The Impact of Clinical Factors, Vitamin B12 and Total Cholesterol on Severity of Anorexia Nervosa: A Multicentric Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Study Design
2.2. Assessment
2.3. Blood Collection
2.4. Statistical Analyses
3. Results
3.1. Descriptive Analyses
3.2. One-Way ANOVAs
3.3. Linear Regression Analyses
4. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Treasure, J.; Duarte, T.A.; Schmidt, U. Seminar Eating disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Silén, Y.; Keski-Rahkonen, A. Worldwide prevalence of DSM-5 eating disorders among young people. Curr. Opin. Psychiatry 2022, 35, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Katzman, D.K. Medical complications in adolescents with anorexia nervosa: A review of the literature. Int. J. Eat. Disord. 2005, 37, S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.; Workman, C.; Mehler, P.S. Medical Complications of Anorexia Nervosa and Bulimia Nervosa. Psychiatr. Clin. N. Am. 2019, 42, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Dakanalis, A.; Alix Timko, C.; Colmegna, F.; Riva, G.; Clerici, M. Evaluation of the DSM-5 severity ratings for anorexia nervosa in a clinical sample. Psychiatry Res. 2018, 262, 124–128. [Google Scholar] [CrossRef]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Duriez, P.; Goueslard, K.; Treasure, J.; Quantin, C.; Jollant, F. Risk of non-fatal self-harm and premature mortality in the three years following hospitalization in adolescents and young adults with an eating disorder: A nationwide population-based study. Int. J. Eat. Disord. 2023, 56, 1534–1543. [Google Scholar] [CrossRef]
- Hanachi, M.; Dicembre, M.; Rives-Lange, C.; Ropers, J.; Bemer, P.; Zazzo, J.F.; Poupon, J.; Dauvergne, A.; Melchior, J.C. Micronutrients Deficiencies in 374 Severely Malnourished Anorexia Nervosa Inpatients. Nutrients 2019, 11, 792. [Google Scholar] [CrossRef]
- Achamrah, N.; Coëffier, M.; Rimbert, A.; Charles, J.; Folope, V.; Petit, A.; Déchelotte, P.; Grigioni, S. Micronutrient Status in 153 Patients with Anorexia Nervosa. Nutrients 2017, 9, 225. [Google Scholar] [CrossRef]
- Strumila, R.; Lengvenyte, A.; Olie, E.; Seneque, M.; Dupuis-Maurin, K.; Alacreu-Crespo, A.; Maimoun, L.; Lefebvre, P.; Renard, E.; Courtet, P.; et al. Selenium deficiency is associated with disease severity, disrupted reward processing, and increased suicide risk in patients with Anorexia Nervosa. Psychoneuroendocrinology 2022, 140, 105723. [Google Scholar] [CrossRef]
- Corbetta, F.; Tremolizzo, L.; Conti, E.; Ferrarese, C.; Neri, F.; Bomba, M.; Nacinovich, R. Paradoxical increase of plasma vitamin B12 and folates with disease severity in anorexia nervosa. Int. J. Eat. Disord. 2015, 48, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Tam, F.I.; Chocholi, I.; Hellerhoff, I.; Kloepfer, M.; Weidner, K.; Roessner, V.; Mirtschink, P.; Poitz, D.M.; Ehrlich, S. Liver and vitamin B(12) parameters in patients with anorexia nervosa before and after short-term weight restoration. Psychiatry Res. 2022, 314, 114673. [Google Scholar] [CrossRef]
