The Tolerance Model of Non-Inflammatory Immune Competence in Acute Pediatric Malnutrition: Origins, Evidence, Test of Fitness and Growth Potential
Abstract
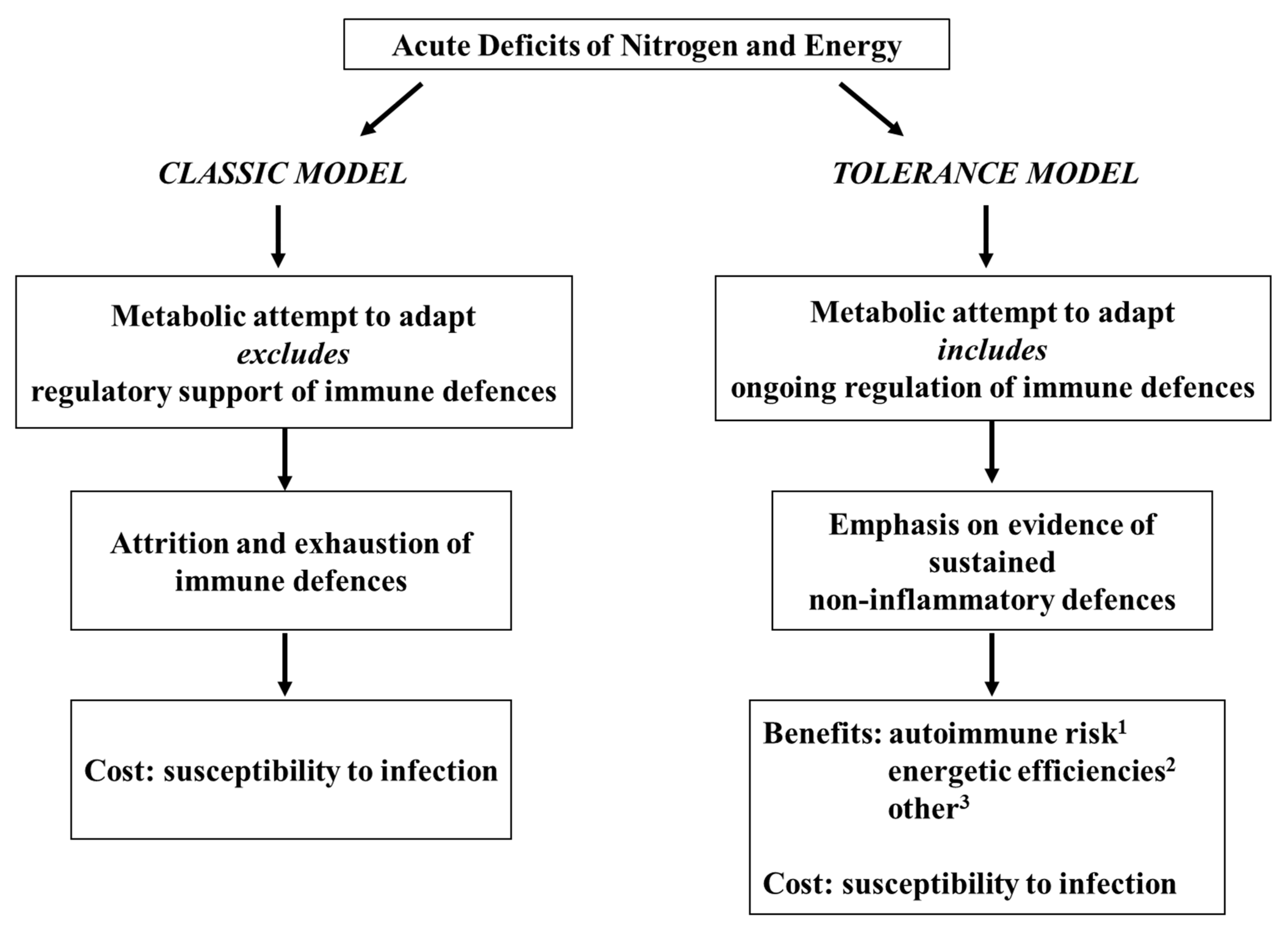
1. Introduction
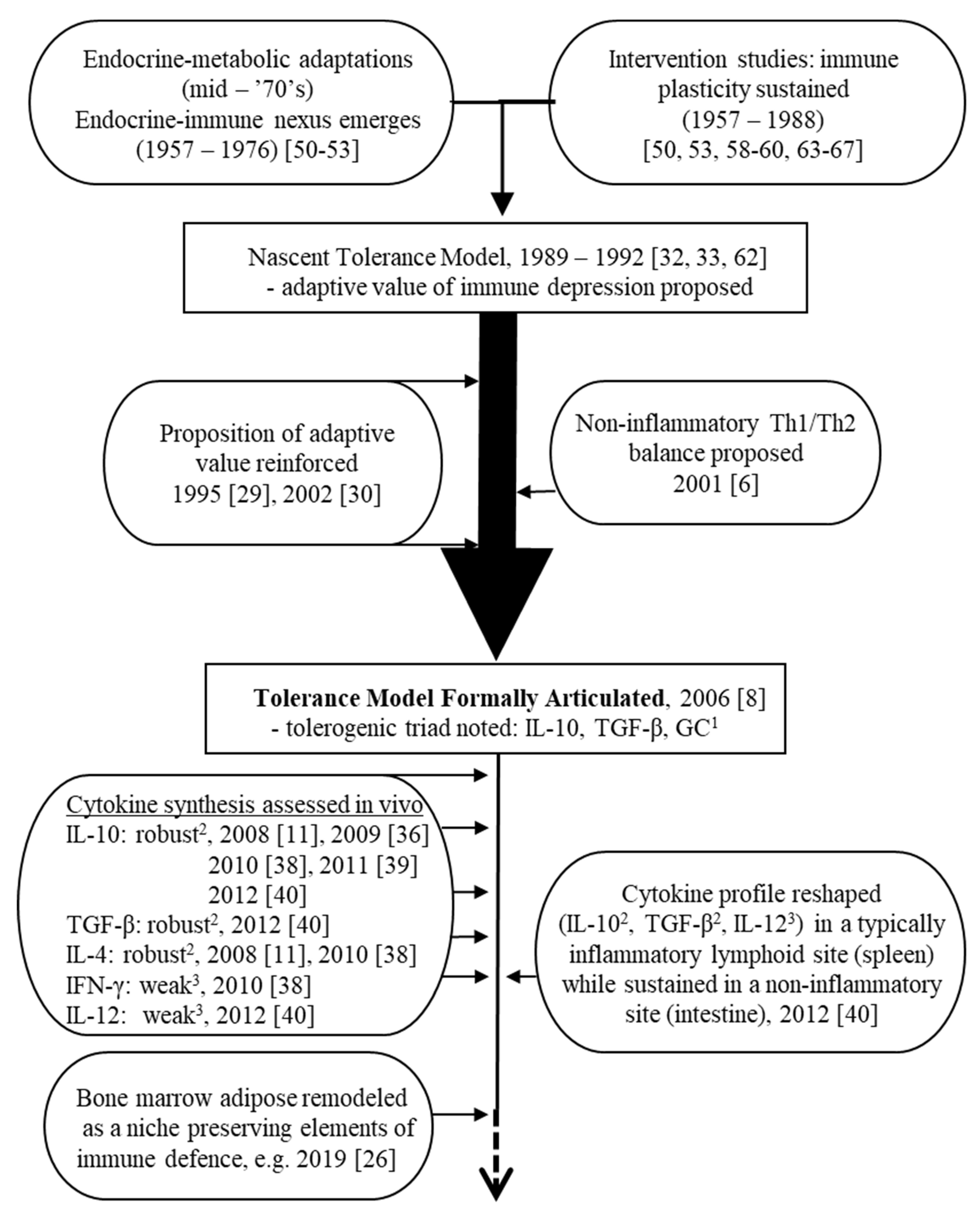
2. First Steps (1950s–1990s): Origins of a Tentative Tolerance-Centered Proposition
2.1. The Endocrine–Immune Nexus
2.2. The Earliest Intervention Studies
2.3. An Adaptive Attempt: Tolerance of Self-Antigens Enters the Discussion
3. The Tolerance Model Takes Shape (1990s–2006)
3.1. T Cell Subset Balance Suggests Quiescence in Acute Pediatric Malnutrition
3.2. Intervention Studies
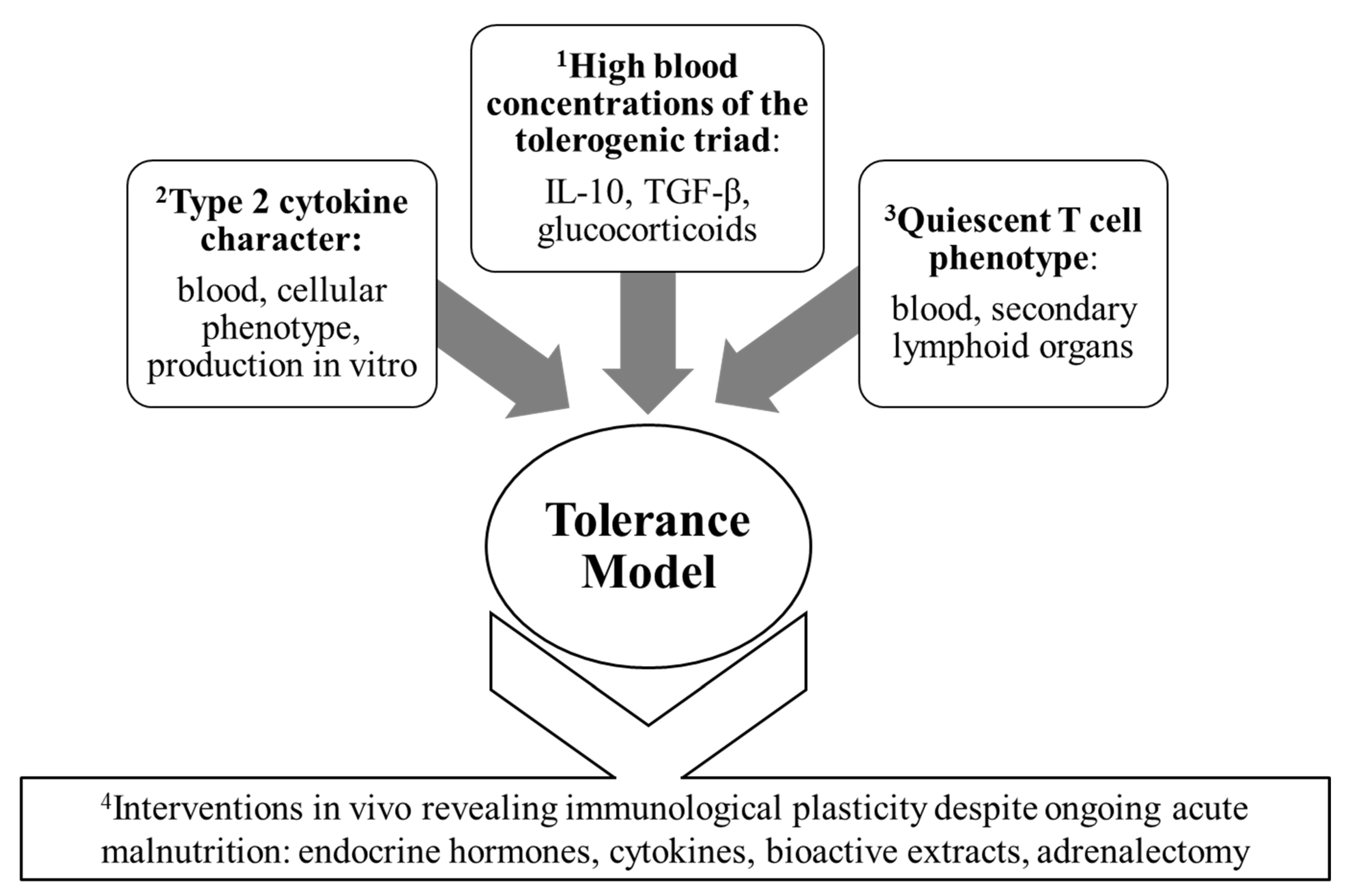
3.3. Cytokine Production and Blood Concentration Profiles: The Tolerance Model Formalized
3.4. Putative Benefits of Inflammatory Immune Depression: Newer Proposals
4. Ongoing Development of an Evidence Base Consistent with the Tolerance Model (2007 to the Present)
4.1. Blood Cytokine Concentrations
4.2. The Cytokine Signature of Mononuclear Cells from the Blood and Lymphoid Organs
4.3. Cytokine Assay Techniques Key to the Development of the Tolerance Model
4.4. Intervention Studies
4.5. Metabolic Indices
5. Fitness Test: Application of the Tolerance Model to a Published Clinical Study of Acutely Malnourished Infants and Children
6. The Tolerance Model: Robustness and Prospects
6.1. Does Acute Pediatric Malnutrition Impose a Risk of Autoimmune Disease?
6.2. Is a High Risk of Autoimmune Pathologies Essential to the Tolerance Model?
6.3. Is an Emphasis on Secondary Immune Deficiency Essential to the Tolerance Model?
6.4. Can the Tolerance Model Accommodate Evidence of an Inflammatory Response?
6.5. Can the Tolerance Model Co-Evolve with Immunobiology?
7. Concluding Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNICEF/WHO/World Bank Group Joint Malnutrition Estimates, May 2023 Edition. Available online: https://data.unicef.org/topic/nutrition/malnutrition/ (accessed on 5 August 2023).
- Dipasquale, V.; Cucinotta, U.; Romano, C. Acute Malnutrition in Children: Pathophysiology, Clinical Effects and Treatment. Nutrients 2020, 12, 2413. [Google Scholar] [CrossRef]
- Michael, H.; Amimo, J.O.; Rajashikara, G.; Saif, L.J.; Vlasova, A.N. Mechanisms of Kwashiorkor-Associated Immune Suppression: Insights from Human, Mouse, and Pig Studies. Front. Immunol. 2022, 13, 826268. [Google Scholar] [CrossRef] [PubMed]
- Woodward, B. Protein, Calories, and Immune Defenses. Nutr. Rev. 1998, 56, S84–S92. [Google Scholar] [CrossRef]
- Rytter, M.J.H.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The Immune System in Children with Malnutrition—A Systematic Review. PLoS ONE 2014, 9, e105017. [Google Scholar] [CrossRef] [PubMed]
- Woodward, B. The Effect of Protein-Energy Malnutrition on Immune Competence. In Nutrition, Immunity, and Infection in Infants and Children; Suskind, R.M., Tontisirin, K., Eds.; Vevey/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 89–120. [Google Scholar]
- Woodward, B. Fidelity in Animal Modeling: Prerequisite for a Mechanistic Research Front Relevant to the Inflammatory Incompetence of Acute Pediatric Malnutrition. Int. J. Molec. Sci. 2016, 17, 541. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, L.; Dao, B.; Niemiec, P.; Lee, S.; Doidge, M.; Bemben, I.; Neyestani, T.; Woodward, B. Elevated Bioactivity of the Tolerogenic Cytokines, Interleukin-10 and Transforming Growth Factor-β, in the Blood of Acutely Malnourished Weanling Mice. Exp. Biol. Med. 2006, 231, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, L.M.; Woodward, B. Acutely Malnourished Weanling Mice Administered Flt3 Ligand Can Support a Cell-Mediated Inflammatory Response. Cytokine 2019, 113, 39–49. [Google Scholar] [CrossRef]
