Effects of Withania somnifera on Cortisol Levels in Stressed Human Subjects: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.2.1. Inclusion Criteria
- Original peer-reviewed articles
- Human studies (randomized controlled trials, prospective or retrospective cohort study, or cross-sectional)
- Studies on adult population (≥18 years)
- Studies including healthy population.
- Studies with the only use of WS.
- Studies including the measurement of cortisol level.
2.2.2. Exclusion Criteria
- Not human studies (cell-based and animal or plant studies).
- Review, case report, and congress abstract.
- Studies including multi-herbal and/or multi-mineral preparations.
- Studies include pharmacological treatments.
- Studies without measurements of cortisol level.
2.3. Data Extraction
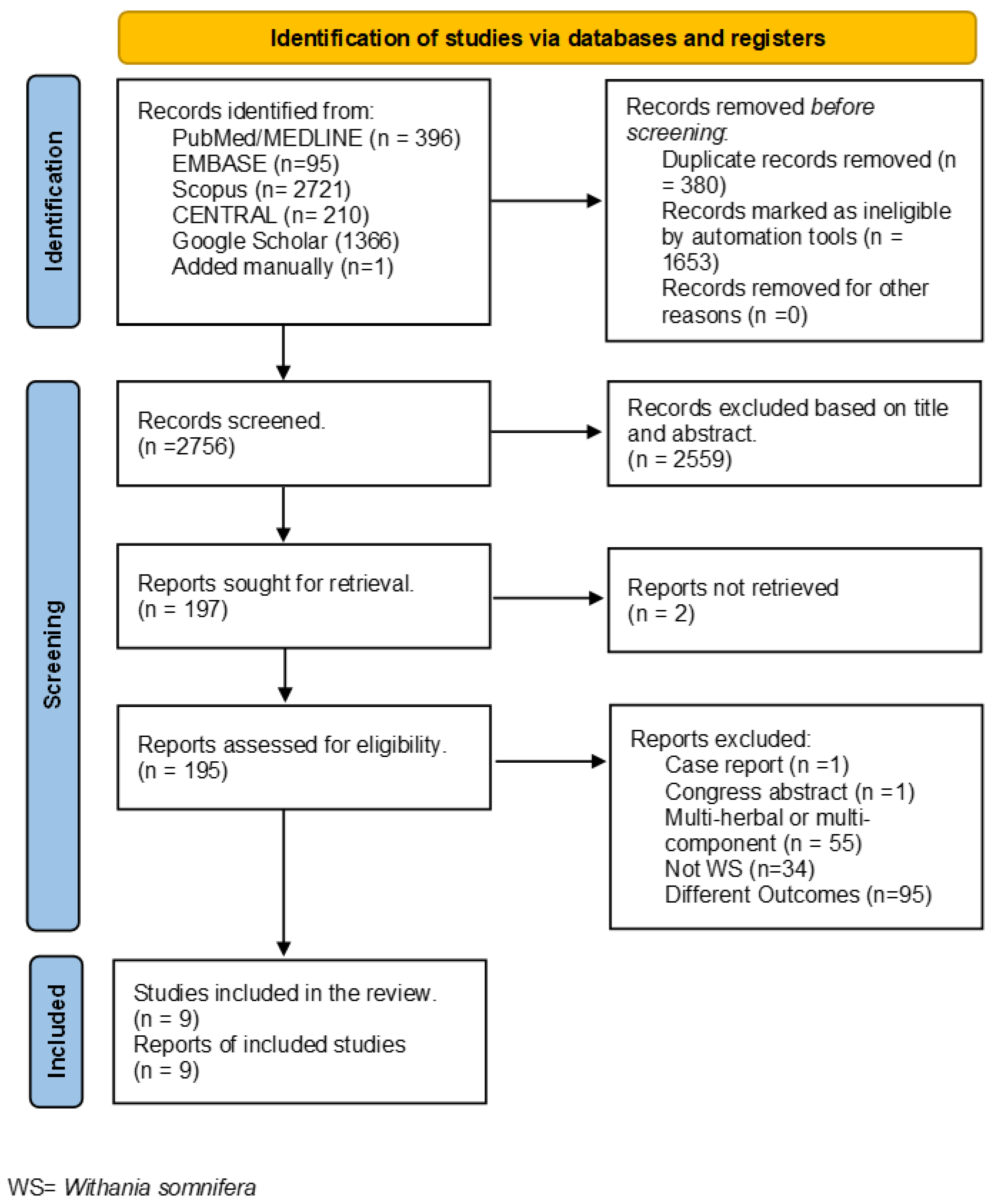
3. Results
3.1. Study Selection
3.2. Results of Individual Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharya, S.K.; Muruganandam, A.V. Adaptogenic activity of Withania somnifera: An experimental study using a rat model of chronic stress. Pharmacol. Biochem. Behav. 2003, 75, 547–555. [Google Scholar] [CrossRef]
- Joshi, V.K.; Joshi, A. Rational use of Ashwagandha in Ayurveda (Traditional Indian Medicine) for health and healing. J. Ethnopharmacol. 2021, 276, 114101. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szeląg, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G.; et al. Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, S.N.; Bossaghzadeh, F.; Malamiri, F.A.; Esmaeili, A.; Moudi, E. The most important medicinal plants affecting sperm and testosterone production: A systematic review. JBRA Assist. Reprod. 2022, 26, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and Safety of Ashwagandha Root Extract in Subclinical Hypothyroid Patients: A Double-Blind, Randomized Placebo-Controlled Trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Loke, W.; Foo, N.X.; Tan, W.J.; Chan, H.W.; Lim, D.Y.; Yeo, W.S. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother. Res. 2020, 34, 583–590. [Google Scholar] [CrossRef]
- Ahmad, M.; Dar, N.J. Withania somnifera: Ethnobotany, Pharmacology, and Therapeutic Functions. In Sustained Energy for Enhanced Human Functions and Activity; Academic Press: Cambridge, MA, USA, 2017; pp. 137–154. [Google Scholar] [CrossRef]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef]
- Dar, P.A.; Singh, L.R.; Kamal, M.A.; Dar, T.A. Unique Medicinal Properties of Withania somnifera: Phytochemical Constituents and Protein Component. Curr. Pharm. Des. 2016, 22, 535–540. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Namdeo, A.G. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J. Diet. Suppl. 2021, 18, 183–226. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress—Protective activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef]
- White, P.T.; Subramanian, C.; Motiwala, H.F.; Cohen, M.S. Natural withanolides in the treatment of chronic diseases. In Advances in Experimental Medicine and Biology; Nature Publishing Group: Cham, Switzerland, 2016; Volume 928, pp. 329–373. [Google Scholar]
- Noushad, S.; Ahmed, S.; Ansari, B.; Mustafa, U.-H.; Saleem, Y.; Hazrat, H. Physiological biomarkers of chronic stress: A systematic review. Int. J. Health Sci. 2021, 15, 46. [Google Scholar]