- Föcker, M.; Timmesfeld, N.; Scherag, S.; Knoll, N.; Singmann, P.; Wang-Sattler, R.; Bühren, K.; Schwarte, R.; Egberts, K.; Fleischhaker, C.; et al. Comparison of metabolic profiles of acutely ill and short-term weight recovered patients with anorexia nervosa reveals alterations of 33 out of 163 metabolites. J. Psychiatr. Res. 2012, 46, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Monteleone, A.M.; Troisi, J.; Dalle Grave, R.; Corrivetti, G.; Calugi, S.; Scala, G.; Patriciello, G.; Zanetti, A.; Maj, M. Metabolomics signatures of acutely ill and short-term weight recovered women with anorexia nervosa. Mol. Psychiatry 2021, 26, 3980–3991. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Hübel, C.; Hindborg, M.; Lindkvist, E.; Kastrup, A.M.; Yilmaz, Z.; Støving, R.K.; Bulik, C.M.; Sjögren, J.M. Increased lipid and lipoprotein concentrations in anorexia nervosa: A systematic review and meta-analysis. Int. J. Eat. Disord. 2019, 52, 611–629. [Google Scholar] [CrossRef]
- Malcolm, A.; Phillipou, A. Current directions in biomarkers and endophenotypes for anorexia nervosa: A scoping review. J. Psychiatr. Res. 2021, 137, 303–310. [Google Scholar] [CrossRef]
- Caldiroli, A.; La Tegola, D.; Affaticati, L.M.; Manzo, F.; Cella, F.; Scalia, A.; Capuzzi, E.; Nicastro, M.; Colmegna, F.; Buoli, M.; et al. Clinical and Peripheral Biomarkers in Female Patients Affected by Anorexia: Does the Neutrophil/Lymphocyte Ratio (NLR) Affect Severity? Nutrients 2023, 15, 1133. [Google Scholar] [CrossRef]
- Speranza, E.; Marra, M.; De Filippo, E.; De Caprio, C.; Sammarco, R.; Morlino, D. Nutritional indicators and metabolic alterations in outpatients with anorexia nervosa: A retrospective study. Eat. Weight Disord. Stud. Anorexia Bulim. Obes. 2021, 26, 2693–2699. [Google Scholar] [CrossRef]
- Słotwińska, S.M.; Słotwiński, R. Immune disorders in anorexia. Cent. J. Immunol. 2017, 42, 294–300. [Google Scholar] [CrossRef]
- Solmi, M.; Veronese, N.; Favaro, A.; Santonastaso, P.; Manzato, E.; Sergi, G.; Correll, C.U. Inflammatory cytokines and anorexia nervosa: A meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology 2015, 51, 237–252. [Google Scholar] [CrossRef]
- Gibson, D.; Mehler, P.S. Anorexia Nervosa and the Immune System—A Narrative Review. J. Clin. Med. 2019, 8, 1915. [Google Scholar] [CrossRef]
- Dalton, B.; Bartholdy, S.; Robinson, L.; Solmi, M.; Ibrahim, M.A.A.; Breen, G.; Schmidt, U.; Himmerich, H. A meta-analysis of cytokine concentrations in eating disorders. J. Psychiatr. Res. 2018, 103, 252–264. [Google Scholar] [CrossRef]
- Abella, E.; Feliu, E.; Granada, I.; Millá, F.; Oriol, A.; Ribera, J.M.; Sánchez-Planell, L.; Berga, L.I.; Reverter, J.C.; Rozman, C. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. Am. J. Clin. Pathol. 2002, 118, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.A.K.; Millischer, V.; Göteson, A.; Hübel, C.; Thornton, L.M.; Bulik, C.M.; Schalling, M.; Landén, M. Aberrant inflammatory profile in acute but not recovered anorexia nervosa. Brain Behav. Imm. 2020, 88, 718–724. [Google Scholar] [CrossRef]
- Morawiecka-Pietrzak, M.; Malczyk, Ż.; Dąbrowska, E.; Blaska, M.; Pietrzak, M.; Gliwińska, A.; Góra, A.; Ziora, K.; Pluskiewicz, W.; Ostrowska, Z. The relationship of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with bone mineral density in adolescent girls suffering from anorexia nervosa. Endokrynol. Pol. 2021, 72, 336–346. [Google Scholar] [CrossRef]
- Legroux-Gérot, I.; Vignau, J.; Viltart, O.; Hardouin, P.; Chauveau, C.; Cortet, B. Adipokines and bone status in a cohort of anorexic patients. Jt. Bone Spine 2019, 86, 95–101. [Google Scholar] [CrossRef]
- Mai, Y.; Zhang, X.; Li, Z.; Wu, X.; Zeng, B.; Fang, Y.; Zou, L.; Zhao, J.; Hummel, T. Olfaction is a Marker of Severity but Not Diagnosis in Anorexia Nervosa: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2020, 30, 251–266. [Google Scholar] [CrossRef]
