Metabolomic Alterations of Volatile Organic Compounds and Bile Acids as Biomarkers of Microbial Shifts in a Murine Model of Short Bowel Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Operation Method
2.3. Euthanasia and Sample Collection
2.4. Gut Permeability
2.5. Cytokines and Chemokines
2.6. Tight Junction Proteins
2.7. Short-Chain Fatty Acids
2.8. Bile Acids in Serum and Fecal Samples
2.9. Fecal Volatile Organic Compounds
2.10. Microbiome
2.11. Statistical Analysis
3. Results
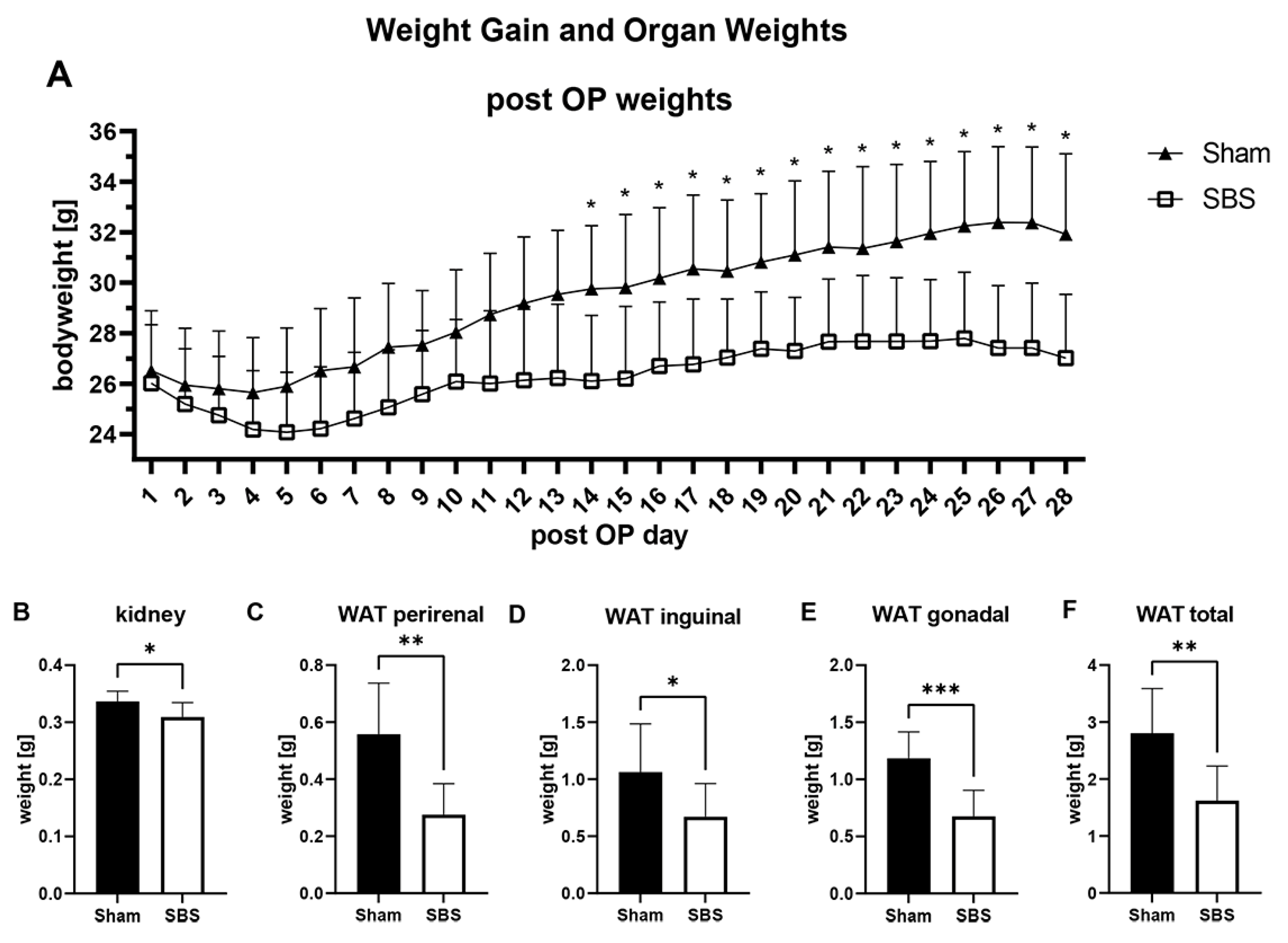
3.1. Operation, Overall Survival and Weight Changes
3.2. Gut Permeability, Cytokines, Chemokines and Tight Junction Proteins
3.3. Short-Chain Fatty Acids (SCFAs)
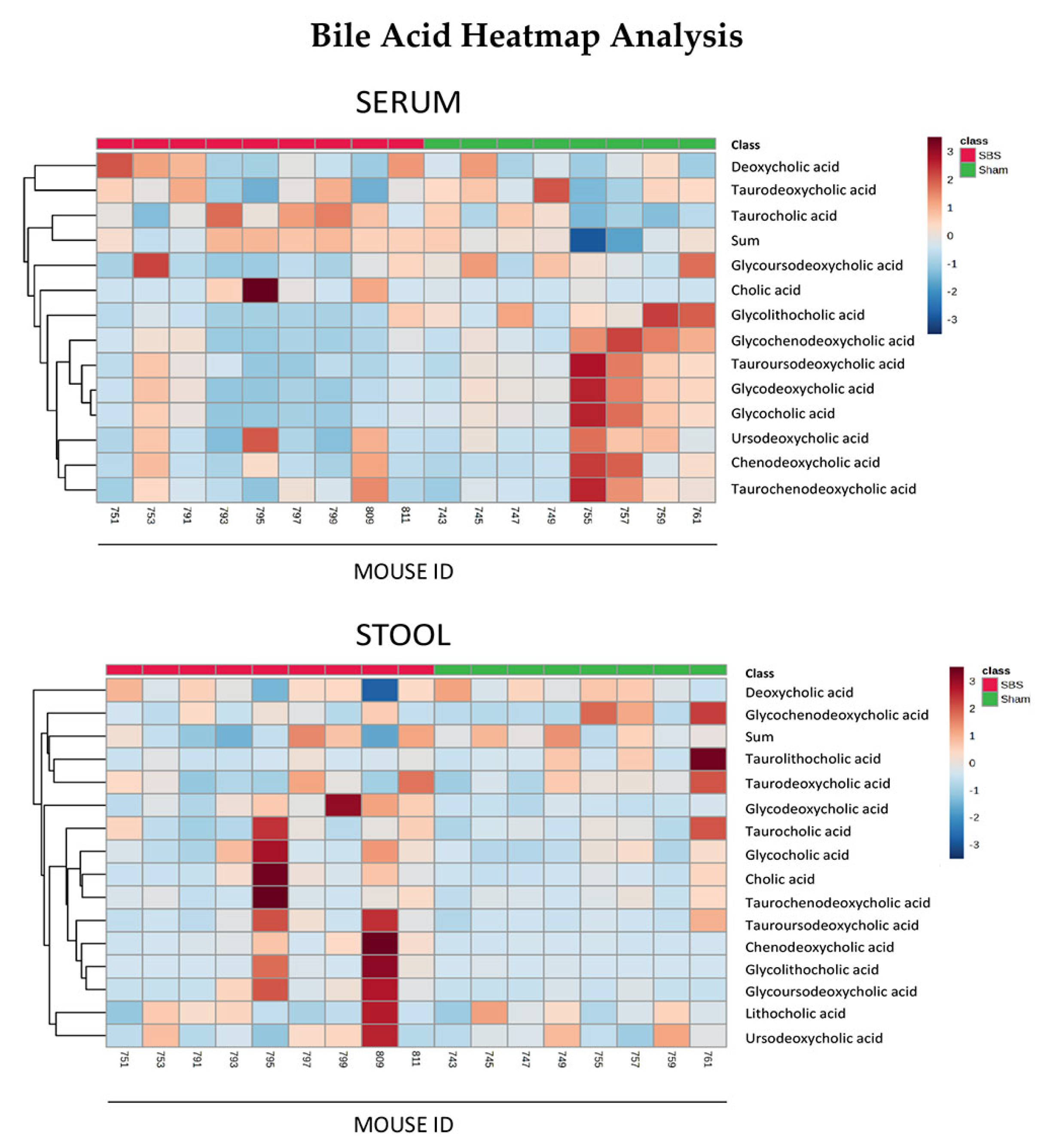
3.4. Bile Acids in Serum and Stool
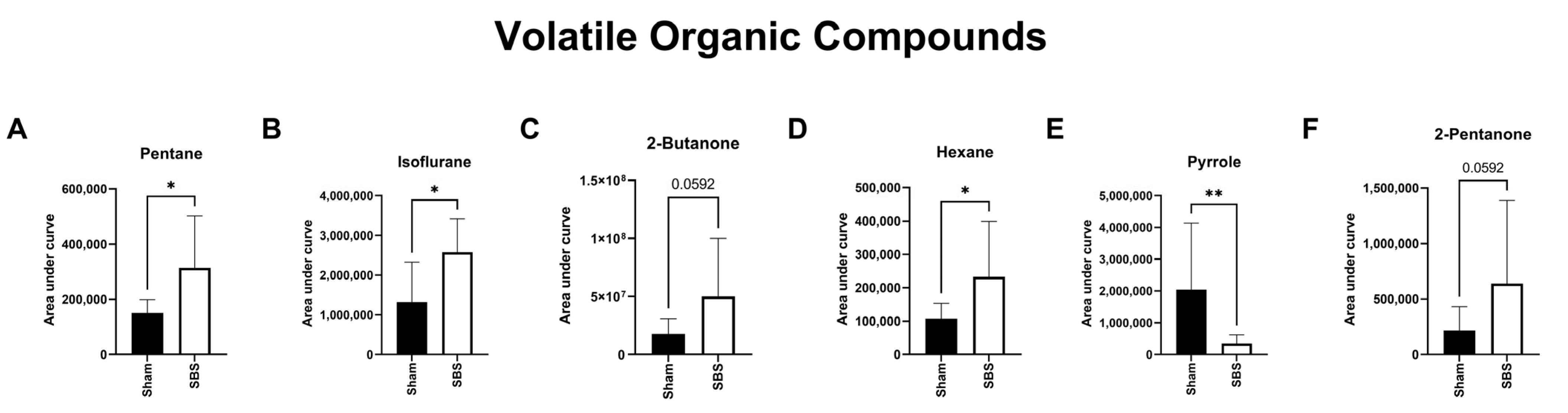
3.5. Volatile Organic Compounds (VOCs)
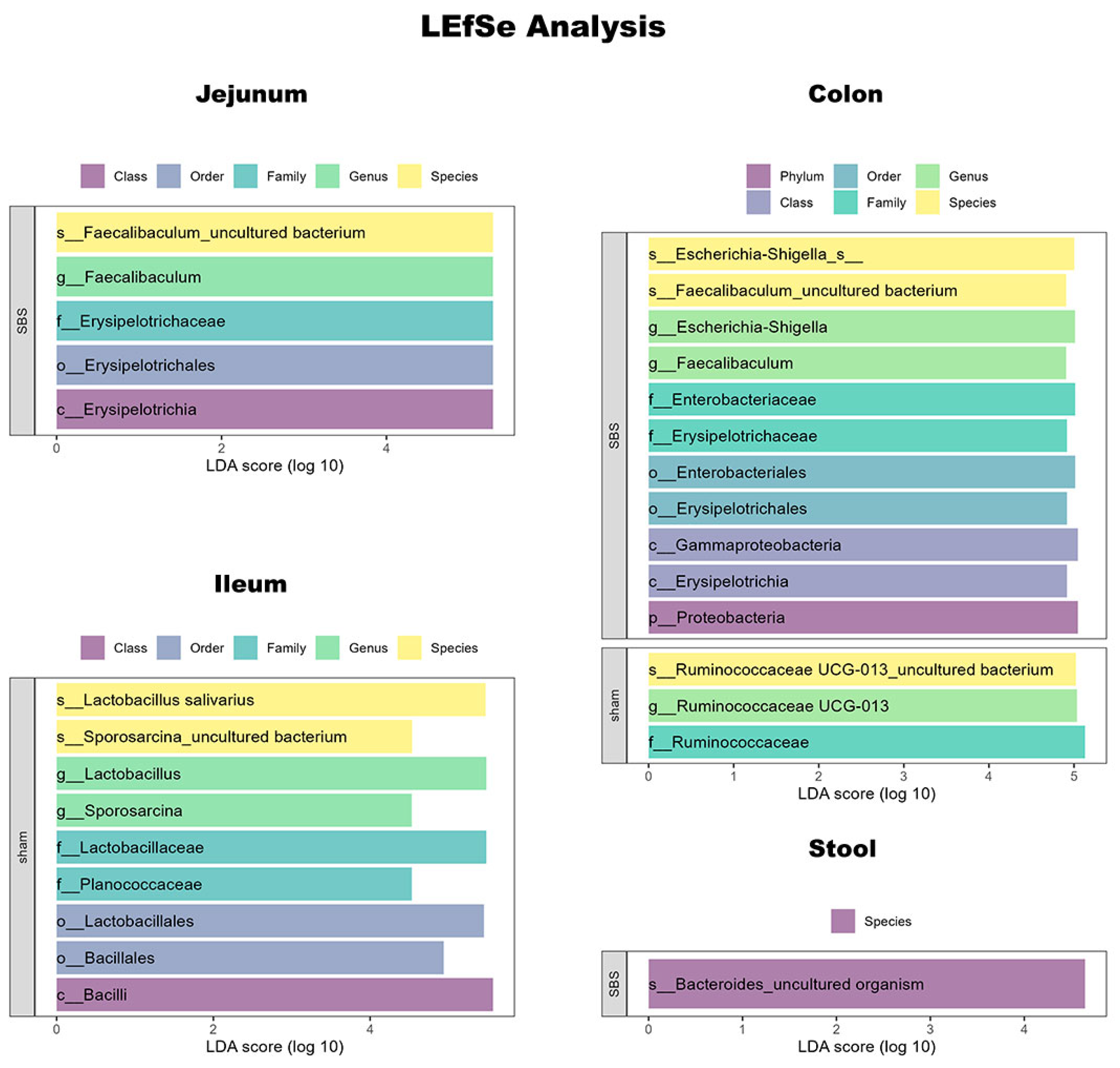
3.6. Microbiome Analysis
3.7. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Channabasappa, N.; Girouard, S.; Nguyen, V.; Piper, H. Enteral Nutrition in Pediatric Short-Bowel Syndrome. Nutr. Clin. Pract. 2020, 35, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130 (Suppl. S1), S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.J.; Cohran, V.; Raphael, B.P.; Sentongo, T.; Volpert, D.; Warner, B.W.; Goday, P.S. Intestinal Rehabilitation Programs in the Management of Pediatric Intestinal Failure and Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 588–596. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benedetti, E.; Schowalter, J.P.; Buchman, A.L. Management and Complications of Short Bowel Syndrome: An Updated Review. Curr. Gastroenterol. Rep. 2016, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Kesavan, A. Current treatment paradigms in pediatric short bowel syndrome. Clin. J. Gastroenterol. 2018, 11, 103–112. [Google Scholar] [CrossRef]
- McGrath, K.H.; Pitt, J.; Bines, J.E. Small intestinal bacterial overgrowth in children with intestinal failure on home parenteral nutrition. JGH Open 2019, 3, 394–399. [Google Scholar] [CrossRef]
- Spencer, A.U.; Kovacevich, D.; McKinney-Barnett, M.; Hair, D.; Canham, J.; Maksym, C.; Teitelbaum, D.H. Pediatric short-bowel syndrome: The cost of comprehensive care. Am. J. Clin. Nutr. 2008, 88, 1552–1559. [Google Scholar] [CrossRef]
- Reddy, V.S.; Patole, S.K.; Rao, S. Role of probiotics in short bowel syndrome in infants and children—A systematic review. Nutrients 2013, 5, 679–699. [Google Scholar] [CrossRef]
- Bohm, M.; Siwiec, R.M.; Wo, J.M. Diagnosis and Management of Small Intestinal Bacterial Overgrowth. Nutr. Clin. Pract. 2013, 28, 289–299. [Google Scholar] [CrossRef]
