Abstract
Hispanics of Mexican descent have disproportionate rates of non-alcoholic fatty liver disease (NAFLD). The purpose of this work is to investigate the association between the traditional Mexican diet score (tMexS) and hepatic steatosis and fibrosis, two NAFLD-related clinical endpoints, in Hispanic adults of Mexican descent. Data from 280 Hispanic adults of Mexican descent (n = 102 men, 178 women) with overweight or obesity enrolled in a cross-sectional observational study were analyzed. The tMexS was calculated from 24 h dietary recalls. Hepatic steatosis and fibrosis measurements were assessed using transient elastography (Fibroscan®). Linear regression models testing the association between tMexS and hepatic steatosis and fibrosis were run individually and through the stratification of significant modifiers. Mean tMexS were 5.9 ± 2.1, hepatic steatosis scores were 288.9 ± 48.9 dB/m, and fibrosis scores were 5.6 ± 2.2 kPa. Among the US-born group, with every point increase in the tMexS, there was a statistically significant 5.7 lower hepatic steatosis point (95% CI: −10.9, −0.6, p-value = 0.07). Higher adherence to a traditional Mexican diet was associated with lower hepatic steatosis in US-born Hispanics of Mexican descent. Findings from the current work may serve to inform future culturally relevant interventions for NAFLD prevention and management in individuals of Mexican descent.
Keywords:
lifestyle; nutrition; liver disease; cancer risk; country of origin; health disparities; Latinos 1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a known risk factor for hepatocellular carcinoma (HCC) [1,2], and its estimated prevalence among Hispanics is 63.7% [3]. These rates are well above those for other racial populations with rates for individuals of Mexican descent being particularly higher than for other Hispanic ethnic heritages [4,5]. NAFLD is on the trajectory to become the fastest growing risk factor for HCC in Hispanics residing in both the US and Mexico [6,7,8,9]. According to the American Association for the Study of Liver Diseases, modification of lifestyle behaviors, including the adoption of healthy eating patterns, is the cornerstone treatment for NAFLD [10,11]. However, there is a paucity of research evaluating the relationship between diet patterns and NAFLD risk or related risk factors and almost none in this high-risk group.
Dietary composition modulates the pathogenesis and trajectory of NAFLD [12,13]. A high intake of caloric-dense diets, particularly those rich in total fat and added sugars, has been implicated in the development and exacerbation of NAFLD [14]. Diets high in saturated and trans fats can contribute to lipid accumulation in the liver, leading to hepatic steatosis, a hallmark of NAFLD [13,15]. Furthermore, the excessive intake of sugars, especially fructose, has been associated with insulin resistance and lipogenesis in the liver [16,17]. In addition to macronutrients, oxidative stress of nutritional origin plays a crucial role in NAFLD [18,19]. The excessive intake of pro-oxidant nutrients, in combination with limited antioxidant defenses, leads to an imbalance and the generation of reactive oxygen species [18]. This oxidative stress contributes to liver inflammation and injury, further promoting the progression of NAFLD to more severe forms, such as non-alcoholic steatohepatitis (NASH) and fibrosis [18,19,20]. Among the behavior-related risk factors that place Hispanics of Mexican descent at greater risk for NAFLD are the high reported intakes of sugar-sweetened beverages and processed meat, which have been shown to increase the risk of developing this chronic condition [21,22,23,24]. However, there is limited research evaluating the relationship between eating patterns and NAFLD in this population despite the significantly elevated incidence of NAFLD and HCC burden. Studies among predominantly non-Hispanic White (NHW) populations support the premise that dietary patterns are a viable target for interventions leading to a reduction in NAFLD [25,26]. Previously, the Dietary Approach to Stop Hypertension (DASH) diet pattern was reported to significantly improve liver enzymes [26], a clinical measure suggestive of ongoing liver disease [6,27,28]. Likewise, robust literature supports the effectiveness of the Mediterranean diet pattern on improving hepatic steatosis and enzymes independent of weight loss [25,29]. Notably, in clinical practice, this dietary pattern has also been found to be the predominant diet recommended by primary care physicians to NAFLD patients [29]. However, potential barriers to consuming a Mediterranean-like diet pattern for Hispanics of Mexican descent have been documented [30], raising questions about the cultural relevance of recommending this diet for this population.
Studies examining more culturally relevant dietary patterns and the impact on any liver-related outcome in Hispanics of Mexican descent are sparse. One randomized, crossover feeding trial found that after 24 days a traditional Mexican dietary pattern intervention significantly improved insulin sensitivity among women of Mexican descent [31]. Epidemiological studies suggest that a higher adherence to a traditional Mexican diet is associated with favorable health outcomes including a reduced risk of obesity [32], obesity-related cancer mortality [33], and key metabolic components associated with NAFLD risk and cancer such as prediabetes [34], inflammation, and insulin resistance [31,35,36]. However, to our knowledge, no research to date has examined the association of this culturally relevant diet pattern and the pathological features of NAFLD in Hispanics of Mexican descent.
The purpose of this study was to examine the association between adherence to a traditional Mexican diet, assessed using the traditional Mexican diet score (tMexS), and hepatic steatosis and fibrosis in a sample of 280 Hispanic adults of Mexican descent with overweight and obesity. Further, we sought to evaluate the potential effect modification by multiple characteristics including sex [37], birthplace [6], and PNPLA3 risk allele carrier status [38,39], factors previously identified to modify the relationship between diet and NAFLD and its risk factors. Findings from this work will contribute to the body of evidence guiding the development of future dietary interventions for NAFLD prevention and clinical management in this high-risk population.
