Outcomes of Maternal Vitamin D Supplementation: Results of an Open-Label, Randomized Controlled Trial of Antenatal Vitamin D Supplementation in Pakistani Women. J. Clin. Endocrinol. Metab. 2014, 99, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D. In Committee to Review Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press Institute of Medicine: Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.F. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, A.L.; Justesen, S.; Volqvartz, T.; Aagaard, S.K.; Andreasen, M.F.; Lesnikova, I.; Uldbjerg, N.; Larsen, A.; Bor, P. Vitamin D insufficiency among Danish pregnant women-Prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet. Gynecol. Scandinavica 2021, 100, 480–488. [Google Scholar] [CrossRef]
- Vestergaard, A.L.; Christensen, M.; Andreasen, M.F.; Larsen, A.; Bor, P. Vitamin D in pregnancy (GRAVITD)—A randomised controlled trial identifying associations and mechanisms linking maternal Vitamin D deficiency to placental dysfunction and adverse pregnancy outcomes—Study protocol. BMC Pregnancy Childbirth 2023, 23, 177. [Google Scholar] [CrossRef]
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Gearing, L.J.; Cumming, H.E.; Chapman, R.; Finkel, A.M.; Woodhouse, I.B.; Luu, K.; Gould, J.A.; Forster, S.C.; Hertzog, P.J. CiiiDER: A tool for predicting and analysing transcription factor binding sites. PLoS ONE 2019, 14, e0215495. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, X.; Bai, G.; Qiang, D.; Zhang, L.; Liu, H.; He, Y.; Tang, Y.; Li, L. Vitamin D deficiency leads to the abnormal activation of the complement system. Immunol. Res. 2023, 71, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Alleyne, D.; Witonsky, D.B.; Mapes, B.; Nakagome, S.; Sommars, M.; Hong, E.; Muckala, K.A.; Di Rienzo, A.; Kupfer, S.S. Colonic transcriptional response to 1α,25(OH)(2) vitamin D(3) in African- and European-Americans. J. Steroid Biochem. Mol. Biol. 2017, 168, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Loda, A.; Heard, E. Xist RNA in action: Past, present, and future. PLoS Genet. 2019, 15, e1008333. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.genecards.org/ (accessed on 29 September 2023).
- Mayeur, G.L.; Fraser, C.S.; Peiretti, F.; Block, K.L.; Hershey, J.W. Characterization of eIF3k: A newly discovered subunit of mammalian translation initiation factor elF3. Eur. J. Biochem. 2003, 270, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Kranzusch, P.J.; Cate, J.H. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 2015, 522, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.J.; Saveliev, A.; Salerno, F.; Matheson, L.S.; Screen, M.; Lawson, H.; Wotherspoon, D.; Kranc, K.R.; Turner, M. A functional screen of RNA binding proteins identifies genes that promote or limit the accumulation of CD138+ plasma cells. Elife 2022, 11, e72313. [Google Scholar] [CrossRef]
- Kind, S.; Merenkow, C.; Büscheck, F.; Möller, K.; Dum, D.; Chirico, V.; Luebke, A.M.; Höflmayer, D.; Hinsch, A.; Jacobsen, F.; et al. Prevalence of Syndecan-1 (CD138) Expression in Different Kinds of Human Tumors and Normal Tissues. Dis. Markers 2019, 2019, 4928315. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Czika, A.; Gorleku, P.N.; Ullah, A.; Panhwar, Z.; Ruan, L.L.; Ding, Y.B.; Wang, Y.X. The Involvement of Cell Adhesion Molecules, Tight Junctions, and Gap Junctions in Human Placentation. Reprod. Sci. 2021, 28, 305–320. [Google Scholar] [CrossRef]