- Till, H.; Castellani, C.; Moissl-Eichinger, C.; Gorkiewicz, G.; Singer, G. Disruptions of the intestinal microbiome in necrotizing enterocolitis, short bowel syndrome, and Hirschsprung’s associated enterocolitis. Front. Microbiol. 2015, 6, 1154. [Google Scholar] [CrossRef]
- Budinska, E.; Gojda, J.; Heczkova, M.; Bratova, M.; Dankova, H.; Wohl, P.; Bastova, H.; Lanska, V.; Kostovcik, M.; Dastych, M.; et al. Microbiome and Metabolome Profiles Associated with Different Types of Short Bowel Syndrome: Implications for Treatment. J. Parenter. Enter. Nutr. 2020, 44, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Mayeur, C.; Bruneau, A.; Noordine, M.-L.; Meylheuc, T.; Langella, P.; Messing, B.; Duée, P.-H.; Cherbuy, C.; Thomas, M. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie 2010, 92, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Sommovilla, J.; Zhou, Y.; Sun, R.C.; Choi, P.M.; Diaz-Miron, J.; Shaikh, N.; Sodergren, E.; Weinstock, G.M.; Tarr, P.I.; Warner, B.W. Small Bowel Resection Induces Long-Term Changes in the Enteric Microbiota of Mice. J. Gastrointest. Surg. 2015, 19, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, A.; Pakarinen, M.P. Featuring molecular regulation of bile acid homeostasis in pediatric short bowel syndrome. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102220. [Google Scholar] [CrossRef]
- Boutte, H.J.; Chen, J.; Wylie, T.N.; Wylie, K.M.; Xie, Y.; Geisman, M.; Prabu, A.; Gazit, V.; Tarr, P.I.; Levin, M.S.; et al. Fecal microbiome and bile acid metabolome in adult short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G154–G168. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.d.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and human health impacts of volatile organic compounds: A perspective review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef]
- Zhang, V.R.; Ramachandran, G.K.; Loo, E.X.L.; Soh, A.Y.S.; Yong, W.P.; Siah, K.T.H. Volatile organic compounds as potential biomarkers of irritable bowel syndrome: A systematic review. Neurogastroenterol. Motil. 2023, 35, e14536. [Google Scholar] [CrossRef]
- Reade, S.; Williams, J.M.; Aggio, R.; Duckworth, C.A.; Mahalhal, A.; Hough, R.; Pritchard, D.M.; Probert, C.S. Potential role of fecal volatile organic compounds as biomarkers of chemically induced intestinal inflammation in mice. FASEB J. 2019, 33, 3129–3136. [Google Scholar] [CrossRef]
- Haxhija, E.Q.; Yang, H.; Spencer, A.U.; Sun, X.; Teitelbaum, D.H. Influence of the site of small bowel resection on intestinal epithelial cell apoptosis. Pediatr. Surg. Int. 2006, 22, 37–42. [Google Scholar] [CrossRef][Green Version]