- Santos, E.W.; Oliveira, D.C.; Silva, G.B.; Tsujita, M.; Beltran, J.O.; Hastreiter, A.; Fock, R.A.; Borelli, P. Hematological Alterations in Protein Malnutrition. Nutr. Rev. 2017, 75, 909–919. [Google Scholar] [CrossRef]
- Fock, R.A.; Vinolo, M.A.R.; Crisma, A.R.; Nakajima, K.; Rogero, M.M.; Borelli, P. Protein-Energy Malnutrition Modifies the Production of Interleukin-10 in Response to Lipopolysaccharide (LPS) in a Murine Model. J. Nutr. Sci. Vitaminol. 2008, 54, 371–377. [Google Scholar] [CrossRef]
- Hughes, S.M.; Amadi, B.; Mwiya, M.; Nkamba, H.; Tomkins, A.; Goldblatt, D. Dendritic Cell Anergy Results from Endotoxemia in Severe Malnutrition. J. Immunol. 2009, 183, 2818–2826. [Google Scholar] [CrossRef]
- Mello, A.S.; de Oliveira, D.C.; Bizzarro, B.; Sa-Nunes, A.; Hastreiter, A.A.; de Oliveira Beltran, J.S.; Xavier, J.G.; Borelli, P.; Fock, R.A. Protein Malnutrition Alters Spleen Cell Proliferation and IL-2 and IL-10 Production by Affecting the STAT-1 and STAT-3 Balance. Inflammation 2014, 37, 2125–2138. [Google Scholar] [CrossRef]
- Bourke, C.D.; Berkley, J.A.; Prendergast, J.A. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of Childhood Malnutrition on Host Defense and Infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef]
- Thaxton, G.E.; Melby, P.C.; Manary, M.J.; Preidis, G.A. New Insights into the Pathogenesis and Treatment of Malnutrition. Gastroenterol. Clin. N. A. 2018, 47, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Paes, T.; Seixas-Costa, P.; Almeida-Amaral, E.E. Validation of a Feed Protocol in a Mouse Model that Mimics Marasmic Malnutrition. Front. Vet. Sci. 2021, 8, 757136. [Google Scholar] [CrossRef]
- Singh, G.; Tucker, E.W.; Rohlwink, U.K. Infection in the Developing Brain: The Role of Unique Systemic Immune Vulnerabilities. Front. Neurol. 2022, 12, 805643. [Google Scholar] [CrossRef]
- Morgan, G. What, If Any, Is the Effect of Malnutrition on Immunological Competence? Lancet 1997, 349, 1693–1695. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin Modulates the T-Cell Immune Response and Reverses Starvation-Induced Immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Lord, G.M.; Matarese, G.; Vendetti, S.; Ghatei, M.A.; Ritter, M.A.; Lechler, R.I.; Bloom, S.R. Leptin Protects Mice from Starvation-Induced Lymphoid Atrophy and Increases Thymic Cellularity in ob/ob Mice. J. Clin. Investig. 1999, 104, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M. The Thymus: A Barometer of Malnutrition. Br. J. Nutr. 1999, 81, 345–347. [Google Scholar] [CrossRef]
- Faggioni, R.; Moser, A.; Feingold, K.R.; Grunfeld, C. Reduced Leptin Levels in Starvation Increase Susceptibility to Endotoxic Shock. Am. J. Pathol. 2000, 156, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, R.; Feingold, K.R.; Grunfeld, C. Leptin Regulation of the Immune Response and the Immunodeficiency of Malnutrition. FASEB J. 2001, 15, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Saucillo, D.C.; Gerriets, V.A.; Sheng, J.; Rathmell, J.C.; MacIver, N.J. Leptin Metabolically Licences T Cells for Activation to Link Nutrition and Immunity. J. Immunol. 2014, 192, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Han, S.-J.; Enamorado, M.; Link, V.M.; Huang, B.; Moseman, E.A.; Kishton, R.J.; Shannon, J.P.; Dixit, D.; Schwab, S.R.; et al. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell 2019, 178, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Nikkanen, J.; Man, K.; Leong, Y.A.; Sogawa, Y.; Maschek, J.A.; van Ry, T.; Chagwedera, D.N.; Cox, J.E.; Chawla, A. Energetic Trade-Offs and Hypometabolic States Promote Disease Tolerance. Cell 2019, 177, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Trim, V.W.; Lynch, L. Immune and Non-Immune Functions of Adipose Tissue Leukocytes. Nature Rev. Immunol. 2022, 22, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Borelli, P.; Mariano, M.; Borojevic, R. Protein Malnutrition: Effect on Myeloid Cell Production and Mobilization into Inflammatory Reactions in Mice. Nutr. Res. 1995, 15, 1477–1485. [Google Scholar] [CrossRef]
- Matarese, G.; La Cava, A.; Sanna, V.; Lord, G.M.; Lechler, R.I.; Fontana, S.; Zappacosta, S. Balancing Susceptibility to Infection and Autoimmunity: A Role for Leptin? Trends Immunol. 2002, 23, 182–187. [Google Scholar] [CrossRef]
- Fock, R.A.; Vinolo, M.A.R.; de Moura Sa Rocha, V.; de Sa Rocha, L.C.; Borelli, P. Protein-Energy Malnutrition Decreases the Expression of TLR-4/MD-2 and CD14 Receptors in Peritoneal Macrophages and Reduces the Synthesis of TNF-α in Response to Lipopolysaccharide in Mice. Cytokine 2007, 40, 105–114. [Google Scholar] [CrossRef]
- Woodward, B.; Filteau, S.M. Immunoenhancement in Wasting Protein-Energy Malnutrition: Assessment of Present Information and Proposal of a New Concept. Adv. Nutr. Res. 1990, 8, 11–34. [Google Scholar] [CrossRef]
- Woodward, B. Influence of Wasting Protein-Energy Malnutrition on Apparent Thymic T Cell Inductive Capacity and on Recirculating Lymphocyte Pool Sizes in the Weanling Mouse. In Nutrition and Immunology; Chandra, R.K., Ed.; ARTS Biomedical Publishers and Distributors: St. John’s, NL, Canada, 1992; pp. 163–177. [Google Scholar]
- Woodward, B.; Monk, J.M. The Tolerance Model: A Non-inflammatory Form of Immune Competence Prevails in Acute Pre-pubescent Malnutrition. In Malnutrition: Risk Factors, Health Effects and Prevention; Knudsen, J.B., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 73–90. [Google Scholar]
- Hillyer, L.M.; Maliwichi, H.E.; Woodward, B. Blood Serum Interferon-γ Bioactivity Is Low in Weanling Mice Subjected to Acute Deficits of Energy or Both Protein and Energy. Br. J. Nutr. 2007, 97, 528–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monk, J.M.; Woodward, B. Elevated Blood Interleukin-10 Levels and Undiminished Systemic Interleukin-10 Production Rate Prevail Throughout Acute Protein-Energy Malnutrition in the Weanling Mouse. Cytokine 2009, 47, 126–131. [Google Scholar] [CrossRef]
- Monk, J.M.; Woodward, B. The Blood Level of Transforming Growth Factor-β Rises in the Early Stages of Acute Protein and Energy Deficit in the Weanling Mouse. Br. J. Nutr. 2010, 103, 886–889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steevels, T.A.M.; Hillyer, L.M.; Monk, J.M.; Fisher, M.E.; Woodward, B.D. Effector/Memory T Cells of the Weanling Mouse Exhibit Type 2 Cytokine Polarization In Vitro and In Vivo in the Advanced Stages of Acute Energy Deficit. J. Nutr. Biochem. 2010, 21, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Steevels, T.A.M.; Hillyer, L.M.; Woodward, B. Constitutive, but Not Challenge-Induced, Interleukin-10 Production Is Robust in Acute Pre-pubescent Protein and Energy Deficits: New Support for the Tolerance Hypothesis of Malnutrition-Associated Immune Depression Based on Cytokine Production In Vivo. Int. J. Environ. Res. Pub. Hlth. 2011, 8, 117–135. [Google Scholar] [CrossRef]