- Gaudio, S.; Quattrocchi, C.C.; Piervincenzi, C.; Zobel, B.B.; Montecchi, F.R.; Dakanalis, A.; Riva, G.; Carducci, F. White matter abnormalities in treatment-naive adolescents at the earliest stages of Anorexia Nervosa: A diffusion tensor imaging study. Psychiatry Res. Neuroimaging 2017, 266, 138–145. [Google Scholar] [CrossRef]
- Kimura, H.; Tonoike, T.; Muroya, T.; Yoshida, K.; Ozaki, N. Age of onset has limited association with body mass index at time of presentation for anorexia nervosa: Comparison of peak-onset and late-onset anorexia nervosa groups. Psychiatry Clin. Neurosci. 2007, 61, 646–650. [Google Scholar] [CrossRef]
- Horváth, Z.; Román, N.; Elekes, Z.; Griffiths, M.D.; Demetrovics, Z.; Urbán, Z. Alcohol consumption and risk for feeding and eating disorders in adolescence: The mediating role of drinking motives. Addict. Behav. 2020, 107, 106431. [Google Scholar] [CrossRef]
- Mellentin, A.I.; Mejldal, A.; Guala, M.M.; Støving, R.K.; Eriksen, L.S.; Stenager, E.; Skøt, L. The Impact of Alcohol and Other Substance Use Disorders on Mortality in Patients with Eating Disorders: A Nationwide Register-Based Retrospective Cohort Study. Am. J. Psychiatry 2022, 179, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.; Leppanen, J.; Campbell, I.C.; Chung, R.; Breen, G.; Schmidt, U.; Himmerich, H. A longitudinal analysis of cytokines in anorexia nervosa. Brain. Behav. Immun. 2020, 85, 88–95. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Calugi, S.; Ricca, V.; Castellini, G.; Lo Sauro, C.; Ruocco, A.; Chignola, E.; El Ghoch, M.; Dalle Grave, R. The eating disorder examination: Reliability and validity of the Italian version. Eat. Weight Disord. 2015, 20, 505–511. [Google Scholar] [CrossRef]
- Buoli, M.; Cesana, B.M.; Fagiolini, A.; Albert, U.; Maina, G.; de Bartolomeis, A.; Pompili, M.; Bondi, E.; Steardo, L., Jr.; Amore, M.; et al. Which factors delay treatment in bipolar disorder? A nationwide study focussed on duration of untreated illness. Early Interv. Psychiatry 2021, 15, 1136–1145. [Google Scholar] [CrossRef]
- Resmark, G.; Herpertz, S.; Herpertz-Dahlmann, B.; Zeeck, A. Treatment of Anorexia Nervosa-New Evidence-Based Guidelines. J. Clin. Med. 2019, 8, 153. [Google Scholar] [CrossRef]
- Feillet, F.; Feillet-Coudray, C.; Bard, J.M.; Parra, H.J.; Favre, E.; Kabuth, B.; Fruchart, J.C.; Vidailhet, M. Plasma cholesterol and endogenous cholesterol synthesis during refeeding in anorexia nervosa. Clin. Chim. Acta 2000, 294, 45–56. [Google Scholar] [CrossRef]
- Weinbrenner, T.; Züger, M.; Jacoby, G.E.; Herpertz, S.; Liedtke, R.; Sudhop, T.; Gouni-Berthold, I.; Axelson, M.; Berthold, H.K. Lipoprotein metabolism in patients with anorexia nervosa: A case-control study investigating the mechanisms leading to hypercholesterolaemia. Br. J. Nutr. 2004, 91, 959–969. [Google Scholar] [CrossRef]
- Rigaud, D.; Tallonneau, I.; Vergès, B. Hypercholesterolaemia in anorexia nervosa: Frequency and changes during refeeding. Diabetes Metab. 2009, 35, 57–63. [Google Scholar] [CrossRef]
- Klinefelter, H.F. Hypercholesterolemia in anorexia nervosa. J. Clin. Endocrinol. Metab. 1965, 25, 1520–1521. [Google Scholar] [CrossRef]
- Crisp, A.H.; Blendis, L.M.; Pawan, G.L. Aspects of fat metabolism in anorexia nervosa. Metabolism 1968, 17, 1109–1118. [Google Scholar] [CrossRef]
- Abuzeid, W.; Glover, C. Acute myocardial infarction and anorexia nervosa. Int. J. Eat. Disord. 2011, 44, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, V.; Slobodianik, N.; Pallaro, A.; Bello, M.; Geissler, C. Risk factors for cardiovascular disease in patients with anorexia nervosa. Int. J. Psychiatr. Nurs. Res. 2007, 13, 1531–1545. [Google Scholar] [PubMed]