- Castellani, C.; Singer, G.; Kaiser, M.; Kaiser, T.; Huang, J.; Sperl, D.; Kashofer, K.; Fauler, G.; Guertl-Lackner, B.; Höfler, G.; et al. Neuroblastoma causes alterations of the intestinal microbiome, gut hormones, inflammatory cytokines, and bile acid composition. Pediatr. Blood Cancer 2017, 64, e26425. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Amplatz, B.; Zöhrer, E.; Haas, C.; Schäffer, M.; Stojakovic, T.; Jahnel, J.; Fauler, G. Bile acid preparation and comprehensive analysis by high performance liquid chromatography–high-resolution mass spectrometry. Clin. Chim. Acta 2017, 464, 85–92. [Google Scholar] [CrossRef]
- Humbert, L.; Maubert, M.A.; Wolf, C.; Duboc, H.; Mahé, M.; Farabos, D.; Seksik, P.; Mallet, J.M.; Trugnan, G.; Masliah, J.; et al. Bile acid profiling in human biological samples: Comparison of extraction procedures and application to normal and cholestatic patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 899, 135–145. [Google Scholar] [CrossRef]
- Bergmann, A.; Trefz, P.; Fischer, S.; Klepik, K.; Walter, G.; Steffens, M.; Ziller, M.; Schubert, J.K.; Reinhold, P.; Köhler, H.; et al. In Vivo Volatile Organic Compound Signatures of Mycobacterium avium subsp. paratuberculosis. PLoS ONE 2015, 10, e0123980. [Google Scholar] [CrossRef]
- Miekisch, W.; Trefz, P.; Bergmann, A.; Schubert, J.K.; Buszewski, B.; Grzywinski, D.; Ligor, T.; Stacewicz, T.; Bielecki, Z.; Wojtas, J.; et al. Microextraction techniques in breath biomarker analysis. Bioanalysis 2014, 6, 1275–1291. [Google Scholar] [CrossRef]
- Obermuller, B.; Singer, G.; Kienesberger, B.; Mittl, B.; Stadlbauer, V.; Horvath, A.; Miekisch, W.; Fuchs, P.; Schweiger, M.; Pajed, L.; et al. Probiotic OMNi-BiOTiC((R)) 10 AAD Reduces Cyclophosphamide-Induced Inflammation and Adipose Tissue Wasting in Mice. Nutrients 2023, 15, 3655. [Google Scholar] [CrossRef]
- Klymiuk, I.; Bilgilier, C.; Stadlmann, A.; Thannesberger, J.; Kastner, M.-T.; Högenauer, C.; Püspök, A.; Biowski-Frotz, S.; Schrutka-Kölbl, C.; Thallinger, G.G.; et al. The Human Gastric Microbiome Is Predicated upon Infection with Helicobacter pylori. Front. Microbiol. 2017, 8, 2508. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, O.F. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