2. Methods and Materials
2.1. Study Sample
This secondary analysis is an ancillary study to an Institutional Review Board (IRB)-approved observational cross-sectional study (IRB #1902380787; 11 May 2021) conducted among men and women of Mexican descent living in Southern Arizona. The details of the primary study have been previously summarized elsewhere [40]. Briefly, to be eligible for the primary study, individuals needed to self-identify as Mexican or from Mexican descent, be aged 18 to 64 years old, and have a body mass index (BMI) ≥25 kg/m2. Participants were excluded if they self-reported a previous diagnosis of liver disease or exceeded alcohol consumption of 21 standard drinks per week for men and 14 drinks per week for women [41]. Alcohol intake is a known independent risk factor for liver disease, and the inclusion of individuals in the study with excessive intake could introduce confounding variables, thereby confounding the nuanced interactions between other dietary factors and liver disease [42,43]. As part of the primary study, participants attended one study visit that took place in a clinic located in Tucson, Arizona, that specializes in the treatment of liver disease. Trained bilingual and bicultural study staff and graduate research assistants collected all study data. All study visits were conducted in the preferred language of each participant (English or Spanish) and, at the end of the study visit, participants were compensated USD 25.00 for their time.
2.2. Dietary Intake Assessment
Dietary intake was assessed through 24 h dietary recalls using the USDA multiple-pass method [44]. These were conducted on three non-sequential days of the week, two weekdays and one weekend day, during a period of two weeks after attending the clinical visit of the primary study. All participants in the current study sample completed all three dietary recalls and used a food amounts booklet provided to them to facilitate reporting of portions and amounts. Participants with an implausible energy intake below <500 and above >4000 were excluded from the analyses [35]. All 24 h recalls were administered by bilingual and bicultural trained personnel in the University of Arizona (UA) Cancer Center Behavioral Measurement and Interventions Shared Resource via telephone. Dietary data were processed using the Nutrition Data System for Research (NDSR-2019) [45]. Participants were compensated an additional USD 25.00 for completion of all three recalls, receiving a total of USD 50.00 if all study measures were completed.
2.3. Diet Pattern Scoring
The traditional Mexican diet pattern was previously identified from a historical review of food composition of traditional Mexican diets in Mexico and the US. It is composed of a mixture of Native Mesoamerican foods (pre-Hispanic) and Hispanic foods, characterized by high amounts of fruits, vegetables, complex carbohydrates, and corn-based dishes cooked with chilies, garlic, onions, herbs, beans, squash, citrus fruits, and rice [46]. From an extensive literature review, a traditional Mexican diet pattern has been converted into a composite score that can be calculated from food frequency data and 24 h dietary recalls [35,47]. The tMexS was based on methods by Santiago-Torres et al. [35] and adapted by Tamez et al. [47]. Briefly, the tMexS comprises 12 food group components (corn tortillas, beans and nuts, fruit, vegetables, rice, Mexican mixed dishes, poultry and eggs, fish, dairy products, red and processed meats, grains, and added sugars) that are categorized under traditional Mexican or US foods and altogether are computed to measure adherence to a traditional Mexican diet. Each component was created by adding the corresponding NDSR food subgroups using the average intake from the three dietary recalls. Each of the 12 components were scored using the sex-specific population median intake. A score of 0 was assigned to participants consuming below the median intake for traditional Mexican food components (corn tortillas, beans and nuts, fruit, vegetables, rice, Mexican mixed dishes, poultry and eggs, fish, and dairy products) or a score of 1 for participants consuming above the median intake. A reverse scoring was used for US food components (red and processed meats, refined grains other than rice, and added sugars). Possible scores for tMexS range from 0 to 12 with higher tMexS representing higher consumption of a more traditional Mexican diet pattern and less US foods. A summary of the scoring system is summarized in Supplementary Table S1.
2.4. Hepatic Steatosis and Fibrosis Assessment
Hepatic steatosis and fibrosis measurements were taken by a trained physician using transient elastography (TE) FibroScan® (Echosens, Paris, France). This method is non-invasive, fast, reliable, and reproducible, with good intra- and interobserver levels of agreement [48] and has been validated against MRI, which is the imaging gold standard for liver disease [48,49]. TE measures hepatic fat infiltration as controlled attenuation parameter (CAP) scores and hepatic fibrosis expressed as kilopascals (kPa). Participants were asked to refrain from food and beverages, with the exception of water and black coffee or tea, for at least three hours before their assessment. A minimum interquartile range CAP score of 30 dB/m was established for each of the ten measurements taken per participant to establish score accuracy and quality assurance [50,51]. At the end of the primary study visit, each participant received a summary of their results and had the opportunity to review their results with a bilingual and bicultural physician or healthcare professional.
2.5. Covariate Assessment
As part of the primary study, participants were asked to complete questionnaires assessing key demographic, socioeconomic, sociocultural, health, behavior, and psychosocial characteristics. Data from these self-reported questionnaires were utilized as covariates to adjust the linear regression models. Anthropometric assessment was conducted by trained staff or graduate research assistant according to standard protocols [52]. Briefly, participant body weight was assessed with street clothing, without shoes, and on a calibrated scale to the nearest 0.1 kg (Tanita WB-100A). Height was assessed to the nearest 0.1 cm using a stadiometer. The measurements for body weight and height were utilized to calculate the participant’s BMI. Waist circumference was measured at the umbilicus using a Gulick measuring tape also recorded to the nearest 0.1 cm.
Two buccal swabs (Whatman, Maidstone, UK, OmniSwab) were collected from each participant to isolate genomic DNA to determine genotype status at the patatin-like phospholipase domain containing 3 (PNPLA3) gene (rs738409), given that this risk allele is highly prevalent in individuals of Mexican descent and associated with NAFLD risk and progression [53]. The UA Genetics Core isolated genomic DNA from buccal swabs, quantified it, and utilized it as a template in a TaqMan® SNP Genotyping assay. This assay aimed to identify the genotype at the rs738409 SNP situated in codon 148 of PNPLA3. PNPLA3 rs738409 genotype was used to define risk allele carrier status, with individuals categorized as CC, CG, and GG genotypes, corresponding to 0, 1, or 2 risk alleles.