- Giovinazzo, S.; Sukkar, S.G.; Rosa, G.M.; Zappi, A.; Bezante, G.P.; Balbi, M.; Brunelli, C. Anorexia nervosa and heart disease: A systematic review. Eat. Weight Disord. 2019, 24, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, R.; Hotta, M.; Oikawa, S.; Takano, K. Etiology of hypercholesterolemia in patients with anorexia nervosa. Int. J. Eat. Disord. 2006, 39, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Glenny, E.M.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.M.; Carroll, I.M. Eating Disorders and the Intestinal Microbiota: Mechanisms of Energy Homeostasis and Behavioral Influence. Curr. Psychiatry Rep. 2017, 19, 51. [Google Scholar] [CrossRef]
- Schwensen, H.F.; Kan, C.; Treasure, J.; Høiby, N.; Sjögren, M. A systematic review of studies on the faecal microbiota in anorexia nervosa: Future research may need to include microbiota from the small intestine. Eat. Weight Disord. 2018, 23, 399–418. [Google Scholar] [CrossRef]
- Shpilberg, O.; Burstein, R.; Epstein, Y.; Suessholz, A.; Getter, R.; Rubinstein, A. Lipid profile in trained subjects undergoing complete food deprivation combined with prolonged intermittent exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 305–308. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Schorr, M.; Miller, K.K. The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nat. Rev. Endocrinol. 2017, 13, 174–186. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Milner, K.L.; Kriketos, A.; Russell, J.; Campbell, L.V. Metabolic dysfunction in anorexia nervosa. Obes. Res. Clin. Pract. 2007, 1, 139–146. [Google Scholar] [CrossRef]
- Kaválková, P.; Dostálová, I.; Haluzíková, D.; Trachta, P.; Hanušová, V.; Lacinová, Z.; Papežová, H.; Domluvilová, D.; Zikán, V.; Haluzík, M. Preadipocyte factor-1 concentrations in patients with anorexia nervosa: The influence of partial realimentation. Physiol. Res. 2012, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.B.; Yang, J.; Morisseau, C.; German, J.B.; Zeeland, A.A.; Armando, A.M.; Quehenberger, O.; Bergen, A.W.; Magistretti, P.; Berrettini, W.; et al. Dysregulation of soluble epoxide hydrolase and lipidomic profiles in anorexia nervosa. Mol. Psychiatry 2016, 21, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Figenschau, Y.; Hutchinson, M.; Emaus, N.; Grimnes, G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur. J. Clin. Nutr. 2010, 64, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Vitezova, A.; Voortman, T.; Zillikens, M.C.; Jansen, P.W.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Kiefte-de Jong, J.C. Bidirectional associations between circulating vitamin D and cholesterol levels: The Rotterdam Study. Maturitas 2015, 82, 411–417. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Brigden, R.; Raman, V.; Cui, X.; Du, Z.; Eyles, D. Vitamin D: A potent regulator of dopaminergic neuron differentiation and function. J. Neurochem. 2023, 166, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Brand-Gothelf, A.; Leor, S.; Apter, A.; Fennig, S. The impact of comorbid depressive and anxiety disorders on severity of anorexia nervosa in adolescent girls. J. Nerv. Ment. Dis. 2014, 202, 759–762. [Google Scholar] [CrossRef]
- Duncan, L.; Yilmaz, Z.; Gaspar, H.; Walters, R.; Goldstein, J.; Anttila, V.; Bulik-Sullivan, B.; Ripke, S.; Eating Disorders Working Group of the Psychiatric Genomics Consortium; Thornton, L.; et al. Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am. J. Psychiatry 2017, 174, 850–858. [Google Scholar] [CrossRef]