- Lapthorne, S.; Pereira-Fantini, P.M.; Fouhy, F.; Wilson, G.; Thomas, S.L.; Dellios, N.L.; Scurr, M.; O’Sullivan, O.; Ross, R.P.; Stanton, C.; et al. Gut microbial diversity is reduced and is associated with colonic inflammation in a piglet model of short bowel syndrome. Gut Microbes 2013, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Tecos, M.E.; Steinberger, A.E.; Guo, J.; Rubin, D.C.; Davidson, N.O.; Warner, B.W. Disruption of Enterohepatic Circulation of Bile Acids Ameliorates Small Bowel Resection Associated Hepatic Injury. J. Pediatr. Surg. 2023, 58, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Fantini, P.M.; E Bines, J.; Lapthorne, S.; Fouhy, F.; Scurr, M.; Cotter, P.D.; Gahan, C.G.; A Joyce, S. Short bowel syndrome (SBS)-associated alterations within the gut-liver axis evolve early and persist long-term in the piglet model of short bowel syndrome. J. Gastroenterol. Hepatol. 2016, 31, 1946–1955. [Google Scholar] [CrossRef]
- Ohkohchi, N.; Andoh, T.; Izumi, U.; Igarashi, Y.; Ohi, R. Disorder of bile acid metabolism in children with short bowel syndrome. J. Gastroenterol. 1997, 32, 472–479. [Google Scholar] [CrossRef]
- Pereira-Fantini, P.M.; Lapthorne, S.; Joyce, S.A.; Dellios, N.L.; Wilson, G.; Fouhy, F.; Thomas, S.L.; Scurr, M.; Hill, C.; Gahan, C.G.; et al. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease. J. Hepatol. 2014, 61, 1115–1125. [Google Scholar] [CrossRef]
- De Lacy Costello, B.; Ewen, R.; Ewer, A.K.; Garner, C.E.; Probert, C.S.; Ratcliffe, N.M.; Smith, S. An analysis of volatiles in the headspace of the faeces of neonates. J. Breath Res. 2008, 2, 037023. [Google Scholar] [CrossRef]
- Dixon, E.; Clubb, C.; Pittman, S.; Ammann, L.; Rasheed, Z.; Kazmi, N.; Keshavarzian, A.; Gillevet, P.; Rangwala, H.; Couch, R.D. Solid-phase microextraction and the human fecal VOC metabolome. PLoS ONE 2011, 6, e18471. [Google Scholar] [CrossRef]
- Garner, C.E.; Smith, S.; de Lacy Costello, B.; White, P.; Spencer, R.; Probert, C.S.J.; Ratcliffem, N.M. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007, 21, 1675–1688. [Google Scholar] [CrossRef]
- E Garner, C.; Ewer, A.K.; Elasouad, K.; Power, F.; Greenwood, R.; Ratcliffe, N.M.; Costello, B.d.L.; Probert, C.S. Analysis of faecal volatile organic compounds in preterm infants who develop necrotising enterocolitis: A pilot study. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Garner, C.; Smith, S.; Bardhan, P.; Ratcliffe, N.; Probert, C. A pilot study of faecal volatile organic compounds in faeces from cholera patients in Bangladesh to determine their utility in disease diagnosis. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Semmler, A.; Beer, C.; Voisey, J. Pyrroles as a Potential Biomarker for Oxidative Stress Disorders. Int. J. Mol. Sci. 2023, 24, 2712. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, A. Malvaria, schizophrenia and the HOD test. Int. J. Neuropsychiatry 1966, 2, 175–178. [Google Scholar] [PubMed]