First, unadjusted models were run, followed by models adjusted for literature-derived covariates (age, sex, BMI, energy intake, and leisure physical activity) (Model 1). Other relevant covariates were tested for substantial effect on model estimates (10% change). These included language spoken at home, income, birthplace, education, sedentary time, and PNPLA3 risk allele (G) carrier status. Covariates with substantial effects on the model were language, birthplace, generation status, education, and PNPLA3 risk allele carrier status. However, to prevent over-adjusting, a correlation analysis was conducted and the final covariates in Model 2 included covariates in Model 1 in addition to birthplace, education, and PNPLA3 carrier status.
2.6. Statistical Analysis
Two-sample t-tests were used to test baseline differences in demographic and clinical characteristics across sex and birthplace and to calculate p-values for continuous variables including hepatic steatosis and fibrosis, age, and tMexS. For categorical variables, such as health insurance, education, language spoken at home, BMI category, PNPLA3 risk allele carrier status, diabetes history, and cancer history, chi-squared tests were used. For variables that were non-normally distributed (BMI, energy intake, leisure activity, and sedentary time), Wilcoxon rank-sum tests were performed to test median differences across groups.
Main outcome variables, hepatic steatosis and fibrosis, were examined as continuous variables in linear regression analysis adjusted for covariates in different models. Effect modification was tested using an interaction term for sex, BMI, birthplace, language, education, income, and PNPLA3 risk allele carrier status, individually. Linear regression models were then run, stratifying by factors that had a significant interaction. As an exploratory analysis, median consumption for each of the 12 tMexS components was examined and tested across groups using the Wilcoxon rank-sum test. All analyses were conducted using STATA/IC version 15.1 (StataCorp, College Station, TX, USA).
3. Results
3.1. Sample Characteristics
A total of 280 Hispanic adults of Mexican descent in Southern Arizona with overweight or obesity were included in this secondary analysis. There were a total of 102 men and 178 women with a mean overall age of 44.8 ± 11.1 years old. Mean hepatic steatosis was 288.9 ± 48.9 dB/m, for which a CAP score of 288 dB/m is equivalent to 5% hepatic steatosis and indicative of NAFLD [51]. Mean hepatic fibrosis was 5.6 ± 2.2 kPa. Participant characteristics by sex and birthplace are summarized in Table 1. When comparing participant characteristics by sex, women were statistically significantly older (p-value = 0.01) with a higher BMI (p-value = 0.04) and reported consuming significantly less calories (p-value < 0.001) compared to men. When further comparing participants by birthplace, both men and women born in Mexico compared to their US-born counterparts had a lower proportion of individuals with health insurance (men: p-value = 0.03, women: p-value < 0.001), and a higher percentage spoke Spanish at home (men: p-value < 0.001, women: p-value < 0.001), had a lower mean BMI (men: p-value = 0.04, women: p-value = 0.003), and had higher tMexS (men: p-value = 0.01, women: p-value < 0.001). Additionally, US-born men were statistically significantly older then Mexican-born men (p-value < 0.001) and US-born women self-reported higher formal education (p-value < 0.001) and higher sedentary time compared to Mexican-born women (p-value < 0.001).

Table 1.
Baseline characteristics of Hispanic adults of Mexican descent in Southern Arizona (N = 280) by sex and birthplace.
3.2. Diet Scores and Liver Steatosis and Fibrosis
Mean tMexS in the study sample were 5.9 ± 2.1. Individual means by sex and birthplace are summarized in Table 1. Overall, US-born men had the lowest tMexS (5.0 ± 2.1), and Mexico-born women had highest scores (6.3 ± 2.0).
Linear regression analyses between tMexS and hepatic steatosis and fibrosis showed no significant associations for any of the models (Table 2). The results showed an inverse association between tMexS and steatosis across all models, though these were not statistically significant.

Table 2.
Linear regression analysis between the traditional Mexican diet Score (tMexS) and hepatic steatosis (CAP, dB/m) and fibrosis (kPa) in a sample of Hispanic adults of Mexican descent in Southern Arizona (N = 280).
Effect modifications between tMexS and hepatic steatosis and fibrosis by sex, BMI, birthplace, language spoken at home, education, income, and PNPLA3 risk allele carrier status were examined. The results showed that sex modified the relationship between tMexS and hepatic steatosis (P-interaction = 0.04), and fibrosis (P-interaction = 0.04). Birthplace (P-interaction = 0.02) and PNPLA3 risk allele carrier status (P-interaction = 0.04) modified the relationship between tMexS and hepatic steatosis but not fibrosis.
Analyses stratified by birthplace showed a statistically significant relationship between tMexS and hepatic steatosis only among the US-born group (Table 3). With every point increase in tMexS, there was a concurrent 5.7-point decrease in hepatic steatosis scores (95% CI: −10.9, −0.6, p-value = 0.03). No other significant findings were observed among the subgroups for liver steatosis or fibrosis.

Table 3.
Relationship between the Traditional Mexican diet Score (tMexS) and hepatic steatosis score (CAP, dB/m) and fibrosis (kPa) stratified by sex, birthplace, and genetic risk allele status in a sample of Hispanic adults of Mexican descent in Southern Arizona (N = 280).
Median intakes and interquartile ranges of each tMexS component by birthplace are summarized in Figure 1. Compared to the US-born group, the Mexico-born group had higher median intakes of corn tortilla (p-value < 0.001), whole fruits (p-value < 0.001), fish and shellfish (p-value = 0.01), and Mexican mixed dishes (p-value = 0.002) and a lower median consumption of refined grains (other than rice) (p-value = 0.02). No other statistically significant differences in median intake values were observed.
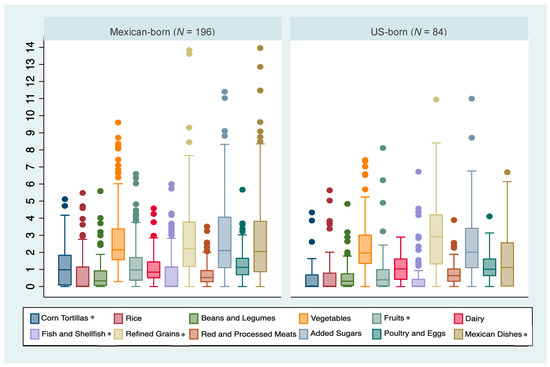
Figure 1.