- Babakhanzadeh, E.; Khodadadian, A.; Rostami, S.; Alipourfard, I.; Aghaei, M.; Nazari, M.; Hosseinnia, M.; Mehrjardi, M.Y.V.; Jamshidi, Y.; Ghasemi, N. Testicular expression of TDRD1, TDRD5, TDRD9 and TDRD12 in azoospermia. BMC Med. Genet. 2020, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.R.; Tokuzawa, Y.; Yang, Z.; Hayashi, E.; Ichisaka, T.; Kajita, S.; Asano, Y.; Kunieda, T.; Sachidanandam, R.; Chuma, S.; et al. Tudor domain containing 12 (TDRD12) is essential for secondary PIWI interacting RNA biogenesis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 16492–16497. [Google Scholar] [CrossRef] [PubMed]
- Handler, D.; Olivieri, D.; Novatchkova, M.; Gruber, F.S.; Meixner, K.; Mechtler, K.; Stark, A.; Sachidanandam, R.; Brennecke, J. A systematic analysis of Drosophila TUDOR domain-containing proteins identifies Vreteno and the Tdrd12 family as essential primary piRNA pathway factors. EMBO J. 2011, 30, 3977–3993. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.R.; Homolka, D.; Olotu, O.; Sachidanandam, R.; Kotaja, N.; Pillai, R.S. Exonuclease Domain-Containing 1 Enhances MIWI2 piRNA Biogenesis via Its Interaction with TDRD12. Cell Rep. 2018, 24, 3423–3432.E4. [Google Scholar] [CrossRef] [PubMed]
- Keighley, L.M.; Lynch-Sutherland, C.F.; Almomani, S.N.; Eccles, M.R.; Macaulay, E.C. Unveiling the hidden players: The crucial role of transposable elements in the placenta and their potential contribution to pre-eclampsia. Placenta 2023, 141, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, J.A.; Hewison, M.; Wagner, C.L.; Bulmer, J.N.; Kilby, M.D. Immunological role of vitamin D at the maternal-fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Ji, J.L.; Muyayalo, K.P.; Zhang, Y.H.; Hu, X.H.; Liao, A.H. Immunological function of vitamin D during human pregnancy. Am. J. Reprod. Immunol. 2017, 78, e12716. [Google Scholar] [CrossRef]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef]
- Benachi, A.; Baptiste, A.; Taieb, J.; Tsatsaris, V.; Guibourdenche, J.; Senat, M.V.; Haidar, H.; Jani, J.; Guizani, M.; Jouannic, J.M.; et al. Relationship between vitamin D status in pregnancy and the risk for preeclampsia: A nested case-control study. Clin. Nutr. 2020, 39, 440–446. [Google Scholar] [CrossRef]
- Smith, T.A.; Kirkpatrick, D.R.; Kovilam, O.; Agrawal, D.K. Immunomodulatory role of vitamin D in the pathogenesis of preeclampsia. Expert. Rev. Clin. Immunol. 2015, 11, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, H.; Litonjua, A.A.; McElrath, T.F.; O’Connor, G.; Lee-Parritz, A.; Iverson, R.; Macones, G.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.; et al. Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Investig. 2016, 126, 4702–4715. [Google Scholar] [CrossRef] [PubMed]
- Yadama, A.P.; Mirzakhani, H.; McElrath, T.F.; Litonjua, A.A.; Weiss, S.T. Transcriptome analysis of early pregnancy vitamin D status and spontaneous preterm birth. PLoS ONE 2020, 15, e0227193. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-González, R.M.; García-Marcos, L.; Morales, E. Prenatal vitamin D status and respiratory and allergic outcomes in childhood: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Loddo, F.; Nauleau, S.; Lapalus, D.; Tardieu, S.; Bernard, O.; Boubred, F. Association of Maternal Gestational Vitamin D Supplementation with Respiratory Health of Young Children. Nutrients 2023, 15, 2380. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Kalish, B.T. The impact of maternal obesity on childhood neurodevelopment. J. Perinatol. 2021, 41, 928–939. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, J.W.; Sen, S.; Chomitz, V.R.; Seidell, J.C.; Leviton, A.; Dammann, O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 2016, 79, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.D.; Mead, M.J.; McWhorter, C.A.; Ebeling, M.D.; Shary, J.R.; Newton, D.A.; Baatz, J.E.; Gregoski, M.J.; Hollis, B.W.; Wagner, C.L. Vitamin D and Child Neurodevelopment-A Post Hoc Analysis. Nutrients 2023, 15, 4250. [Google Scholar] [CrossRef]