- Capuzzi, E.; Caldiroli, A.; Capellazzi, M.; Tagliabue, I.; Auxilia, A.; Ghilardi, G.; Buoli, M.; Clerici, M. Exploring the role of serum lipid profile and neutrophil-to-lymphocyte ratio in violent suicide attempters: A cross sectional study. CNS Spectr. 2022, 27, 362–368. [Google Scholar] [CrossRef]
- Papillard-Marechal, S.; Sznajder, M.; Hurtado-Nedelec, M.; Alibay, Y.; Martin-Schmitt, C.; Dehoux, M.; Westerman, M.; Beaumont, C.; Chevallier, B.; Puy, H.; et al. Iron metabolism in patients with anorexia nervosa: Elevated serum hepcidin concentrations in the absence of inflammation. Am. J. Clin. Nutr. 2012, 95, 548–554. [Google Scholar] [CrossRef]
- Ermens, A.A.M.; Vlasveld, L.T.; Lindemans, J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin. Biochem. 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Arendt, J.F.B.; Nexo, E. Cobalamin related parameters and disease patterns in patients with increased serum cobalamin levels. PLoS ONE 2012, 7, e45979. [Google Scholar] [CrossRef]
- Dou, J.; Xu, W.; Ye, B.; Zhang, Y.; Mao, W. Serum vitamin B12 levels as indicators of disease severity and mortality of patients with acute-on-chronic liver failure. Clin. Chim. Acta 2012, 413, 23–24, 1809–1812. [Google Scholar] [CrossRef]
- Kumar, G.P.; Bhaumik, P.; Chakraborty, A.; Ramchandradasar. Vitamin B12 as Severity and Prognostic Marker in Chronic Liver Disease. J. Assoc. Physicians India 2023, 71, 1. [Google Scholar]
- Tsukamoto, M.; Tanaka, A.; Arai, M.; Ishii, N.; Ohta, D.; Horiki, N.; Fujita, Y. Hepatocellular injuries observed in patients with an eating disorder prior to nutritional treatment. Intern. Med. 2008, 47, 1447–1450. [Google Scholar] [CrossRef]
- Harris, R.H.; Sasson, G.; Mehler, P.S. Elevation of liver function tests in severe anorexia nervosa. Int. J. Eat. Disord. 2013, 46, 369–374. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.; Wan, Z.; Lu, Q.; Zhang, X.; Qiu, Z.; Li, L.; Zhu, K.; Liu, L.; Pan, A.; et al. Associations of Serum Folate and Vitamin B12 Levels with Cardiovascular Disease Mortality among Patients with Type 2 Diabetes. JAMA Netw. Open 2022, 5, e2146124. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Heiner-Fokkema, M.R.; Green, R.; Gans, R.O.B. Relationship between serum B12 concentrations and mortality: Experience in NHANES. BMC Med. 2020, 18, 307. [Google Scholar] [CrossRef]
- Mendonça, N.; Jagger, C.; Granic, A.; Martin-Ruiz, C.; Mathers, J.C.; Seal, C.J.; Hill, T.R. Elevated Total Homocysteine in All Participants and Plasma Vitamin B12 Concentrations in Women Are Associated with All-Cause and Cardiovascular Mortality in the Very Old: The Newcastle 85+ Study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1258–1264. [Google Scholar] [CrossRef]
- Heinz, J.; Kropf, S.; Luley, C.; Dierkes, J. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: A meta-analysis. Am. J. Kidney Dis. 2009, 54, 478–489. [Google Scholar] [CrossRef]
- Saposnik, G.; Ray, J.G.; Sheridan, P.; McQueen, M.; Lonn, E.; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: Additional findings from the HOPE 2 trial. Stroke 2009, 40, 1365–1372. [Google Scholar] [CrossRef]
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Eating Disorders, 4th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2023. [Google Scholar]
- Davis, H.; Attia, E. Pharmacotherapy of eating disorders. Curr. Opin. Psychiatry 2017, 30, 452–457. [Google Scholar] [CrossRef]