Distribution of servings for each Traditional Mexican Diet (tMexS) score category by birthplace in a sample of Hispanic adults of Mexican descent in Southern Arizona (n = 280). * Indicates statistically significant differences across birthplace groups at p-value < 0.05.
4. Discussion
The current study investigated the relationship between a culturally relevant diet score, the tMexS, which measures adherence to a traditional Mexican diet, and hepatic steatosis and fibrosis in a sample of Hispanic adults of Mexican descent with overweight or obesity living in Southern Arizona. The results demonstrate a non-significant inverse association between the tMexS and hepatic steatosis in the total study sample. When investigating the potential effect modification, sex modified the association between tMexS and hepatic steatosis and fibrosis, while birthplace and PNPLA3 risk allele carrier status modified the relationship between tMexS and hepatic steatosis only. Stratified analyses showed a statistically significant relationship between tMexS and hepatic steatosis only for US-born men. To our knowledge, this is the first study to investigate the relationship between a diet pattern score and hepatic steatosis and fibrosis, two clinical endpoints of NAFLD, in a sample of Hispanic adults of Mexican descent, a population consistently shown to have some of the highest rates of this disease.
The mean tMexS in the current analysis are comparable to those previously reported by Tamez et al. [47] and Santiago-Torres and colleagues [35] with mean scores in the current study being slightly higher. In the work by Tamez and colleagues including data from 3542 Mexican heritage individuals from the Hispanic Community Health Study/Study of Latinos, the mean traditional Mexican diet score was 5.8 ± 0.05. In the work by Santiago-Torres and colleagues, which utilized data from 493 postmenopausal women of Mexican decent, the mean traditional Mexican diet scores were 5.8 ± 2.1. In the current study, mean scores were 5.9 ± 2.1 in the overall sample and 6.3 ± 2.0 among Mexican-born women. Scores in our study, particularly those for women, may be slightly higher than those in prior work due to the geographical location of the study participants, which is in close proximity to Mexico and in a highly dense Hispanic–Mexican community. Regarding hepatic steatosis and fibrosis, the results are comparable to those reported in a study of the Cameron County Hispanic Cohort by Watt and colleagues [54]. In their study of Mexican American Hispanics, men and women had a median hepatic steatosis score of 306 dB/m and 277 dB/m, respectively, comparable to the men (291.0 dB/m) and women (287.6 dB/m) in the current study. It should be noted that Watt et al. [55] presented medians versus means; therefore, direct comparisons may be hindered.
The literature on diet and NAFLD for Hispanic individuals is sparse and further limited for Hispanics of Mexican descent, making the direct comparison of results challenging. The majority of studies have evaluated dietary patterns like the DASH pattern [56,57,58], Mediterranean pattern [25,57,59,60,61], or variations of the Healthy Eating Index [56,59], in predominantly NHW populations [25,59,60,61,62], with few among diverse populations [56,57,58]. In these studies, NAFLD-related outcomes have varied with outcomes including liver fat [25,59], liver damage [60], liver enzymes [26,60], and odds or the risk for NAFLD [56,58,61,62]. Consistent with previous research [37,63], the mean hepatic steatosis was higher among men compared to women. There was a stronger relationship between tMexS and hepatic steatosis among men, although these results were not statistically significant, possibly due to sample size. Birthplace significantly modified the relationship between tMexS and hepatic steatosis. Consistently, the literature highlights differences in the prevalence and presence of NAFLD risk factors for individuals born in Mexico versus the US [6,64,65,66]. One study by Flores and colleagues compared the prevalence of chronic liver disease risk factors in a sample of Mexican individuals born and residing in Mexico vs. the US [6]. Findings from this work indicate that US-born Mexicans have a 1.4 (1.1–1.9) higher odds of having metabolic syndrome, 3.0 (1.9–4.8) higher odds of having diabetes, 3.9 (3.1–4.9) higher odds for obesity, and 5.4 (2.9–10.1) higher odds of having abdominal obesity, compared to Mexico-born individuals. Similar to the current study, US-born individuals had statistically significant higher BMIs compared to their Mexico-born counterparts. Interestingly, our results indicate that US-born individuals of Mexican descent may benefit more from following a more traditional Mexican diet than their Mexican-born counterparts. A potential explanation for this may be that the adoption of healthier diet habits may lead to better health outcomes, especially for individuals who start off with poorer eating habits [67]. For example, a study by Grafenauer et al. demonstrated that individuals who started off with unhealthier diet habits benefitted more from weight loss interventions as compared to those who started with healthier food choices [68]. Similarly, in the current study, it may be that adopting a traditional Mexican diet pattern may be particularly beneficial for US-born individuals who may have unhealthy dietary habits reflective of higher acculturation as compared to healthier dietary habits for less acculturated, Mexico-born individuals. However, more research is needed to better understand how and why this may occur.
In the current analysis, a significant interaction was found for PNPLA3 rs738409 risk allele carrier status in that it modified the relationship between tMexS and hepatic steatosis, although no significant relationship was observed once stratification analyses were conducted. The rs738409 (C > G) single-nucleotide polymorphism has been shown to increase the susceptibility and severity of NAFLD [53]. Our results are consistent with those in the literature who have found that PNPLA3 risk allele carrier status modified the relationship between certain dietary factors and hepatic steatosis [38,69]. Although precision nutrition in the clinical setting remains a theoretical practice, reports indicate approximately 75,000 genetic tests currently on the market with around 10 new ones entering the market daily [70]. The current findings emphasize the viability of integrating a culturally pertinent dietary paradigm as a prospective approach to reduce the incidence of NAFLD and HCC among US-born Hispanic individuals. Additionally, these results may catalyze the development of culturally targeted lifestyle interventions and dietary recommendations, potentially heightening the overall interest and engagement of individuals in seeking consultations with dietitians.