- Oyerinde, A.S.; Selvaraju, V.; Babu, J.R.; Geetha, T. Potential Role of Oxidative Stress in the Production of Volatile Organic Compounds in Obesity. Antioxidants 2023, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Dryahina, K.; Smith, D.; Bortlik, M.; Machkova, N.; Lukas, M.; Spanel, P. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn’s disease and ulcerative colitis. J. Breath Res. 2017, 12, 016002. [Google Scholar] [CrossRef] [PubMed]
- Pelli, M.A.; Trovarelli, G.; Capodicasa, E.; De Medio, G.E.; Bassotti, G. Breath alkanes determination in ulcerative colitis and Crohn’s disease. Dis. Colon Rectum 1999, 42, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, J.; Nelson, R.L.; Swedler, W.I.; Skosey, J.; Abcarian, H. Determination of inflammatory bowel disease activity by breath pentane analysis. Dis. Colon Rectum 1993, 36, 597–601. [Google Scholar] [CrossRef]
- Moran-Lev, H.; Kocoshis, S.A.; Oliveira, S.B.; Helmrath, M.; Cole, C.R. Chronic Mucosal Inflammation in Pediatric Intestinal Failure Patients—A Unique Phenomenon. J. Pediatr. Gastroenterol. Nutr. 2023, 77, e29–e35. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Ben-Shahar, Y.; Pollak, Y.; Cohen, S.; Guz-Mark, A.; Shamir, R. Anti-TNF-α Therapy Exerts Intestinal Anti-inflammatory and Anti-apoptotic Effects After Massive Bowel Resection in a Rat. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 49–55. [Google Scholar] [CrossRef]
- Lilja, H.E.; Wefer, H.; Nyström, N.; Finkel, Y.; Engstrand, L. Intestinal dysbiosis in children with short bowel syndrome is associated with impaired outcome. Microbiome 2015, 3, 18. [Google Scholar]
- Davidovics, Z.H.; Carter, B.A.; Luna, R.A.; Hollister, E.B.; Shulman, R.J.; Versalovic, J. The Fecal Microbiome in Pediatric Patients with Short Bowel Syndrome. JPEN J. 2015, 40, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, F.; Li, Y.; Wang, J.; Li, J. Fecal microbiota signatures of adult patients with different types of short bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Neelis, E.G.; de Koning, B.A.E.; Hulst, J.M.; Papadopoulou, R.; Kerbiriou, C.; Rings, E.H.H.M.; Wijnen, R.M.H.; Nichols, B.; Gerasimidis, K. Gut microbiota and its diet-related activity in children with intestinal failure receiving long-term parenteral nutrition. J. Parenter. Enter. Nutr. 2022, 46, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Klymiuk, I.; Singer, G.; Castellani, C.; Trajanoski, S.; Obermüller, B.; Till, H. Characterization of the Luminal and Mucosa-Associated Microbiome along the Gastrointestinal Tract: Results from Surgically Treated Preterm Infants and a Murine Model. Nutrients 2021, 13, 1030. [Google Scholar] [CrossRef] [PubMed]