- Monk, J.M.; Richard, C.L.; Woodward, B. A Non-inflammatory Form of Immune Competence Prevails in Acute Pre-pubescent Malnutrition: New Evidence Based on Critical mRNA Transcripts in the Mouse. Br. J. Nutr. 2012, 107, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.J. Evolution of kwashiorkor and marasmus. Lancet 1974, 303, 709–711. [Google Scholar] [CrossRef]
- Hansen, J.D.L. Endocrines and Malnutrition. In Protein-Calorie Malnutrition; Olson, R.E., Ed.; Academic Press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1975; pp. 229–241. [Google Scholar]
- Pimstone, B. Endocrine Function in Protein-Calorie Malnutrition. Clin. Endocrinol. 1976, 5, 79–95. [Google Scholar]
- Anonymous. Adaptation in Protein Calorie Malnutrition (PCM). Nutr. Rev. 1979, 37, 250–252. [Google Scholar]
- Brasel, J.A. Endocrine Adaptation to Malnutrition. Pediatr. Res. 1980, 14, 1299–1303. [Google Scholar] [CrossRef]
- Becker, D.J. The Endocrine Responses to Protein Calorie Malnutrition. Ann. Rev. Nutr. 1983, 3, 187–212. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentic, S.; Sestan, M.; Wensveen, T.T.; Polic, B. Interactions Between Adipose Tissue and the Immune System in Health and Malnutrition. Seminars Immunol. 2015, 27, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Goldstein, J.L.; Shi, X.; Liang, G.; Brown, M.S. Unexpected Role for IGF-1 in Starvation: Maintenance of Blood Glucose. Proc. Nat. Acad. Sci. USA 2022, 119, e2208855119. [Google Scholar] [CrossRef]
- Savino, W.; Duraes, J.; Maldonado-Galdeano, C.; Perdigon, G.; Mendes-da-Cruz, D.A.; Cuervo, P. Thymus, Undernutrition, and Infection: Approaching Cellular and Molecular Interactions. Frontiers Nutr. 2022, 9, 948488. [Google Scholar] [CrossRef] [PubMed]
- Aschkenasy, A. On the Pathogenesis of Anemias and Leukopenias Induced by Dietary Protein Deficiency. Am. J. Clin. Nutr. 1957, 5, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Schonland, M.M.; Shanley, B.C.; Loening, W.E.K.; Parent, M.A.; Coovadia, H.M. Plasma Cortisol and Immunosuppression in Protein-Calorie Malnutrition. Lancet 1972, 300, 435–436. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.; Hamid, J. Cell-Mediated Immune Response in Malnutrition. Clin. Exp. Immunol. 1973, 13, 153–164. [Google Scholar] [PubMed]
- Bell, R.G.; Hazell, L.A.; Price, P. Influence of Dietary Protein Restriction on Immune Competence II. Effect on Lymphoid Tissue. Clin. Exp. Immunol. 1976, 26, 314–326. [Google Scholar]
- Thurstans, S.; Opondo, C.; Seal, A.; Wells, J.C.; Khara, T.; Dolan, C.; Briend, A.; Myatt, M.; Garenne, M.; Mertens, A.; et al. Understanding Sex Differences in Childhood Undernutrition: A Narrative Review. Nutrients 2022, 14, 948. [Google Scholar] [CrossRef]
- Lee, A.H.; Dixit, V.D. Dietary Regulation of Immunity. Immunity 2020, 53, 510–523. [Google Scholar] [CrossRef]
- Chandra, R.K.; Newberne, P.M. Immunocompetence in Undernutrition. In Nutrition, Immunity, and Infection; Plenum Press: New York, NY, USA, 1977; pp. 67–126. [Google Scholar]
- Gross, R.L.; Newberne, P.M. Role of Nutrition in Immunologic Function. Physiol. Rev. 1980, 60, 188–302. [Google Scholar] [CrossRef] [PubMed]
- Filteau, S.M.; Perry, K.J.; Woodward, B. Triiodothyronine Improves the Primary Antibody Response to Sheep Red Blood Cells in Severely Undernourished Weanling Mice. Proc. Soc. Exp. Biol. Med. 1987, 185, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Filteau, S.M.; Berdusco, E.; Perry, K.J.; Woodward, B. The Effect of Triiodothyronine on Evanescent Delayed Hypersensitivity to Sheep Red Blood Cells and on the Primary Antibody Response to Trinitrophenylated Brucella abortus in Severely Undernourished Weanling Mice. Int. J. Immunopharmac. 