- Sander, J.; Moessner, M.; Bauer, S. Depression, Anxiety and Eating Disorder-Related Impairment: Moderators in Female Adolescents and Young Adults. Int. J. Environ. Res. Public Health 2021, 18, 5. [Google Scholar] [CrossRef]
- Fennig, S.; Hadas, A. Suicidal behavior and depression in adolescents with eating disorders. Nord. J. Psychiatry 2010, 64, 32–39. [Google Scholar] [CrossRef]
- Pollice, C.; Kaye, W.H.; Greeno, C.G.; Weltzin, T.E. Relationship of depression, anxiety, and obsessionality to state of illness in anorexia nervosa. Int. J. Eat. Disord. 1997, 21, 367–376. [Google Scholar] [CrossRef]
- Laessle, R.G.; Schweiger, U.; Pirke, K.M. Depression as a correlate of starvation in patients with eating disorders. Biol. Psychiatry 1988, 23, 719–725. [Google Scholar] [CrossRef]
- Meehan, K.G.; Loeb, K.L.; Roberto, C.A.; Attia, E. Mood change during weight restoration in patients with anorexia nervosa. Int. J. Eat. Disord. 2006, 39, 587–589. [Google Scholar] [CrossRef]
- Kaufmann, L.K.; Moergeli, H.; Milos, G.F. Lifetime Weight Characteristics of Adult Inpatients with Severe Anorexia Nervosa: Maximal Lifetime BMI Predicts Treatment Outcome. Front. Psychiatry 2021, 12, 682952. [Google Scholar] [CrossRef]
- Attia, E.; Kaplan, A.S.; Walsh, B.T.; Gershkovich, M.; Yilmaz, Z.; Musante, D.; Wang, Y. Olanzapine versus placebo for out-patients with anorexia nervosa. Psychol. Med. 2011, 41, 2177–2182. [Google Scholar] [CrossRef]
- Attia, E.; Steinglass, J.E.; Walsh, B.T.; Wang, Y.; Wu, P.; Schreyer, C.; Wildes, J.; Yilmaz, Z.; Guarda, A.S.; Kaplan, A.S.; et al. Olanzapine Versus Placebo in Adult Outpatients with Anorexia Nervosa: A Randomized Clinical Trial. Am. J. Psychiatry 2019, 176, 449–456. [Google Scholar] [CrossRef]
- Chiu, H.P.; Huang, M.W.; Tsai, S.Y.; Hsu, C.Y. A retrospective study of pharmacological treatment in anorexia nervosa: 6-month and 12-month follow-up. BMC Psychiatry 2023, 23, 126. [Google Scholar] [CrossRef]
- Blanchet, C.; Guillaume, S.; Bat-Pitault, F.; Carles, M.E.; Clarke, J.; Dodin, V.; Duriez, P.; Gerardin, P.; Hanachi-Guidoum, M.; Iceta, S.; et al. Medication in AN: A Multidisciplinary Overview of Meta-Analyses and Systematic Reviews. J. Clin. Med. 2019, 8, 278. [Google Scholar] [CrossRef]
- Boast, N.; Coker, E.; Wakeling, A. Anorexia nervosa of late onset. Br. J. Psychiatry 1992, 160, 257–260. [Google Scholar] [CrossRef]
- Fichter, M.M.; Quadflieg, N.; Crosby, R.D.; Koch, S. Long-term outcome of anorexia nervosa: Results from a large clinical longitudinal study. Int. J. Eat. Disord. 2017, 50, 1018–1030. [Google Scholar] [CrossRef]
- Nexo, E.; Hoffmann-Lücke, E. Holotranscobalamin, a marker of vitamin B-12 status: Analytical aspects and clinical utility. Am. J. Clin. Nutr. 2011, 94, 359S–365S. [Google Scholar] [CrossRef]
- Valente, E.; Scott, J.M.; Ueland, P.M.; Cunningham, C.; Casey, M.; Molloy, A.M. Diagnostic accuracy of holotranscobalamin, methylmalonic acid, serum cobalamin, and other indicators of tissue vitamin B₁₂ status in the elderly. Clin. Chem. 2011, 57, 856–863. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Brito, A.; Miller, J.W.; Green, R.; Allen, L.H. Combined indicator of vitamin B12 status: Modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin. Chem. Lab. Med. 2015, 53, 1215–1225. [Google Scholar] [CrossRef]
- Green, R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am. J. Clin. Nutr. 2011, 94, 666S–672S. [Google Scholar] [CrossRef]