While national dietary guidelines for the treatment of NAFLD do not currently exist, the EASL-EASD-EASO Clinical Practice Guidelines have recommended the Mediterranean diet as the diet of choice for treatment of NAFLD [29]. The Mediterranean diet has been found to be among the most effective diets to improve cardio-metabolic factors associated with NAFLD and to induce weight loss [71], the most effective way to reduce hepatic steatosis and improve histopathological features of NASH [11]. However, barriers to engage in such dietary modifications and long-term weight loss maintenance have been difficult among Hispanic patients for a variety of reasons [30,72]. First, the cultural relevance and overall acceptability of the Mediterranean diet among Hispanics may be questionable. A study by Estrada Del Campo and colleagues reported that Hispanic American women who engaged in a Mediterranean diet intervention aimed at reducing cardiometabolic factors reported that they or their families disliked the suggested foods or that the cost of the suggested foods was too high [30]. A lack of cultural relevance of lifestyle programs and dietary recommendations may also have an impact on the overall interest of patients in procuring dietician consults. A study by Saeed et al. reported that provider referral to dietetic services for the management of NAFLD was hindered by several factors, including patients’ overall low interest in acquiring such services and the availability of dieticians and lifestyle programs [73]. In this regard, a traditional Mexican diet poses a promising dietary strategy for NAFLD prevention and management while sustaining traditional and familiar dietary choices and thus addressing acceptability concerns observed for other dietary recommendations such as with the Mediterranean diet.
A traditional Mexican diet holds promise in the prevention and management of NAFLD through the modulation of various molecular pathways. One potential mechanism of action could be through the diet’s high fiber content, derived from fruits, vegetables, and whole grains, which may influence gut microbiota composition and function, thereby impacting short-chain fatty acid production [74]. These fatty acids, in turn, have been linked to anti-inflammatory and lipid-metabolism-modulating effects [75], potentially mitigating hepatic lipid accumulation and the inflammation characteristic of liver disease [76]. Furthermore, traditional Mexican foods contain bioactive compounds, such as polyphenols and flavonoids, which may exert antioxidant properties, counteracting oxidative stress implicated in NAFLD pathogenesis [20,77]. The diet’s well-balanced macronutrient profile of healthy fats from sources like avocados and omega-3 fatty acids from fish might contribute to lipid homeostasis that benefits hepatic steatosis [78]. The potential interplay of these molecular pathways underscores the scientific rationale supporting further research efforts to more granularly understand the preventive effects of a traditional Mexican diet on NAFLD.
Strengths and Limitations
Several strengths exist in the current study. First, this analysis addresses a significant gap in the literature given that the evidence on diet and NAFLD-related outcomes among Hispanics of Mexican descent is scarce. Second, we expand on the existing literature by including a culturally relevant dietary score for a population underrepresented in NAFLD research [79] despite their disproportionate burden of this disease. This work represents a steppingstone for building the necessary evidence for the implementation of culturally relevant dietary guidelines to address the NAFLD disparities observed for this group. However, future research should evaluate the effectiveness and clinical utility of current NAFLD management guidelines for this at-risk population to ensure that efforts to manage this condition are equitable and accessible to those who need them most. Lastly, the study sample in the current study was well characterized and allowed for the appropriate evaluation of covariates. While the strengths of this study are evident, limitations of the current work also exist. First, while dietary studies are crucial for understanding the impact of nutrition on various health outcomes, these face significant limitations due to the inherent challenges in accurately measuring individuals’ dietary exposures. The self-reported dietary intake assessment with 24 h dietary recalls is subject to recall and reporting biases, which may have affected the accuracy of our data. Additionally, the inclusion of only individuals with overweight or obesity hinders our ability to account for a wider range of exposures and interactions in the current analysis including the generalizability to “lean” NAFLD patients [80]. Future research should comprise a larger and more clinically diverse sample that includes normal-weight participants with a variety of body compositions to allow for adequately powered sub-analyses. For example, this would allow an evaluation of the relationship between a traditional Mexican diet and hepatic steatosis among US-born individuals that are not carriers of the PNPLA3 risk allele.
5. Conclusions
Results of this study indicate that the interplay of birthplace and the presence of the PNPLA3 risk allele in overweight or obese Hispanic adults of Mexican descent could influence the association between the consumption of a traditional Mexican diet and hepatic steatosis. This work contributes to the sparse body of literature exploring the relationship between dietary patterns and NAFLD outcomes in Hispanic adults of Mexican descent. Findings from the current study seek to guide the development of forthcoming culturally targeted interventions aimed at preventing and managing NAFLD within this specific population.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu15234997/s1: Supplementary Table S1. Description of the traditional Mexican diet score (tMexS).
Author Contributions
The authors confirm contributions to the paper as follows: study conception and design: M.L.-P., M.T., C.A.T. and D.O.G.; data collection: M.L.-P. and D.O.G.; analysis and interpretation of results: M.L.-P., M.T., J.M., E.T.J., C.A.T. and D.O.G.; draft manuscript preparation: M.L.-P. All authors reviewed the results and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research reported in this publication was supported by the National Cancer Institute under award number T32CA251064; the National Institute on Minority Health and Health Disparities of the National Institutes of Health (award numbers F31MD016283 and 1 K01 MD014761-01), the Harvard National Heart, Lung, Blood Institute Training Grant (T32 HL 098048); the University of Arizona Cancer Center’s Behavioral Measurements and Intervention Shared Resources (P30 CA023074); and the University of Arizona Health Sciences, Center for Health Disparities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by The University of Arizona Institutional Review Board (IRB #1902380787; 11 May 2021). Written informed consent was obtained from all participants involved in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
The authors thank the participants who participated in this study and all study staff and students that participated in the development, implementation, and data collection for the study.
Conflicts of Interest
The authors declare no potential conflict of interest.