1987, 9, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Perry, K.J.; Filteau, S.M.; Woodward, B. Dissociation of Immune Capacity from Nutritional Status by Triiodothyronine Supplements in Severe Protein Deficiency. FASEB J. 1988, 2, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.W.; Woodward, B.D. Enhancement of Primary Systemic Acquired Immunity by Exogenous Triiodothyronine in Wasted, Protein-Energy Malnourished Weanling Mice. J. Nutr. 1991, 121, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Woodward, W.D. Morphometric and Functional Studies of the Thymus and Production of Cytokines in Protein-Energy Malnutrition. In Proceedings of the XIV International Congress of Nutrition, Seoul, Republic of Korea, 20–25 August 1989; Volume I, pp. 281–284. [Google Scholar]
- Hoffman-Goetz, L.; Kluger, M.J. Protein Deprivation: Its Effect on Fever and Plasma Iron During bacterial Infection in Rabbits. J. Physiol. 1979, 295, 419–430. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ishii, S.; Nishioka, K. Heightened Resistance Against Listeria monocytogenes Infection in Malnourished Rats After Lentinan Treatment: Correlation with C3 Levels. Nutr. Res. 1983, 3, 705–718. [Google Scholar] [CrossRef]
- Moldawer, L.L.; Hamawy, K.J.; Bistrian, B.R.; Giorgieff, M.; Drabik, M.; Dinarello, C.A.; Blackburn, G.L. A Therapeutic Use for Interleukin-1 in the Protein-Depleted Animal. Br. J. Rheumatol. 1985, 24 (Suppl. S1), 220–223. [Google Scholar] [CrossRef]
- Watson, R.R.; Chien, G.; Chung, C. Thymosin Treatment: Serum Corticosterone and Lymphocyte Mitogenesis in Moderately and Severely Protein-Malnourished Mice. J. Nutr. 1983, 113, 483–493. [Google Scholar] [CrossRef]
- Watson, R.R.; Lim, T.S. Thymosin Fraction 5: Effects on T Cell Functions in Mice Immunosuppressed by Severe Dietary Protein Deficiency. Int. J. Immunopharmacol. 1986, 8, 545–552. [Google Scholar] [CrossRef]
- Craddock, C.G. Corticosteroid-Induced Lymphopenia, Immunosuppression, and Body Defense. Ann. Intern. Med. 1978, 88, 564–566. [Google Scholar] [CrossRef]
- Baker, C.C. Immune Mechanisms and Host Resistance in the Trauma Patient. Yale J. Biol. Med. 1986, 59, 387–393. [Google Scholar] [PubMed]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An Emerging Frontier. Nat. Rev. Immunol. 2011, 11, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Woodward, B. The Cellular Basis for Depressed Acquired Immunity in Wasting Protein-Energy Malnutrition: Assessment of Experimental Evidence with Emphasis on Cellular Numbers and Subset Imbalances. J. Chinese Nutr. Soc. 1994, 19, 101–123. [Google Scholar]
- Woodward, B.; Miller, R.G. Depression of Thymus-Dependent Immunity in Wasting Protein-Energy Malnutrition Does Not Depend on an Altered Ratio of Helper (CD4+) to Suppressor (CD8+) T Cells or on a Disproportionately Large Atrophy of the T Cell Relative to the B Cell Pool. Am. J. Clin. Nutr. 1991, 53, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Woodward, B.D.; Bezanson, K.D.; Hillyer, L.M.; Lee, W.-H. The CD45RA+ (Quiescent) Cellular Phenotype Is Overabundant Relative to the CD45RA− Phenotype Within the Involuted Splenic T Cell Population of Weanling Mice Subjected to Wasting Protein-Energy Malnutrition. J. Nutr. 1995, 125, 2471–2482. [Google Scholar] [PubMed]
- Lee, W.-H.; Woodward, B. The CD4/CD8 Ratio in the Blood Does Not Reflect the Response of This Index in Secondary Lymphoid Organs of Weanling Mice in Models of Protein-Energy Malnutrition Known to Depress Thymus-Dependent Immunity. J. Nutr. 1996, 126, 849–859. [Google Scholar] [CrossRef]