- Dakanalis, A.; Gaudio, S.; Serino, S.; Clerici, M.; Carrà, G.; Riva, G. Body-image distortion in anorexia nervosa. Nat. Rev. Dis. Primers 2016, 2, 1–2. [Google Scholar] [CrossRef]
- Rodan, S.C.; Bryant, E.; Le, A.; Maloney, D.; Touyz, S.; McGregor, I.S.; Maguire, S.; National Eating Disorder Research Consortium. Pharmacotherapy, alternative and adjunctive therapies for eating disorders: Findings from a rapid review. J. Eat. Disord. 2023, 11, 112. [Google Scholar] [CrossRef]
- Márquez, M.C.; Sánchez, J.M.; Salazar, A.M.; Martínez, C.V.; Valderrama, F.; Rojas-Gualdrón, D.F. Efficacy and Safety of Antipsychotics and Antidepressants in the Treatment of Anorexia Nervosa: A Systematic Review. Eficacia y seguridad de antipsicóticos y antidepresivos en el tratamiento de la anorexia nerviosa: Revisión sistemática. Rev. Colomb. Psiquiatr. 2022, 51, 227–235. [Google Scholar] [CrossRef] [PubMed]
| Variables | N (%) | BMI (kg/m2) (Mean ± SD) | F * | p | |
|---|---|---|---|---|---|
| Setting | Inpatients | 12 (15.4%) | 15.7 ± 2.21 | 4.66 | 0.03 |
| Outpatients | 66 (84.6%) | 17.4 ± 2.15 | |||
| Sex | Male | 1 (1.3%) | 16.8 ± 0.0 | 0.03 | 0.85 |
| Female | 77 (98.7%) | 17.2 ± 2.22 | |||
| Being in a relationship | Yes | 20 (29.9%) | 16.9 ± 1.83 | 1.37 | 0.25 |
| No | 47 (70.1%) | 17.6 ± 2.37 | |||
| Missing | 11 | ||||
| Occupational status | Student | 50 (67%) | 17.4 ± 2.31 | 0.28 | 0.76 |
| Unemployed | 13 (17%) | 16.9 ± 2.48 | |||
| Worker | 12 (16%) | 17.1 ± 1.65 | |||
| Missing | 3 | ||||
| Substance misuse | Yes | 9 (11.8%) | 16.3 ± 1.81 | 1.77 | 0.19 |
| No | 67 (88.2%) | 17.4 ± 2.25 | |||
| Missing | 2 | ||||
| Main substance of misuse | None | 67 (89.3%) | 17.4 ± 2.25 | 0.75 | 0.53 |
| Alcohol | 3 (4.0%) | 16.8 ± 3.03 | |||
| Cannabinoids | 4 (5.3%) | 15.7 ± 0.93 | |||
| Others | 1 (1.4%) | 15.5 ± 0.0 | |||
| Missing | 3 | ||||
| Poly-substance misuse | Yes | 3 (4.0%) | 16.0 ± 0.71 | 0.63 | 0.43 |
| No | 72 (96.0%) | 17.3 ± 2.26 | |||
| Missing | 3 | ||||
| Family history of mental disorders | Yes | 27 (35.5%) | 17.0 ± 2.64 | 0.45 | 0.50 |
| No | 49 (64.5%) | 17.4 ± 1.95 | |||
| Missing | 2 | ||||
| Type of family history for mental disorders | None | 49 (64.5%) | 17.4 ± 1.95 | 0.79 | 0.58 |
| Anxiety disorders | 4 (5.3%) | 17.7 ± 1.29 | |||
| Bipolar disorder | 1 (1.3%) | 17.1 ± 0.0 | |||
| MDD | 12 (15.8%) | 17.6 ± 3.59 | |||
| Eating disorders | 8 (10.5%) | 16.4 ± 1.36 | |||
| SUD | 1 (1.3%) | 14.0 ± 0.0 | |||
| Others | 1 (1.3%) | 15.2 ± 0.0 | |||
| Missing | 2 | ||||
| Psychiatric comorbidity | Yes | 27 (34.6%) | 17.6 ± 2.72 | 1.13 | 0.29 |
| No | 51 (65.4%) | 17.0 ± 1.85 | |||
| Type of psychiatric comorbidity | None | 51 (65.4%) | 17.0 ± 1.85 | 1.45 | 0.22 |
| Anxiety disorder | 8 (10.3%) | 19.1 ± 3.57 | |||
| MDD | 11 (14.1%) | 16.8 ± 2.54 | |||
| OCD | 3 (3.8%) | 17.4 ± 0.51 | |||
| Personality disorder | 4 (5.1%) | 16.7 ± 1.76 | |||
| Others | 1 (1.3%) | 16.9 ± 0.0 | |||
| Presence of multiple psychiatric comorbidities | Yes | 8 (10.3%) | 15.8 ± 2.52 | 2.07 | 0.16 |
| No | 70 (89.7%) | 17.3 ± 2.17 | |||
| Type of comorbid personality disorder | None | 65 (89.0%) | 17.4 ± 2.21 | 1.31 | 0.28 |
| Borderline | 4 (5.5%) | 15.2 ± 1.77 | |||
| Schizoid | 1 (1.4%) | 16.9 ± 0.0 | |||
| Schizotypal | 2 (2.7%) | 14.6 ± 2.8 | |||
| NOS | 1 (1.4%) | 19.0 ± 0.0 | |||
| Missing | 5 | ||||
| Current psychotherapy | Yes | 4 (6.5%) | 15.5 ± 1.94 | 2.80 | 0.10 |
| No | 58 (93.5%) | 17.5 ± 2.29 | |||
| Missing | 16 | ||||
| Current pharmacological treatment | Yes | 29 (37.2%) | 16.2 ± 1.75 | 5.73 | 0.02 |