- Piper, H.G.; Coughlin, L.A.; Nguyen, V.; Channabasappa, N.; Koh, A.Y. A comparison of small bowel and fecal microbiota in children with short bowel syndrome. J. Pediatr. Surg. 2020, 55, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Yan, W.; Lu, L.; Tao, Y.; Jia, J.; Cai, W. Microbial alteration of small bowel stoma effluents and colonic feces in infants with short bowel syndrome. J. Pediatr. Surg. 2020, 55, 1366–1372. [Google Scholar] [CrossRef]
- Peters, F.B.; Bone, J.N.; Van Oerle, R.; Albersheim, S.; Casey, L.; Piper, H. The Importance of the ileocecal valve and colon in achieving intestinal independence in infants with short bowel syndrome. J. Pediatr. Surg. 2022, 57, 117–121. [Google Scholar] [CrossRef]
- Smith, A.; Namjoshi, S.; Kerner, J.A.; Dunn, J.C. Importance of Ileum and Colon in Children with Short Bowel Syndrome. J. Pediatr. Surg. 2023, 58, 1258–1262. [Google Scholar] [CrossRef]
- Berlin, P.; Barrantes, I.; Reiner, J.; Schröder, E.; Vollmar, B.; Bull, J.; Kreikemeyer, B.; Lamprecht, G.; Witte, M. Dysbiosis and reduced small intestinal function are required to induce intestinal insufficiency in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G10–G23. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, A.; Guo, F.; Wang, J.; Li, Y. Severe Intestinal Dysbiosis in Rat Models of Short Bowel Syndrome with Ileocecal Resection. Dig. Dis. Sci. 2020, 65, 431–441. [Google Scholar] [CrossRef] [PubMed]
- McKee, R.F.; Knight, K.; Leitch, E.F.; Stevens, P. The role of surgery in weaning patients from home parenteral support—A cohort study. Color. Dis. 2022, 24, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.K.; Al-Beltagi, M.; Bediwy, A.S.; El-Sawaf, Y.; Toema, O. Gut microbiota in various childhood disorders: Implication and indications. World J. Gastroenterol. 2022, 28, 1875–1901. [Google Scholar] [CrossRef] [PubMed]


| Citrulline, Membrane Proteins, Chemokine/Cytokines and Tight Junction Proteins | |||
|---|---|---|---|
| Sham | SBS | p-Value | |
| Citrulline (ng/mL) | 254.0 ± 205.7 | 339.1 ± 142.8 | 0.370 |
| Occludin (ng/mL) | 8.508 ± 3.383 | 10.710 ± 3.483 | 0.167 |
| Claudin 2 (ng/mL) | 12.43 ± 2.913 | 14.03 ± 3.406 | 0.370 |
| ENA-78 (pg/mL) | 1149.0 ± 572.7 | 1492 ± 634.8 | 0.423 |
| G-CSF (pg/mL) | 12.74 ± 6.885 | 10.21 ± 2.782 | 0.815 |
| GM-CSF (pg/mL) | 24.23 ± 5.605 | 19.37 ± 6.924 | 0.276 |
| IFN beta (pg/mL) | 61.95 ± 18.93 | 43.35 ± 20.52 | 0.114 |
| IFN gamma (pg/mL) | 1.666 ± 0.627 | 1.220 ± 0.562 | 0.191 |
| IL-1 beta (pg/mL) | 1.890 ± 1.902 | 0.641 ± 0.859 | 0.043 |
| IL-10 (pg/mL) | 13.09 ± 7.802 | 11.74 ± 7.452 | 0.943 |