- Woodward, B.; Hillyer, L.; Hunt, K. T Cells with a Quiescent Phenotype (CD45RA+) Are Overabundant in the Blood and Involuted Lymphoid Tissues in Wasting Protein and Energy Deficiencies. Immunology 1999, 96, 246–253. [Google Scholar] [CrossRef]
- Ha, C.-L.; Wong, S.S.-L.; Gray, M.M.; Watt, J.; Hillyer, L.M.; Woodward, B. Overabundance of CD45RA+ (Quiescent-Phenotype) Cells Within the Involuted CD4+ T Cell Population Follows Immune Depression in the Energy-Deficient Weanling Mouse and Reflects Involution Exclusive to the CD45RA− Subset. J. Nutr. 2001, 131, 1812–1818. [Google Scholar] [CrossRef]
- ten Bruggencate, S.J.M.; Hillyer, L.M.; Woodward, B.D. The Proportion of CD45RA+CD62L+ (Quiescent-Phenotype) T Cells within the CD8+ Subset Increases in Advanced Weight Loss in the Protein- or Energy-Deficient Weanling Mouse. J. Nutr. 2001, 131, 3266–3269. [Google Scholar] [CrossRef]
- Najera, O.; Gonzalez, C.; Cortes, E.; Toledo, G.; Ortiz, R. Effector T Lymphocytes in Well-Nourished and Malnourished Infected Children. Clin. Exp. Immunol. 2007, 148, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Ingram, K.G.; Crouch, D.A.; Douez, D.L.; Croy, B.A.; Woodward, B. Effects of Triiodothyronine Supplements on Splenic Natural Killer Cells in Malnourished Weanling Mice. Int. J. Immunopharmac. 1995, 17, 21–32. [Google Scholar] [CrossRef]
- Hill, A.D.K.; Naama, H.; Shou, J.; Calvano, S.E.; Daly, J.M. Antimicrobial Effects of Granulocyte-Macrophage Colony-Stimulating Factor in Protein-Energy Malnutrition. Arch. Surg. 1995, 130, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.D.K.; Naama, H.A.; Gallagher, H.J.; Shou, J.; Calavano, S.E.; Daly, J.M. Glucocorticoids Mediate Macrophage Dysfunction in Protein Calorie Malnutrition. Surgery 1995, 118, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Takahashi, K.; Naito, M.; Fujiyama, S. The Role of Macrophage Colony-Stimulating Factor in the Differentiation and Proliferation of Kupffer Cells in the Liver of Protein-Deprived Mice. Lab. Investig. 1995, 72, 696–706. [Google Scholar]
- Hidemura, A.; Saito, H.; Fukatsu, K.; Matsuda, T.; Kitayama, J.; Ikeda, S.; Kang, W.; Nagawa, H. Oral Administration of Bifidobacterium longum Culture Condensate in a Diet-Restricted Murine Peritonitis Model Enhances Polymorphonuclear Neutrophil Recruitment into the Local Inflammatory Site. Nutrition 2003, 19, 270–274. [Google Scholar] [CrossRef]
- Mengheri, E.; Nobili, F.; Crocchioni, G.; Lewis, J.A. Protein Starvation Impairs the Ability of Activated Lymphocytes to Produce Interferon-γ. J. Interferon Res. 1992, 12, 17–21. [Google Scholar] [CrossRef]
- Hagel, I.; Lynch, N.R.; Di Prisco, M.C.; Sanchez, J.; Perez, M. Nutritional Status and the IgE Response Against Ascaris Lumbricoides in Children from a Tropical Slum. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 562–565. [Google Scholar] [CrossRef]
- Chan, J.; Tian, Y.; Tanaka, K.E.; Tsang, M.S.; Yu, K.; Salgame, P.; Carroll, D.; Kress, Y.; Teitelbaum, R.; Bloom, B.R. Effects of Protein Calorie Malnutrition on Tuberculosis in Mice. Proc. Natl. Acad. Sci. USA 1996, 93, 14857–14861. [Google Scholar] [CrossRef]
- McMurray, D.N. Impact of Nutritional Deficiencies on Resistance to Experimental Pulmonary Tuberculosis. Nutr. Rev. 1998, 56, S147–S152. [Google Scholar] [CrossRef]
- Palacio, A.; Lopez, M.; Perez-Bravo, F.; Monkeberg, F.; Schlesinger, L. Leptin Levels Are Associated with Immune Response in Malnourished Infants. J. Clin. Endocrinol. Metab. 2002, 87, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Gonzalez, C.; Flores, L.; Jimenez-Zamudio, L.; Graniel, J.; Ortiz, R. Assessment by Flow Cytometry of Cytokine Production in Malnourished Children. Clin. Diagn. Lab. Immunol. 2005, 12, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Westermann, J.; Pabst, R. Distribution of lymphocytes and natural killer cells in the human body. Clin. Investig. 1992, 70, 539–544. [Google Scholar] [CrossRef]