- Kaushik, M.K.; Kaul, S.C.; Wadhwa, R.; Yanagisawa, M.; Urade, Y. Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction. PLoS ONE 2017, 12, e0172508. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, E.M.K.; Xu, Y.-M.; Scherz-Shouval, R.; Marron, M.T.; Rocha, D.D.; Liu, M.X.; Costa-Lotufo, L.V.; Santagata, S.; Lindquist, S.; Whitesell, L.; et al. Structure−Activity Relationships for Withanolides as Inducers of the Cellular Heat-Shock Response. J. Med. Chem. 2014, 57, 2851–2863. [Google Scholar] [CrossRef]
- Bharti, V.K.; Malik, J.K.; Gupta, R.C. Ashwagandha: Multiple Health Benefits. In Nutraceuticals; Academic Press: Cambridge, MA, USA, 2016; pp. 717–733. [Google Scholar] [CrossRef]
- Crane, E.A.; Heydenreuter, W.; Beck, K.R.; Strajhar, P.; Vomacka, J.; Smiesko, M.; Mons, E.; Barth, L.; Neuburger, M.; Vedani, A.; et al. Profiling withanolide A for therapeutic targets in neurodegenerative diseases. Bioorg. Med. Chem. 2019, 27, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- Berghe, W.V.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef]
- Kaur, P.; Mathur, S.; Sharma, M.; Tiwari, M.; Sdvastava, K.K.; Chandra, R. A Biologically active constituent of Withania somnifera (Ashwagandha) with anti-stress activity. Indian J. Clin. Biochem. 2001, 16, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Rauf, A.; Akhter, S.; Islam, M.N.; Emran, T.B.; Mitra, S.; Khan, I.N.; Mubarak, M.S. Role of Withaferin A and Its Derivatives in the Management of Alzheimer’s Disease: Recent Trends and Future Perspectives. Molecules 2021, 26, 3696. [Google Scholar] [CrossRef]
- Tetali, S.D.; Acharya, S.; Ankari, A.B.; Nanakram, V.; Raghavendra, A.S. Metabolomics of Withania somnifera (L.) Dunal: Advances and applications. J. Ethnopharmacol. 2021, 267, 113469. [Google Scholar] [CrossRef]
- Dhar, N.; Razdan, S.; Rana, S.; Bhat, W.W.; Vishwakarma, R.; Lattoo, S.K. A decade of molecular understanding of withanolide biosynthesis and in vitro studies in Withania somnifera (L.) dunal: Prospects and perspectives for pathway engineering. Front. Plant Sci. 2015, 6, 1031. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, Q.; Zou, H.; Zhao, J.; Zhang, J.; Ren, L. Withaferin A: A potential selective glucocorticoid receptor modulator with anti-inflammatory effect. Food Chem. Toxicol. 2023, 179, 113949. [Google Scholar] [CrossRef]
- Dey, A.; Chatterjee, S.; Kumar, V. Triethylene glycol-like effects of Ashwagandha (Withania somnifera (L.) Dunal) root extract devoid of withanolides in stressed mice. AYU (An Int. Q. J. Res. Ayurveda) 2018, 39, 230. [Google Scholar] [CrossRef]
- Van Cauter, E.A.; Spiegel, K.B.; Tasali, E.A.; Leproult, R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008, 9, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Irani, N.; Balkrishnan, R.; Benny, I.R. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020, 72, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study. Cureus 2019, 11, e5797. [Google Scholar] [CrossRef] [PubMed]
- Langade, D.; Thakare, V.; Kanchi, S.; Kelgane, S. Clinical evaluation of the pharmacological impact of ashwagandha root extract on sleep in healthy volunteers and insomnia patients: A double-blind, randomized, parallel-group, placebo-controlled study. J. Ethnopharmacol. 2021, 264, 113276. [Google Scholar] [CrossRef]