References
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Lee, M.J.; Chang, H.S.; Bae, I.Y.; Kim, H.K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2017, 68, 140–146. [Google Scholar] [CrossRef]
- Dhamija, E.; Paul, S.B.; Kedia, S. Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: An increasing concern. Indian J. Med. Res. 2019, 149, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Heredia, N.I.; Balakrishnan, M.; Thrift, A.P. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: Results from NHANES 2017–2018. PLoS ONE 2021, 16, e0252164. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Manne, V.; Nieto, J.; Schwimmer, J.B.; Chalasani, N.P. Nonalcoholic Fatty Liver Disease in Latinos. Clin. Gastroenterol. Hepatol. 2016, 14, 5–12; quiz e9–e10. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Goding Sauer, A.; Ortiz, A.P.; Fedewa, S.A.; Pinheiro, P.S.; Tortolero-Luna, G.; Martinez-Tyson, D.; Jemal, A.; Siegel, R.L. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J. Clin. 2018, 68, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Flores, Y.N.; Zhang, Z.F.; Bastani, R.; Leng, M.; Crespi, C.M.; Ramirez-Palacios, P.; Stevens, H.; Salmeron, J. Risk factors for liver disease among adults of Mexican descent in the United States and Mexico. World J. Gastroenterol. 2018, 24, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, M.W.; Budoff, M.; Zeb, I.; Li, D.; Foster, T. NAFLD prevalence differs among hispanic subgroups: The Multi-Ethnic Study of Atherosclerosis. World J. Gastroenterol. 2014, 20, 4987–4993. [Google Scholar] [CrossRef]
- Kallwitz, E.R.; Daviglus, M.L.; Allison, M.A.; Emory, K.T.; Zhao, L.; Kuniholm, M.H.; Chen, J.; Gouskova, N.; Pirzada, A.; Talavera, G.A.; et al. Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/Latino individuals differs by heritage. Clin. Gastroenterol. Hepatol. 2015, 13, 569–576. [Google Scholar] [CrossRef]
- Streba, L.A.; Vere, C.C.; Rogoveanu, I.; Streba, C.T. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: An open question. World J. Gastroenterol. 2015, 21, 4103–4110. [Google Scholar] [CrossRef]
- Munteanu, M.A.; Nagy, G.A.; Mircea, P.A. Current Management of NAFLD. Med. Pharm. Rep. 2016, 89, 19–23. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Rauf, N.; Nabi, G.; Ullah, H.; Shen, Y.; Zhou, Y.D.; Fu, J. Role of Nutrition in the Pathogenesis and Prevention of Non-alcoholic Fatty Liver Disease: Recent Updates. Int. J. Biol. Sci. 2019, 15, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Martin, F.; Bugianesi, E.; Soria, B. Nutrition could prevent or promote non-alcoholic fatty liver disease: An opportunity for intervention. BMJ 2023, 383, e075179. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, M.; Neuschwander-Tetri, B.A. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterol. Hepatol. 2006, 2, 282–291. [Google Scholar]
- Spruss, A.; Bergheim, I. Dietary fructose and intestinal barrier: Potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2009, 20, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.; Yki-Jarvinen, H.; Hawkins, M. Out of the frying pan: Dietary saturated fat influences nonalcoholic fatty liver disease. J. Clin. Investig. 2017, 127, 454–456. [Google Scholar] [CrossRef]
- Hong, T.; Chen, Y.; Li, X.; Lu, Y. The Role and Mechanism of Oxidative Stress and Nuclear Receptors in the Development of NAFLD. Oxidative Med. Cell. Longev. 2021, 2021, 6889533. [Google Scholar] [CrossRef]
- Rives, C.; Fougerat, A.; Ellero-Simatos, S.; Loiseau, N.; Guillou, H.; Gamet-Payrastre, L.; Wahli, W. Oxidative Stress in NAFLD: Role of Nutrients and Food Contaminants. Biomolecules 2020, 10, 1702. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Li, Z.; Lam, C.W.K.; Xiao, Y.; Wu, Q.; Zhang, W. Consumption of Sugar-Sweetened Beverages Has a Dose-Dependent Effect on the Risk of Non-Alcoholic Fatty Liver Disease: An Updated Systematic Review and Dose-Response Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2192. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Zelber-Sagi, S.; Wilkens, L.R.; Porcel, J.; Boushey, C.J.; Le Marchand, L.; Rosen, H.R.; Setiawan, V.W. Diet Associations With Nonalcoholic Fatty Liver Disease in an Ethnically Diverse Population: The Multiethnic Cohort. Hepatology 2020, 71, 1940–1952. [Google Scholar] [CrossRef]
- Sharkey, J.R.; Johnson, C.M.; Dean, W.R. Nativity is associated with sugar-sweetened beverage and fast-food meal consumption among Mexican-origin women in Texas border colonias. Nutr. J. 2011, 10, 101. [Google Scholar] [CrossRef]
- Ogden, C.L.; Kit, B.K.; Carroll, M.D.; Park, S. Consumption of Sugar Drinks in the United States, 2005–2008. In NCHS Data Brief; NCH: Highlandsville, MD, USA, 2011; pp. 1–8. [Google Scholar]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Razavi Zade, M.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2016, 36, 563–571. [Google Scholar] [CrossRef]
- Alazmi, W.M.; Regev, A.; Molina, E.G.; Schiff, E.R. Predictors of cirrhosis in Hispanic patients with nonalcoholic steatohepatitis. Dig. Dis. Sci. 2006, 51, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, D.; Mukherjee, P.; Raychaudhuri, M.; Ghosh, S.; Mukherjee, S.; Chowdhury, S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J. Endocrinol. Metab. 2015, 19, 597–601. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949. [Google Scholar] [CrossRef]