- Bienvenu, J.A.D.; Monneret, G.; Gutowski, M.C.L.; Fabien, N. Cytokine Assays in Human Sera and Tissues. Toxicology 1998, 129, 55–61. [Google Scholar] [CrossRef]
- Dai, G.; McMurray, D.N. Altered Cytokine Production and Impaired Antimycobacterial Immunity in Protein-Malnourished Guinea Pigs. Infect. Immun. 1998, 66, 3562–3568. [Google Scholar] [CrossRef]
- Woodward, B. Depressed Adaptive Immune Competence in Acute Protein-Energy Malnutrition: A Regulated Pathophysiology Controlled by Endocrine Hormones and Cytokines. In Nutrition and Immunology in the 21st Century; Chandra, R.K., Ed.; TSAR Health: Toronto, ON, Canada, 2004; pp. 23–38. [Google Scholar]
- Kuby, J. Hypersensitivity Reactions. In Immunology, 3rd ed.; W.H. Freeman and Co.: New York, NY, USA, 1997; pp. 413–439. [Google Scholar]
- Schaible, U.E.; Kaufmann, S.H.E. Malnutrition and Infection: Complex Mechanisms and Global Impacts. PLoS Med. 2007, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Huang, Z.; Liang, W.-C.; Yin, J.; Lin, W.Y.; Wu, J.; Vernes, J.-M.; Lutman, J.; Caplazi, P.; Jeet, S.; et al. TGFβ2 and TGFβ3 Isoforms Drive Fibrotic Disease Pathogenesis. Science Translat. Med. 2021, 13, eabe0407. [Google Scholar] [CrossRef]
- Monk, J.M.; Makinen, K.; Shrum, B.; Woodward, B. Blood Corticosterone Concentration Reaches Critical Illness Levels Early During Acute Malnutrition in the Weanling Mouse. Exp. Biol. Med. 2006, 231, 264–268. [Google Scholar] [CrossRef]
- Gonzalez-Torres, C.; Gonzalez-Martinez, H.; Miliar, A.; Najera, O.; Graniel, J.; Firo, V.; Alvarez, C.; Bonilla, E.; Rodriguez, L. Effect of Malnutrition on the Expression of Cytokines Involved in Th1 Cell Differentiation. Nutrients 2013, 5, 579–593. [Google Scholar] [CrossRef]
- Badr, G.; Sayed, D.; Ibrahim, M.A.; Elsayh, K.I.; Ahmed, E.A.; Alwasel, S.H. T Lymphocytes from Malnourished Infants Are Short-Lived and Dysfunctional Cells. Immunobiology 2011, 216, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune Regulation by Glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Shipp, K.; Woodward, B. A Simple Exsanguination Method That Minimizes Acute Pre-Anesthesia Stress in the Mouse: Evidence Based on Serum Corticosterone Concentrations. Contemp. Top. Lab. Anim. Sci. 1998, 37, 73–77. [Google Scholar]
- Sauerwein, R.W.; Mulder, J.H.A.; Mulder, L.; Lowe, B.; Peshu, N.; Demacker, P.N.; van der Meer, J.W.; Marsh, K. Inflammatory Mediators in Children with Protein-Energy Malnutrition. Am. J. Clin. Nutr. 1997, 65, 1534–1539. [Google Scholar] [CrossRef]
- Dulger, H.; Arik, M.; Sekeroglu, M.R.; Tarakcioglu, M.; Noyan, T.; Cesur, Y.; Balahoroglu, R. Pro-Inflammatory Cytokines in Turkish Children with Protein-Energy Malnutrition. Mediators Inflamm. 2002, 11, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Bartz, S.; Mody, A.; Hornik, C.; Bain, J.; Meuhlbauer, M.; Kiyimba, T.; Kiboneka, E.; Stevens, R.; Bartlett, J.; St. Peter, J.V.; et al. Severe Acute Malnutrition in Childhood: Hormonal and Metabolic Status at Presentation, Response to Treatment, and Predictors of Mortality. J. Clin. Endocrinol. Metab. 2014, 99, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.T.; Osorio, E.Y.; Peniche, A.; Dann, S.M.; Cordova, E.; Preidis, G.A.; Suh, J.H.; Ito, I.; Saldarriaga, O.A.; Loeffelholz, M.; et al. Pathologic Inflammation in Malnutrition Is Driven by Proinflammatory Intestinal Microbiota, Large Intestine Barrier Dysfunction, and Translocation of Bacterial Lipopolysaccharide. Front. Immunol. 