- Murthy, S.V.; Fathima, S.N.; Mote, R. Hydroalcoholic Extract of Ashwagandha Improves Sleep by Modulating GABA/Histamine Receptors and EEG Slow-Wave Pattern in In Vitro—In Vivo Experimental Models. Prev. Nutr. Food Sci. 2022, 27, 108–120. [Google Scholar] [CrossRef]
- Mehta, A.K.; Binkley, P.; Gandhi, S.S.; Ticku, M.K. Pharmacological effects of Withania somnifera root extract on GABAA receptor complex. Indian J. Med. Res. 1991, 8, 312–315. [Google Scholar] [CrossRef]
- Candelario, M.; Cuellar, E.; Reyes-Ruiz, J.M.; Darabedian, N.; Feimeng, Z.; Miledi, R.; Russo-Neustadt, A.; Limon, A. Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABAA and GABAρ receptors. J. Ethnopharmacol. 2015, 171, 264–272. [Google Scholar] [CrossRef]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on stress and the stressrelated neuropsychiatric disorders anxiety, depression, and insomnia. Curr. Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of Ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress through Treatment with Ashwagandha Root Extract: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Evid.-Based Complement. Altern. Med. 2017, 22, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-blind, Randomized, Placebo-controlled Clinical Study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R.; Wane, D.; Chandrasekhar, K.; Kapoor, J.; Anishetty, S.; Chengappa, K.N.R.; Bowie, C.R.; et al. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2019, 13, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Auddy, B.; Hazra, J.; Mitra, A.; Abedon, B.; Ghosal, S. A Standardized Withania somnifera Extract Significantly Reduces Stress-Related Parameters in Chronically Stressed Humans: A Double-Blind, Randomized, Placebo-Controlled Study. JANA 2008, 11, 51–57. [Google Scholar]
- Remenapp, A.; Coyle, K.; Orange, T.; Lynch, T.; Hooper, D.; Hooper, S.; Conway, K.; Hausenblas, H.A. Efficacy of Withania somnifera supplementation on adult’s cognition and mood. J. Ayurveda Integr. Med. 2022, 13, 100510. [Google Scholar] [CrossRef] [PubMed]
- Gopukumar, K.; Thanawala, S.; Somepalli, V.; Rao, T.S.S.; Thamatam, V.B.; Chauhan, S. Efficacy and Safety of Ashwagandha Root Extract on Cognitive Functions in Healthy, Stressed Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Evidence-based Complement. Altern. Med. 2021, 2021, 8254344. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Drummond, P.D.; Smith, S.J. A Randomized, Double-Blind, Placebo-Controlled, Crossover Study Examining the Hormonal and Vitality Effects of Ashwagandha (Withania somnifera) in Aging, Overweight Males. Am. J. Mens. Health 2019, 13, 1557988319835985. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Rajender, S.; Shankhwar, S.N.; Singh, V.; Dalela, D. Withania somnifera improves semen quality in stress-related male fertility. Evid.-Based Complement. Altern. Med. 2011, 2011, 576962. [Google Scholar] [CrossRef]
- Tandon, N.; Yadav, S.S. Safety and clinical effectiveness of Withania somnifera (Linn.) Dunal root in human ailments. J. Ethnopharmacol. 2020, 255, 112768. [Google Scholar] [CrossRef]