| IL-17F (pg/mL) | 16.42 ± 5.279 | 11.75 ± 5.593 | 0.167 |
| IL-2 (pg/mL) | 10.72 ± 4.825 | 9.227 ± 5.457 | 0.743 |
| IL-2R (pg/mL) | 39.10 ± 19.30 | 62.42 ± 19.31 | 0.036 |
| IL-6 (pg/mL) | 65.97 ± 22.74 | 46.97 ± 25.71 | 0.200 |
| M-CSF (pg/mL) | 1.449 ± 0.516 | 1.056 ± 0.518 | 0.146 |
| MCP-1 (pg/mL) | 28.11 ± 8.401 | 21.77 ± 7.265 | 0.139 |
| MCP-5 (pg/mL) | 4.740 ± 2.892 | 3.065 ± 2.188 | 0.167 |
| MIP-1 alpha (pg/mL) | 0.419 ± 0.323 | 0.420 ± 0.389 | 0.586 |
| MIP-1 beta (pg/mL) | 1.808 ± 0.574 | 2.158 ± 0.713 | 0.384 |
| TNF alpha (pg/mL) | 10.91 ± 3.670 | 5.403 ± 4.020 | 0.014 |
| CRP (µg/mL) | 133.3 ± 68.67 | 134.4 ± 43.75 | 0.673 |
| Bile Acids | ||||
|---|---|---|---|---|
| Type | Serum Quantitative | |||
| Sham | SBS | p-Value | ||
| Chenodeoxycholic acid | primary | 0.0177 ± 0.0100 | 0.1164 ± 0.2268 | 0.0927 |
| Cholic acid | primary | 0.0126 ± 0.0227 | 2.1760 ± 4.8080 | 0.0993 |
| Tauroursodeoxycholic acid | primary | 0.0000 ± 0.0000 | 0.1793 ± 0.3470 | 0.2059 |
| Taurochenodeoxycholic acid | primary | 0.0000 ± 0.0000 | 0.0765 ± 0.0944 | 0.0294 |
| Taurocholic acid | primary | 0.3905 ± 0.4495 | 5.9760 ± 8.8150 | 0.0360 |
| Glycochenodeoxycholic acid | primary | 0.0371 ± 0.0135 | 0.0343 ± 0.0068 | 0.9626 |
| Sum primary BAs | primary | 0.4578 ± 0.4527 | 8.559 ± 12.28 | 0.0464 |
| Lithocholic acid | secondary | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 | 1.0000 |
| Deoxycholic acid | secondary | 0.1292 ± 0.1295 | 0.5447 ± 0.3098 | 0.0080 |
| Glycolithocholic acid | secondary | 0.1082 ± 0.1157 | 0.0241 ± 0.0721 | 0.0905 |
| Glycoursodeoxycholic acid | secondary | 0.0686 ± 0.0734 | 0.1064 ± 0.0603 | 0.7517 |
| Glycocholic acid | secondary | 0.1105 ± 0.0140 | 0.1438 ± 0.0620 | 0.2359 |
| Taurolithocholic acid | secondary | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 | 1.0000 |
| Taurodeoxycholic acid | secondary | 0.1322 ± 0.1089 | 0.4403 ± 0.2887 | 0.3334 |
| Glycodeoxycholic acid | secondary | 0.1703 ± 0.0023 | 0.1711 ± 0.0025 | 0.6058 |
| Sum secondary BAs | secondary | 0.719 ± 0.2963 | 1.43 ± 0.838 | 0.0464 |
| Ursodeoxycholic acid | tertiary | 0.0510 ± 0.0107 | 0.4350 ± 1.0120 | 0.0592 |
| Total Sum | 1.2270 ± 0.6919 | 10.4200 ± 13.2400 | 0.0152 | |
| Type | stool quantitative | |||
| sham | SBS | p-value | ||
| Chenodeoxycholic acid | primary | 0.0193 ± 0.0052 | 0.0371 ± 0.0423 | 0.2359 |
| Cholic acid | primary | 0.2823 ± 0.3232 | 0.2876 ± 0.2570 | 0.8148 |
| Tauroursodeoxycholic acid | primary | 0.0440 ± 0.0423 | 0.0317 ± 0.0260 | 0.1996 |
| Taurochenodeoxycholic acid | primary | 0.0185 ± 0.0112 | 0.0125 ± 0.0072 | 0.3213 |