| No | 49 (62.8%) | 17.5 ± 2.31 | |||
| Presence of poly-therapy | Yes | 12 (19.4%) | 15.7 ± 2.21 | 5.64 | 0.02 |
| No | 50 (80.6%) | 17.7 ± 2.22 | |||
| Missing | 16 | ||||
| Medical comorbidity | Yes | 21 (27.6%) | 17.3 ± 1.73 | 0.00 | 0.97 |
| No | 55 (72.4%) | 17.2 ± 2.42 | |||
| Missing | 2 | ||||
| Medical poly-comorbidity | Yes | 3 (4.1%) | 16.9 ± 2.65 | 0.08 | 0.78 |
| No | 71 (95.9%) | 17.3 ± 2.23 | |||
| Missing | 4 |
| Variables | Mean ± SD | Pearson’s Correlation (r) | p |
|---|---|---|---|
| Age (years) | 23.2 ± 8.32 | −0.281 | 0.015 |
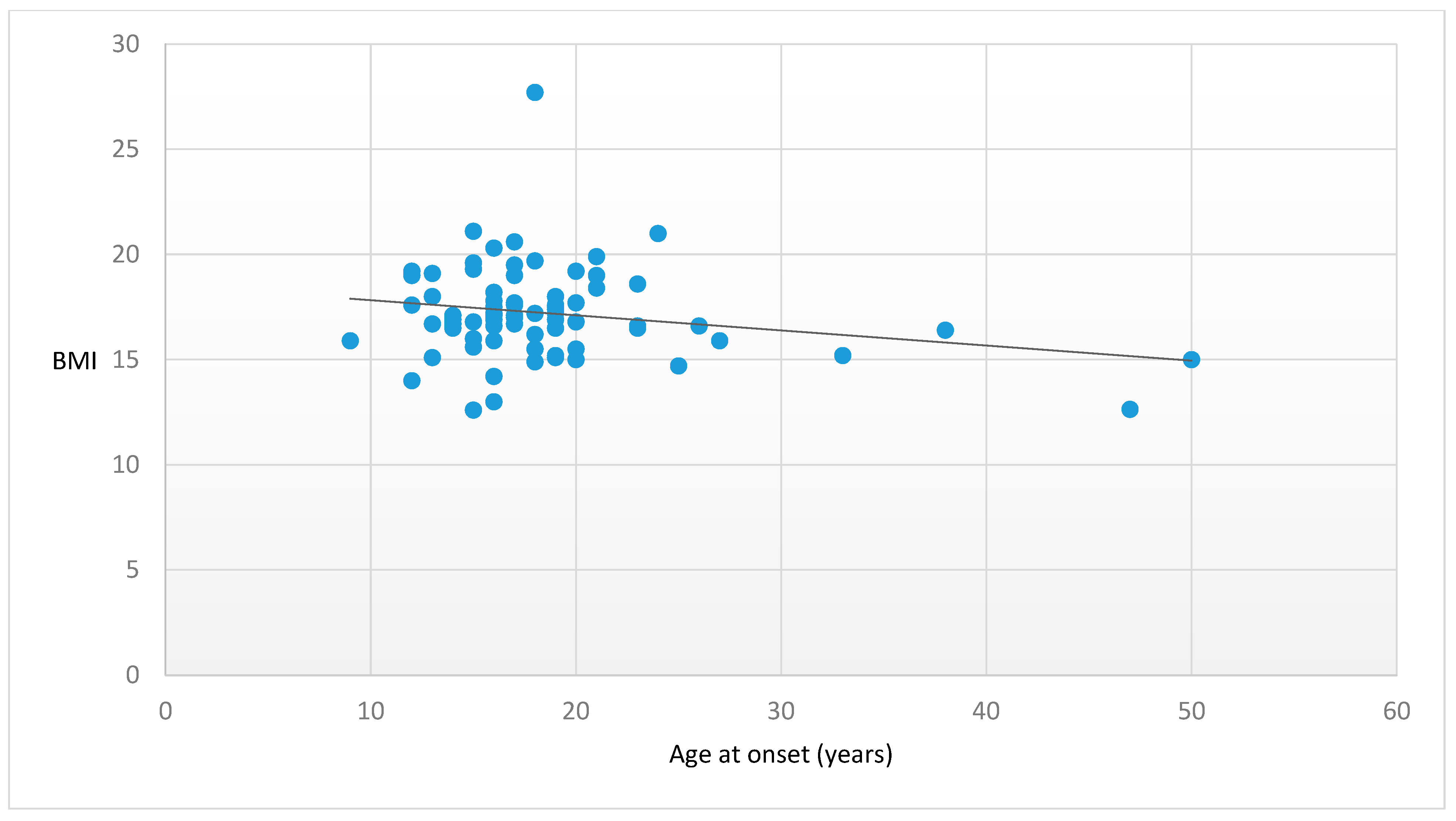
| Age at onset (years) | 18.6 ± 6.61 | −0.22 | 0.06 |
| DUI (years) | 2.1 ± 2.69 | −0.104 | 0.384 |
| Duration of illness (years) | 4.1 ± 5.77 | −0.143 | 0.228 |
| Red Cells (1012/L) | 4.3 ± 0.44 | 0.173 | 0.442 |
| (n.v. 4.50–6.00 × 1012/L) | |||
| Red cell volume (fL) | 90.8 ± 5.47 | 0.276 | 0.214 |
| (n.v. 80.0–99.0 fL) | |||
| Hemoglobin (g/dL) | 13.0 ± 1.26 | 0.232 | 0.299 |
| (n.v. 14.0–18.0 g/dL) | |||
| White cells (109/L) | 5.7 ± 1.74 | 0.165 | 0.166 |
| (n.v. 4.00–11.00 × 109/L) | |||
| Lymphocytes (109/L) | 2.1 ± 0.74 | −0.013 | 0.916 |
| (n.v. 1.00–5.00 × 109/L) | |||
| Neutrophils (109/L) | 3.2 ± 1.67 | 0.03 | 0.84 |
| (n.v. 1.50–7.50 × 109/L) | |||
| Platelets (109/L) | 228.2 ± 49.01 | 0.071 | 0.551 |
| (n.v. 140.0–440.0 × 109/L) | |||
| NLR | 1.5 ± 0.92 | 0.09 | 0.539 |
| (n.v. 1–2) | |||
| PLR | 117.5 ± 41.47 | 0.051 | 0.675 |
| (n.v. 90–210) | |||
| Iron (mcg/dL) | 106.6 ± 74.72 | −0.005 | 0.971 |
| (n.v. 65.0–178.0 mcg/dL) | |||
| Vitamin D (ng/mL) | 30.4 ± 14.14 | −0.061 | 0.714 |
| (n.v. 20.0–40.0 ng/mL) | |||
| Folate (ng/mL) | 8.9 ± 6.24 | −0.247 | 0.081 |
| (n.v. 2–20 ng/mL) | |||
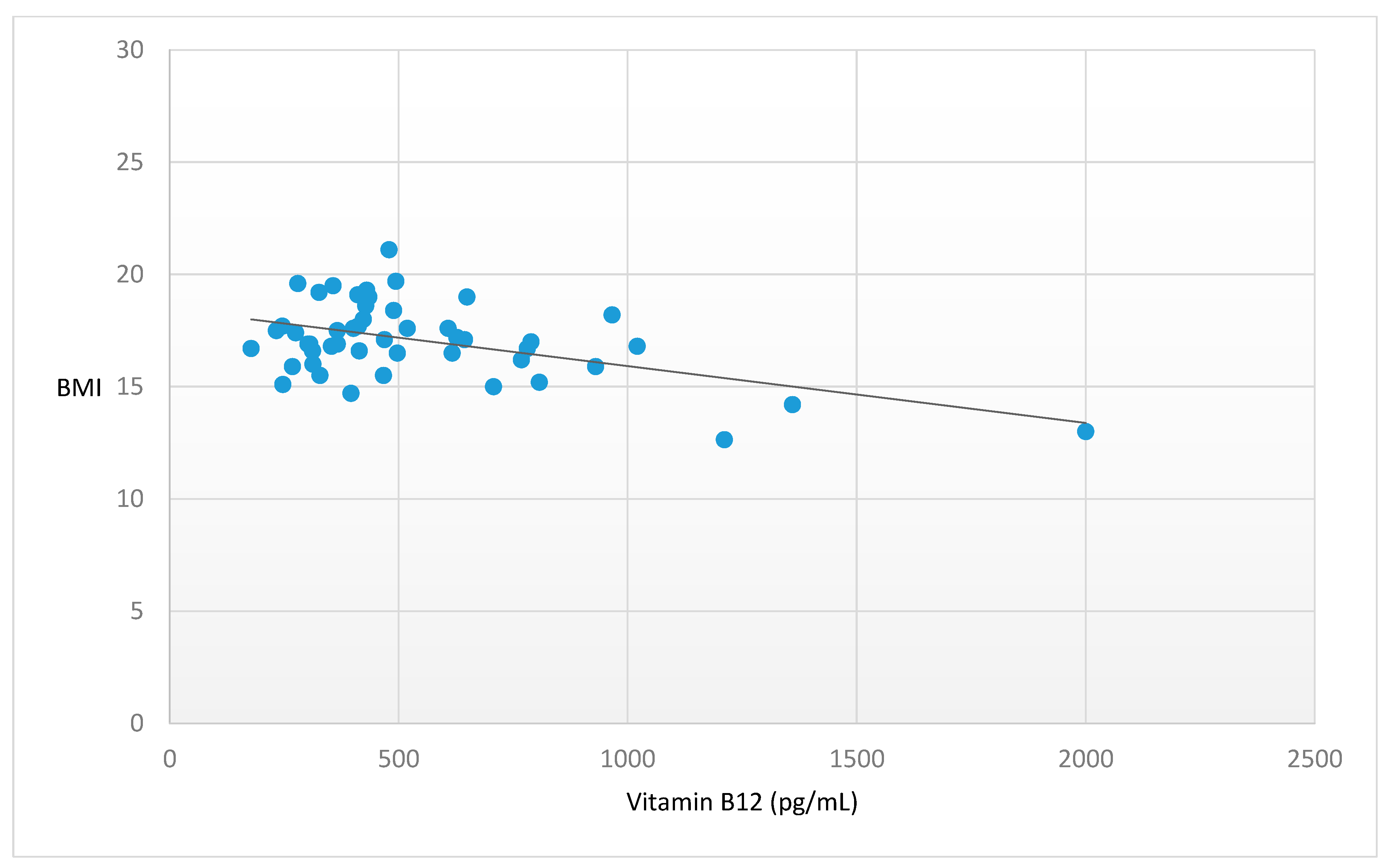
| Vitamin B12 (pg/mL) | 529.4 ± 325.68 | −0.499 | <0.001 |
| (n.v. 200.0–900.0 pg/mL) | |||
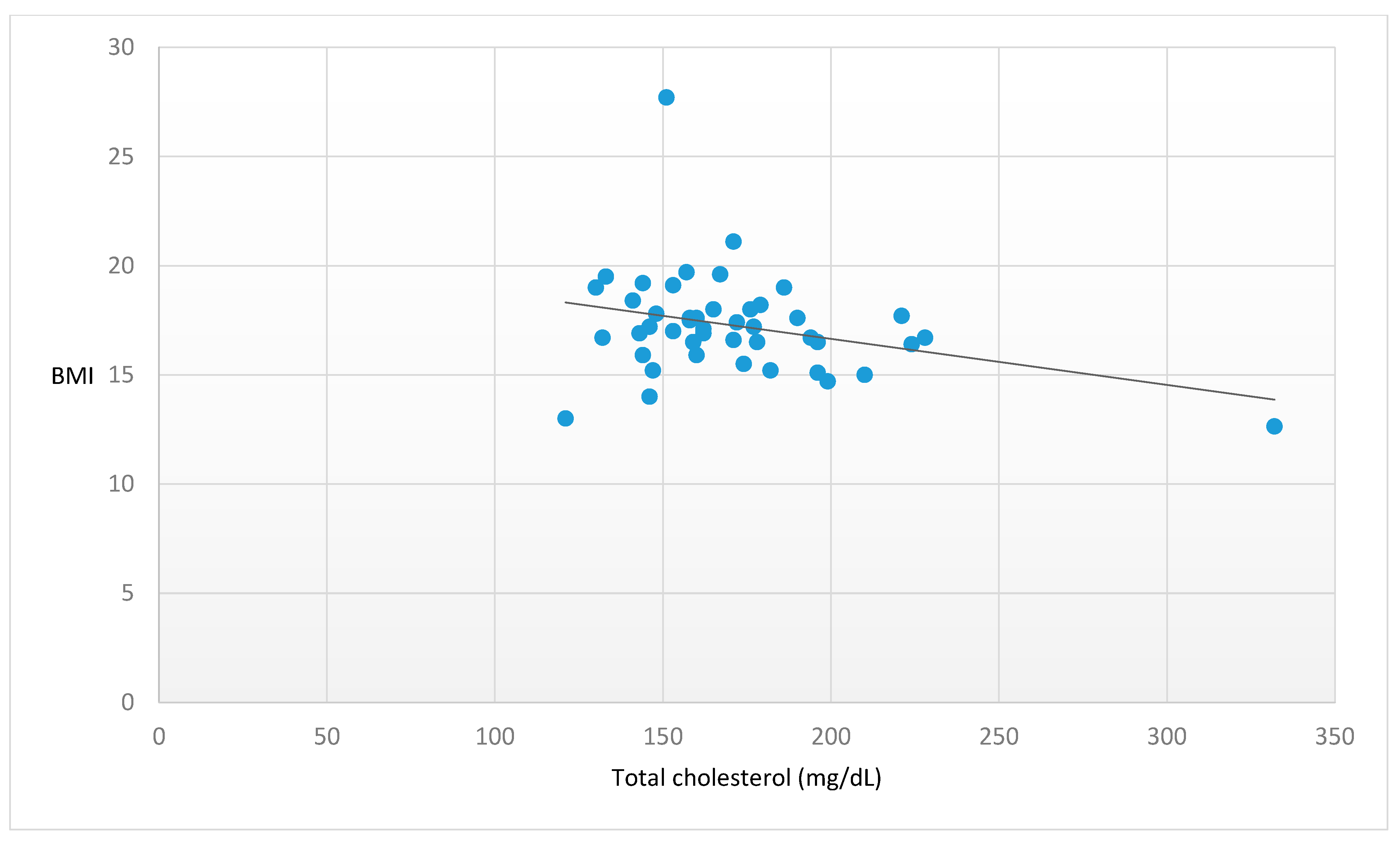
| Cholesterol (mg/dL) | 172.3 ± 34.60 | −0.315 | 0.035 |
| (n.v. < 190 mg/dL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affaticati, L.M.; Buoli, M.; Vaccaro, N.; Manzo, F.; Scalia, A.; Coloccini, S.; Zuliani, T.; La Tegola, D.; Capuzzi, E.; Nicastro, M.; et al. The Impact of Clinical Factors, Vitamin B12 and Total Cholesterol on Severity of Anorexia Nervosa: A Multicentric Cross-Sectional Study. Nutrients 2023, 15, 4954. https://doi.org/10.3390/nu15234954
Affaticati LM, Buoli M, Vaccaro N, Manzo F, Scalia A, Coloccini S, Zuliani T, La Tegola D, Capuzzi E, Nicastro M, et al. The Impact of Clinical Factors, Vitamin B12 and Total Cholesterol on Severity of Anorexia Nervosa: A Multicentric Cross-Sectional Study. Nutrients. 2023; 15(23):4954. https://doi.org/10.3390/nu15234954
Chicago/Turabian StyleAffaticati, Letizia Maria, Massimiliano Buoli, Nadia Vaccaro, Francesca Manzo, Alberto Scalia, Sara Coloccini, Tommaso Zuliani, Davide La Tegola, Enrico Capuzzi, Monica Nicastro, and et al. 2023. "The Impact of Clinical Factors, Vitamin B12 and Total Cholesterol on Severity of Anorexia Nervosa: A Multicentric Cross-Sectional Study" Nutrients 15, no. 23: 4954. https://doi.org/10.3390/nu15234954
APA StyleAffaticati, L. M., Buoli, M., Vaccaro, N., Manzo, F., Scalia, A., Coloccini, S., Zuliani, T., La Tegola, D., Capuzzi, E., Nicastro, M., Colmegna, F., Clerici, M., Dakanalis, A., & Caldiroli, A. (2023). The Impact of Clinical Factors, Vitamin B12 and Total Cholesterol on Severity of Anorexia Nervosa: A Multicentric Cross-Sectional Study. Nutrients, 15(23), 4954. https://doi.org/10.3390/nu15234954