- Berger, I.; Werdermann, M.; Bornstein, S.R.; Steenblock, C. The adrenal gland in stress—Adaptation on a cellular level. J. Steroid Biochem. Mol. Biol. 2019, 190, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Stag, M.; Bozsik, M.; Karow, C.; Wertz, D.; Kloehn, I.; Pillai, S.; Gasser, P.J.; Gilmartin, M.R.; Evans, J.A. Chronic stress alters adrenal clock function in a sexually dimorphic manner. J. Mol. Endocrinol. 2018, 60, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Debono, M. Review: Replication of cortisol circadian rhythm: New advances in hydrocortisone replacement therapy. Ther. Adv. Endocrinol. Metab. 2010, 1, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Ghobadi, C.; Rostami-Hodjegan, A.; Huatan, H.; Campbell, M.J.; Newell-Price, J.; Darzy, K.; Merke, D.P.; Arlt, W.; Ross, R.J. Modified-Release Hydrocortisone to Provide Circadian Cortisol Profiles. J. Clin. Endocrinol. Metab. 2009, 94, 1548–1554. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Frank, F.; Ortlund, E.A.; Liu, X. Structural insights into glucocorticoid receptor function. Biochem. Soc. Trans. 2021, 49, 2333–2343. [Google Scholar] [CrossRef]
- Cato, A.C.B.; Nestl, A.; Mink, S. Rapid Actions of Steroid Receptors in Cellular Signaling Pathways. Sci. STKE 2002, 2002, re9. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in psychiatric disorders: What comes first? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Hu, F.; Miller, A.H. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain. Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry 2003, 160, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, H.K.; Björnsson, E.S.; Avula, B.; Khan, I.A.; Jonasson, J.G.; Ghabril, M.; Hayashi, P.H.; Navarro, V. Ashwagandha-induced liver injury: A case series from Iceland and the US Drug-Induced Liver Injury Network. Liver Int. 2020, 40, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Ahamed, R.; Rajesh, S.; George, T.; Mohanan, M.; Augustine, P. Comprehensive review of hepatotoxicity associated with traditional Indian Ayurvedic herbs. World J. Hepatol. 2020, 12, 574–595. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.I.; Patel, K.; Brdak, A.; Heffernan, J.; Ahmad, N. Ashwagandha as a Unique Cause of Thyrotoxicosis Presenting with Supraventricular Tachycardia. Cureus 2022, 14, e23494. [Google Scholar] [CrossRef]
- Fry, C.H.; Fluck, D.; Han, T.S. Adrenal hypofunction associated with ashwagandha (Withania somnifera) supplementation: A case report. Toxicol. Environ. Health Sci. 2022, 14, 141–145. [Google Scholar] [CrossRef]
| Study | Number of Subjects * | Type of Study | Conditions | Type of Extract | Dose/ Posology | Treatment Duration (Days) | Effects | Comments |
|---|---|---|---|---|---|---|---|---|
| Study 1 Chandrasekhar et al. 2012 [34] | 61 (WS = 30; P = 31) | RCT DB parallel | Healthy subjects Higher level of stress (score WHO-5 < 15) | WS root extract full spectrum (5% withanolides) | 300 mg/b.i.d | 60 | ↓ Plasma cortisol level by 27.9% from baseline | |
| Study 2 Choudhary et al. 2017 [35] | 50 (WS = 25; P = 25) | RCT DB parallel | Healthy subjects with PSS ≥ 20 | WS root extract full spectrum (5% withanolides) | 300 mg/b.i.d | 56 | ↓ Plasma cortisol level by 22.7% from baseline | |
| Study 3 Salve et al. 2019 [36] | 58 (WS1 = 19; WS2 = 20; P = 20) | RCT DB parallel | Healthy subjects with PSS ≥ 20 | WS root extract full spectrum (5% withanolides) | 125 mg/b.i.d. WS1; 300 mg/b.i.d. WS2 | 56 | ↓ Plasma cortisol level by 16.5% and 32.63% from baseline, respectively, in WS1 and WS2 | Reduction in HAM-A only for WS2 treatment |
| Study 4 Mahdi et al. 2011 [42] | 121 (WS = 60; C = 60) | CT without P, parallel | Healthy men with 24 ≥ mHAM-A ≤ 42. NS men, NS infertile men; NS-stressed men; NS-smoker men | WS root powder | 5 g/die | 90 | ↓ Plasma cortisol level in infertile NS, NS-smokers, NS-stressed, respectively, by 11%, 28%, and 32% | |