- Estrada Del Campo, Y.; Cubillos, L.; Vu, M.B.; Aguirre, A.; Reuland, D.S.; Keyserling, T.C. Feasibility and acceptability of a Mediterranean-style diet intervention to reduce cardiovascular risk for low income Hispanic American women. Ethn. Health 2019, 24, 415–431. [Google Scholar] [CrossRef]
- Santiago-Torres, M.; Kratz, M.; Lampe, J.W.; De Dieu, T.J.; Breymeyer, K.L.; Levy, L.; Villasenor, A.; Wang, C.Y.; Song, X.; Neuhouser, M.L. Metabolic responses to a traditional Mexican diet compared with a commonly consumed US diet in women of Mexican descent: A randomized crossover feeding trial. Am. J. Clin. Nutr. 2016, 103, 366–374. [Google Scholar] [CrossRef]
- Flores, M.; Macias, N.; Rivera, M.; Lozada, A.; Barquera, S.; Rivera-Dommarco, J.; Tucker, K.L. Dietary patterns in Mexican adults are associated with risk of being overweight or obese. J. Nutr. 2010, 140, 1869–1873. [Google Scholar] [CrossRef]
- Lopez-Pentecost, M.; Crane, T.E.; Garcia, D.O.; Kohler, L.N.; Wertheim, B.C.; Hebert, J.R.; Steck, S.E.; Shivappa, N.; Santiago-Torres, M.; Neuhouser, M.L.; et al. Role of dietary patterns and acculturation in cancer risk and mortality among postmenopausal Hispanic women: Results from the Women’s Health Initiative (WHI). J. Public Health 2020, 30, 811–822. [Google Scholar] [CrossRef]
- Robles-Ordaz, M.D.; Gallegos-Aguilar, A.C.; Urquidez-Romero, R.; Diaz-Zavala, R.G.; Lavandera-Torres, M.G.; Esparza-Romero, J. Prevalence of prediabetes and modifiable factors in an ethnic group of Mexico: The Comcaac Project. Public Health Nutr. 2018, 21, 333–338. [Google Scholar] [CrossRef]
- Santiago-Torres, M.; Tinker, L.F.; Allison, M.A.; Breymeyer, K.L.; Garcia, L.; Kroenke, C.H.; Lampe, J.W.; Shikany, J.M.; Van Horn, L.; Neuhouser, M.L. Development and Use of a Traditional Mexican Diet Score in Relation to Systemic Inflammation and Insulin Resistance among Women of Mexican Descent. J. Nutr. 2015, 145, 2732–2740. [Google Scholar] [CrossRef]
- Valerino-Perea, S.; Armstrong, M.E.G.; Papadaki, A. Adherence to a traditional Mexican diet and non-communicable disease-related outcomes: Secondary data analysis of the cross-sectional Mexican National Health and Nutrition Survey. Br. J. Nutr. 2022, 129, 1266–1279. [Google Scholar] [CrossRef]
- Pan, J.J.; Fallon, M.B. Gender and racial differences in nonalcoholic fatty liver disease. World J. Hepatol. 2014, 6, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Valenti, L. A Nutrigenomic Approach to Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2017, 18, 1534. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Le, K.A.; Walker, R.W.; Vikman, S.; Spruijt-Metz, D.; Weigensberg, M.J.; Allayee, H.; Goran, M.I. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am. J. Clin. Nutr. 2010, 92, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.O.; Morrill, K.E.; Lopez-Pentecost, M.; Villavicencio, E.A.; Vogel, R.M.; Bell, M.L.; Klimentidis, Y.C.; Marrero, D.G.; Thomson, C.A. Nonalcoholic Fatty Liver Disease and Associated Risk Factors in a Community-Based Sample of Mexican-Origin Adults. Hepatol. Commun. 2022, 6, 1322–1335. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Choi, J.H.; Sohn, W.; Cho, Y.K. The effect of moderate alcohol drinking in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2020, 26, 662–669. [Google Scholar] [CrossRef]
- Roerecke, M.; Vafaei, A.; Hasan, O.S.M.; Chrystoja, B.R.; Cruz, M.; Lee, R.; Neuman, M.G.; Rehm, J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1574–1586. [Google Scholar] [CrossRef]
- Blanton, C.A.; Moshfegh, A.J.; Baer, D.J.; Kretsch, M.J. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J. Nutr. 2006, 136, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Schakel, S.F.; Buzzard, I.; Gebhardt, S.E. Procedures for Estimating Nutrient Values for Food Composition Databases. J. Food Compos. Anal. 1997, 10, 102–114. [Google Scholar] [CrossRef]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Tamez, M. A Traditional Mexican Diet Score, Diet Quality Scores, and Risk of Hypertension Among U.S. Adults of Mexican Heritage. Ph.D. Thesis, Harvard T.H. Chan School of Public Health, Boston, MA, USA, 2020. [Google Scholar]
- Mikolasevic, I.; Orlic, L.; Franjic, N.; Hauser, G.; Stimac, D.; Milic, S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease—Where do we stand? World J. Gastroenterol. 2016, 22, 7236–7251. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530–542. [Google Scholar] [CrossRef]
- Jang, H.W.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Han, K.H.; Chon, C.Y.; Park, Y.N.; Choi, E.H.; Kim, D.Y. How many valid measurements are necessary to assess liver fibrosis using FibroScan® in patients with chronic viral hepatitis? An analysis of subjects with at least 10 valid measurements. Yonsei Med. J. 2012, 53, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018, 67, 1348–1359. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Dai, G.; Liu, P.; Li, X.; Zhou, X.; He, S. Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease (NAFLD) susceptibility and severity: A meta-analysis. Medicine 2019, 98, e14324. [Google Scholar] [CrossRef]
- Watt, G.P.; De La Cerda, I.; Pan, J.J.; Fallon, M.B.; Beretta, L.; Loomba, R.; Lee, M.; McCormick, J.B.; Fisher-Hoch, S.P. Elevated Glycated Hemoglobin Is Associated With Liver Fibrosis, as Assessed by Elastography, in a Population-Based Study of Mexican Americans. Hepatol. Commun. 2020, 4, 1793–1801. [Google Scholar] [CrossRef]