| Taurocholic acid | primary | 0.4305 ± 0.3866 | 0.2554 ± 0.2598 | 0.1672 |
| Glycochenodeoxycholic acid | primary | 0.0003 ± 0.0005 | 0.0001 ± 0.0002 | 0.5629 |
| Sum primary BAs | primary | 0.7949 ± 0.7529 | 0.6244 ± 0.4628 | 0.5414 |
| Lithocholic acid | secondary | 2.0650 ± 1.4540 | 0.9991 ± 0.7162 | 0.0745 |
| Deoxycholic acid | secondary | 21.3900 ± 4.2740 | 14.5700 ± 10.5800 | 0.2359 |
| Glycolithocholic acid | secondary | 0.0004 ± 0.0007 | 0.0006 ± 0.0007 | 0.4923 |
| Glycoursodeoxycholic acid | secondary | 0.0004 ± 0.0008 | 0.0013 ± 0.001 | 0.1332 |
| Glycocholic acid | secondary | 0.0041 ± 0.0032 | 0.0032 ± 0.0020 | 0.8884 |
| Taurolithocholic acid | secondary | 0.0013 ± 0.0021 | 0.0003 ± 0.0004 | 0.2176 |
| Taurodeoxycholic acid | secondary | 0.1080 ± 0.0774 | 0.0791 ± 0.0796 | 0.4234 |
| Glycodeoxycholic acid | secondary | 0.0060 ± 0.0027 | 0.0122 ± 0.0209 | 0.9626 |
| Sum secondary BAs | secondary | 23.71 ± 5.354 | 15.66 ± 11.01 | 0.1672 |
| Ursodeoxycholic acid | tertiary | 1.1780 ± 0.6763 | 0.8077 ± 0.7047 | 0.1996 |
| Total Sum | 25.5500 ± 5.7610 | 17.1000 ± 11.9200 | 0.1388 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfschluckner, V.; Obermüller, B.; Horvath, A.; Rodriguez-Blanco, G.; Fuchs, P.; Miekisch, W.; Mittl, B.; Flucher, C.; Till, H.; Singer, G. Metabolomic Alterations of Volatile Organic Compounds and Bile Acids as Biomarkers of Microbial Shifts in a Murine Model of Short Bowel Syndrome. Nutrients 2023, 15, 4949. https://doi.org/10.3390/nu15234949
Wolfschluckner V, Obermüller B, Horvath A, Rodriguez-Blanco G, Fuchs P, Miekisch W, Mittl B, Flucher C, Till H, Singer G. Metabolomic Alterations of Volatile Organic Compounds and Bile Acids as Biomarkers of Microbial Shifts in a Murine Model of Short Bowel Syndrome. Nutrients. 2023; 15(23):4949. https://doi.org/10.3390/nu15234949
Chicago/Turabian StyleWolfschluckner, Vanessa, Beate Obermüller, Angela Horvath, Giovanny Rodriguez-Blanco, Patricia Fuchs, Wolfram Miekisch, Barbara Mittl, Christina Flucher, Holger Till, and Georg Singer. 2023. "Metabolomic Alterations of Volatile Organic Compounds and Bile Acids as Biomarkers of Microbial Shifts in a Murine Model of Short Bowel Syndrome" Nutrients 15, no. 23: 4949. https://doi.org/10.3390/nu15234949
APA StyleWolfschluckner, V., Obermüller, B., Horvath, A., Rodriguez-Blanco, G., Fuchs, P., Miekisch, W., Mittl, B., Flucher, C., Till, H., & Singer, G. (2023). Metabolomic Alterations of Volatile Organic Compounds and Bile Acids as Biomarkers of Microbial Shifts in a Murine Model of Short Bowel Syndrome. Nutrients, 15(23), 4949. https://doi.org/10.3390/nu15234949