- Pasing, Y.; Fenton, C.G.; Jorde, R.; Paulssen, R.H. Changes in the human transcriptome upon vitamin D supplementation. J. Steroid Biochem. Mol. Biol. 2017, 173, 93–99. [Google Scholar] [CrossRef]
- Verburg, P.E.; Tucker, G.; Scheil, W.; Erwich, J.J.; Dekker, G.A.; Roberts, C.T. Sexual Dimorphism in Adverse Pregnancy Outcomes—A Retrospective Australian Population Study 1981–2011. PLoS ONE 2016, 11, e0158807. [Google Scholar] [CrossRef]
| 90 µg of Vitamin D n = 34 | 10 µg of Vitamin D n = 36 | p-Value | |
|---|---|---|---|
| Maternal age (years), mean (± SD) | 30.4 (5.2) | 29.1 (4.1) | 0.237 |
| BMI (kg/m2) mean (±SD) | 24.8 (4.8) | 25.9 (5.9) | 0.372 |
| Parity n (%) | 0.520 | ||
| Nulliparous | 17 (50.0) | 16 (44.4) | |
| Primiparous | 10 (29.4) | 15 (41.7) | |
| Multiparous | 7 (20.6) | 5 (13.9) | |
| Season at enrolment n (%) | 0.814 | ||
| Winter | 9 (26.5) | 7 (19.4) | |
| Spring | 9 (26.5) | 8 (22.2) | |
| Summer | 8 (23.5) | 11 (30.6) | |
| Autumn | 8 (23.5) | 10 (27.8) | |
| Gestational age at enrolment (week + day) mean (±SD) | 12 + 6 (0.8) | 12 + 6 (1.1) | 0.938 |
| First trimester 25(OH)D (nmol/L) mean (±SD) | 69.9 (22.8) | 76.4 (18.3) | 0.203 |
| Third trimester 25(OH)D (nmol/L) mean (±SD) * | 124.2 (26.2) | 86.5 (20.7) | <0.0001 |
| First trimester vitamin D status n (%) | 0.074 | ||
| Deficient < 50 nmol/L | 10 (30.3) | 3 (8.6) | |
| Insufficient 50–74 nmol/L | 6 (18.2) | 9 (25.7) | |
| Sufficient ≥ 75 nmol/L | 17 (51.5) | 23 (65.7) | |
| Third trimester vitamin D status n (%) * | 0.059 | ||
| Deficient < 50 nmol/L | 0 (0.0) | 1 (5.3) | |
| Insufficient 50–74 nmol/L | 1 (4.3) | 5 (26.3) | |
| Sufficient ≥ 75 nmol/L | 22 (95.7) | 13 (68.4) | |
| Gestational age at delivery (week + day), mean (±SD) | 39 + 6 (1.0) | 40 + 0 (1.2) | 0.395 |
| Mode of delivery n (%) | 0.313 | ||
| Vaginal | 28 (82.4) | 26 (72.2) | |
| Caesarean section | 6 (17.7) | 10 (27.8) | |
| Sex of the baby n (%) | 0.794 | ||
| Female | 19 (55.9) | 19 (52.8) | |
| Male | 15 (44.1) | 17 (47.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vestergaard, A.L.; Andersen, M.K.; Olesen, R.V.; Bor, P.; Larsen, A. High-Dose Vitamin D Supplementation Significantly Affects the Placental Transcriptome. Nutrients 2023, 15, 5032. https://doi.org/10.3390/nu15245032
Vestergaard AL, Andersen MK, Olesen RV, Bor P, Larsen A. High-Dose Vitamin D Supplementation Significantly Affects the Placental Transcriptome. Nutrients. 2023; 15(24):5032. https://doi.org/10.3390/nu15245032
Chicago/Turabian StyleVestergaard, Anna Louise, Matilde K. Andersen, Rasmus V. Olesen, Pinar Bor, and Agnete Larsen. 2023. "High-Dose Vitamin D Supplementation Significantly Affects the Placental Transcriptome" Nutrients 15, no. 24: 5032. https://doi.org/10.3390/nu15245032
APA StyleVestergaard, A. L., Andersen, M. K., Olesen, R. V., Bor, P., & Larsen, A. (2023). High-Dose Vitamin D Supplementation Significantly Affects the Placental Transcriptome. Nutrients, 15(24), 5032. https://doi.org/10.3390/nu15245032