- Watt, G.P.; Lee, M.; Pan, J.J.; Fallon, M.B.; Loomba, R.; Beretta, L.; McCormick, J.B.; Fisher-Hoch, S.P. High Prevalence of Hepatic Fibrosis, Measured by Elastography, in a Population-Based Study of Mexican Americans. Clin. Gastroenterol. Hepatol. 2019, 17, 968–975. [Google Scholar] [CrossRef]
- Park, S.Y.; Noureddin, M.; Boushey, C.; Wilkens, L.R.; Setiawan, V.W. Diet Quality Association with Nonalcoholic Fatty Liver Disease by Cirrhosis Status: The Multiethnic Cohort. Curr. Dev. Nutr. 2020, 4, nzaa024. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Guo, B.; Xiao, X.; Yin, J.; Wang, Z.; Jiang, X.; Li, J.; Long, L.; Zhou, J.; Zhang, N.; et al. Healthy dietary patterns and metabolic dysfunction-associated fatty liver disease in less-developed ethnic minority regions: A large cross-sectional study. BMC Public Health 2022, 22, 118. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.L.; Lin, J.S.; Li, Y.H.; Liu, M.; Deng, Y.Y.; Wang, C.Y.; Chen, Y.M. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and elderly adults. Public Health Nutr. 2020, 23, 674–682. [Google Scholar] [CrossRef]
- Ma, J.; Hennein, R.; Liu, C.; Long, M.T.; Hoffmann, U.; Jacques, P.F.; Lichtenstein, A.H.; Hu, F.B.; Levy, D. Improved Diet Quality Associates With Reduction in Liver Fat, Particularly in Individuals With High Genetic Risk Scores for Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cantero, I.; Abete, I.; Babio, N.; Aros, F.; Corella, D.; Estruch, R.; Fito, M.; Hebert, J.R.; Martinez-Gonzalez, M.A.; Pinto, X.; et al. Dietary Inflammatory Index and liver status in subjects with different adiposity levels within the PREDIMED trial. Clin. Nutr. 2018, 37, 1736–1743. [Google Scholar] [CrossRef]
- Entezari, M.R.; Talenezhad, N.; Mirzavandi, F.; Rahimpour, S.; Mozaffari-Khosravi, H.; Fallahzadeh, H.; Hosseinzadeh, M. Mediterranean dietary pattern and non-alcoholic fatty liver diseases: A case-control study. J. Nutr. Sci. 2021, 10, e55. [Google Scholar] [CrossRef]
- Salehi-Sahlabadi, A.; Sadat, S.; Beigrezaei, S.; Pourmasomi, M.; Feizi, A.; Ghiasvand, R.; Hadi, A.; Clark, C.C.T.; Miraghajani, M. Dietary patterns and risk of non-alcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 41. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Lopez, L.; Peralta, C.A.; Lee, A.; Zeki Al Hazzouri, A.; Haan, M.N. Impact of acculturation on cardiovascular risk factors among elderly Mexican Americans. Ann. Epidemiol. 2014, 24, 714–719. [Google Scholar] [CrossRef]
- Afable-Munsuz, A.; Mayeda, E.R.; Perez-Stable, E.J.; Haan, M.N. Immigrant generation and diabetes risk among Mexican Americans: The Sacramento Area Latino Study on Aging. Am. J. Public Health 2013, 103, e45–e52. [Google Scholar] [CrossRef]
- Akresh, I.R. Overweight and obesity among foreign-born and U.S.-born Hispanics. Biodemography Soc. Biol. 2008, 54, 183–199. [Google Scholar] [CrossRef]
- Tapsell, L.C. Dietary behaviour changes to improve nutritional quality and health outcomes. Chronic Dis. Transl. Med. 2017, 3, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Grafenauer, S.J.; Tapsell, L.C.; Beck, E.J.; Batterham, M.J. Baseline dietary patterns are a significant consideration in correcting dietary exposure for weight loss. Eur. J. Clin. Nutr. 2013, 67, 330–336. [Google Scholar] [CrossRef]
- Santoro, N.; Savoye, M.; Kim, G.; Marotto, K.; Shaw, M.M.; Pierpont, B.; Caprio, S. Hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS ONE 2012, 7, e37827. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Deverka, P.A.; Hooker, G.W.; Douglas, M.P. Genetic Test Availability And Spending: Where Are We Now? Where Are We Going? Health Aff. 2018, 37, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017, 49, 471–483. [Google Scholar] [CrossRef]
- Morrill, K.E.; Lopez-Pentecost, M.; Molina, L.; Pfander, J.L.; Hingle, M.D.; Klimentidis, Y.C.; Thomson, C.A.; Garcia, D.O. Weight Loss Interventions for Hispanic Women in the United States: A Systematic Review. J. Environ. Public Health 2021, 2021, 8714873. [Google Scholar] [CrossRef]
- Saeed, N.; Glass, L.M.; Habbal, H.; Mahmood, A.; Sengstock, D.; Saini, S.D.; Tincopa, M.A. Primary care and referring physician perspectives on non-alcoholic fatty liver disease management: A nationwide survey. Ther. Adv. Gastroenterol. 2021, 14, 17562848211042200. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef]
- Lopez-Pentecost, M.; Hallmark, B.; Thomson, C.A.; Chilton, F.; Garcia, D.O. Association between Dietary Fatty Acid Intake and Liver Steatosis and Fibrosis in a Sample of Mexican-Origin Hispanic Adults with Overweight or Obesity. Int. J. Environ. Res. Public Health 2023, 20, 3103. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Jin, L.; Qin, X.; He, B. Natural flavonoids: Potential therapeutic strategies for non-alcoholic fatty liver disease. Front. Pharmacol. 2022, 13, 1005312. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, S.; Li, J.; Wang, J.; Zhang, R.; Zhou, Y.; Yin, Q.; Zheng, Y.; Wang, F.; Xia, Y.; et al. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1459790. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Muller, C.; Paul, S. Racial disparities in nonalcoholic fatty liver disease clinical trial enrollment: A systematic review and meta-analysis. World J. Hepatol. 2020, 12, 506–518. [Google Scholar] [CrossRef]
- Chrysavgis, L.; Ztriva, E.; Protopapas, A.; Tziomalos, K.; Cholongitas, E. Nonalcoholic fatty liver disease in lean subjects: Prognosis, outcomes and management. World J. Gastroenterol. 2020, 26, 6514–6528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).