| Study 5 Lopresti et al. 2019 a [37] | 60 (WS = 30; P = 30) | RCT DB parallel | Healthy subjects with 6 ≥ HAM-A ≤ 17 | WS leaves and roots ethanol/water 70:30 extract (35% withanolide glycosides) | 240 mg/die | 60 | ↓ Plasma cortisol level by 23.39% from baseline | ↑ testosterone and DHEA level, respectively, by 11% by 8% from baseline |
| Study 6 Lopresti et al. 2019 b [41] | 43 (WS = 23; P = 20) | RCT DB Cross-over | Healthy overweight subjects POMS above 50th percentiles | WS leaves and roots ethanol/water 70:30 extract (35% withanolide glycosides) | 60 mg/die | 112 (56 + 56) | No significant changes in salivary cortisol | ↑ salivary testosterone and DHEA level, respectively, by 14.7% and 18% |
| Study 7 Auddy 2008 et al. [38] | 98 (WS1 = 19; WS2 = 30; WS3 = 34; P = 15) | RCT DB parallel | Healthy subjects with 24 ≥ mHAM-A ≤ 42 | WS leaves and roots water extract (11.90% withanolide glycosides; 1.05% withaferin A; 40.25% oligosaccharides) | 125 mg/q. d. WS1; 125 mg/b.i.d. WS2; 250 mg/b.i.d. WS3 | 60 | ↓ Plasma cortisol level by 14.5%, 24.2 and 30.5 from baseline, respectively, in WS1, WS2 and WS3 | ↑ serum DHEA and decrease in CRP; FBG; and TC |
| Study 8 Remenapp 2021 et al. [39] | 57 (WS1 = 19; WS2 = 19; P = 19) | RCT DB parallel | Healthy subjects with PSS ≥ 14 | WS leaves and root extract (3.5% withanolides) | 225 mg/die WS1; 400 mg/die WS2 | 30 | ↓ salivary cortisol only for WS1 group | Improvement of cognitive ability |
| Study 9 Gopukumar 2021 et al. [40] | 125 (WS = 62; P = 63) | RCT DB parallel | Healthy subjects with 14 ≥ PSS ≥ 24 | WS Butanol/water extract (5% withanolides) | 300 mg/die | 90 | ↓ Plasma cortisol level by 29.87% from baseline | No change in serum BDNF |
| Study | Time of Cortisol Measurement | Time of WS Administration |
|---|---|---|
| 1 [34] | Morning, not specified | 2 times a day, after food, not specified |
| 2 [35] | Not specified | No controlled |
| 3 [36] | Morning, not specified | 2 times a day, after food, not specified |
| 4 [42] | 8.00 a.m. | Not specified |
| 5 [37] | Morning, not specified | After dinner, not specified. |
| 6 [41] | 6.00–8.00 a.m. | After dinner, not specified. |
| 7 [38] | 9.00–11.00 a.m. | Before lunch, not specified |
| 8 [39] | Morning not specified. | No controlled |
| 9 [40] | 9.00–11.00 a.m. | After breakfast, not specified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Porta, M.; Maier, J.A.; Cazzola, R. Effects of Withania somnifera on Cortisol Levels in Stressed Human Subjects: A Systematic Review. Nutrients 2023, 15, 5015. https://doi.org/10.3390/nu15245015
Della Porta M, Maier JA, Cazzola R. Effects of Withania somnifera on Cortisol Levels in Stressed Human Subjects: A Systematic Review. Nutrients. 2023; 15(24):5015. https://doi.org/10.3390/nu15245015
Chicago/Turabian StyleDella Porta, Matteo, Jeanette A. Maier, and Roberta Cazzola. 2023. "Effects of Withania somnifera on Cortisol Levels in Stressed Human Subjects: A Systematic Review" Nutrients 15, no. 24: 5015. https://doi.org/10.3390/nu15245015
APA StyleDella Porta, M., Maier, J. A., & Cazzola, R. (2023). Effects of Withania somnifera on Cortisol Levels in Stressed Human Subjects: A Systematic Review. Nutrients, 15(24), 5015. https://doi.org/10.3390/nu15245015