2022, 13, 846155. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The Master Regulator of Immunity to Infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Carlos, A.R.; Weis, S.; Soares, M.P. Cross-Talk Between Iron and Glucose Metabolism in the Establishment of Disease Tolerance. Front. Immunol. 2018, 9, 2498. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.D.; James, K.M.; Ippolito, J.R.; Barney, D.E., Jr.; Miller, K.M.; Murphy, N.E.; Gwin, J.A.; Pasiakos, S.M.; McClung, J.P.; Margolis, L.M.; et al. Mild to Moderate Food Deprivation Increases Hepcidin and Results in Hypoferremia and Tissue Iron Sequestration in Mice. J. Nutr. 2022, 152, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.-P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of Adipose Tissue Inflammation by Interleukin 6. Proc. Nat. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef]
- Zganiacz, A.; Santosuosso, M.; Wang, J.; Yang, T.; Chen, L.; Anzulovic, M.; Alexander, S.; Gicquel, B.; Wan, Y.; Bramson, J.; et al. TNF-α Is a Critical Negative Regulator of Type 1 Immune Activation During Intracellular Bacterial Infection. J. Clin. Investig. 2004, 113, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-Mediated Inflammatory Disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor Necrosis Factor α and Regulatory T Cells in Oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Graniel, J.; Ortiz, R. Effect of Leptin on Activation and Cytokine Synthesis in Peripheral Blood Lymphocytes of Malnourished Infected Children. Clin. Exp. Immunol. 2007, 148, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, H.; Rodriguez, L.; Najera, O.; Cruz, D.; Miliar, A.; Dominguez, A.; Sanchez, F.; Graniel, J.; Gonzalez-Torres, M.C. Expression of Cytokine mRNA in Lymphocytes of Malnourished Children. J. Clin. Immunol. 2008, 28, 593–599. [Google Scholar] [CrossRef]
- Mire-Sluis, A.R.; Gaines-Das, R.; Thorpe, R. Immunoassays for Detecting Cytokines: What Are They Really Measuring? J. Immunol. Methods 1995, 186, 157–160. [Google Scholar] [CrossRef]
- Malone, D.; Napolitano, L.M.; Genuit, T.; Bochicchio, G.V.; Kole, K.; Scalea, T.M. Total Cytokine Immunoassay: A More Accurate Method of Cytokine Measurement? J. Trauma. 2001, 50, 821–825. [Google Scholar] [CrossRef]
- Hillyer, L.M.; Woodward, B. Interleukin-10 Concentration Determined by Sandwich Enzyme-Linked Immunosorbent Assay Is Unrepresentative of Bioactivity in Murine Blood. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1514–R1519. [Google Scholar] [CrossRef]
- Khan, S.A.; Joyce, J.; Tsuda, T. Quantification of Active and Total Transforming Growth Factor-β Levels in Serum and Solid Organ Tissues by Bioassay. BMC Res. Notes 2012, 5, 636. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Morris, S.C. Development of an Assay to Measure In Vivo Cytokine Production in the Mouse. Int. Immunol. 1999, 11, 1811–1818. [Google Scholar] [CrossRef]
- Hillyer, L.; Whitley, C.; Olver, A.; Webster, M.; Steevels, T.; Woodward, B. Adoptively Transferred Dendritic Cells Restore Primary Cell-Mediated Inflammatory Competence to Acutely Malnourished Weanling Mice. Am. J. Pathol. 2008, 172, 378–385. [Google Scholar] [CrossRef]
- Tanaka, M.; Suganami, T.; Kim-Saijo, M.; Toda, C.; Tsuiji, M.; Ochi, K.; Kamei, Y.; Minokoshi, Y.; Ogawa, Y. Role of Central Leptin Signaling in the Starvation-Induced Alteration of B-Cell Development. J. Neurosci. 2011, 31, 8373–8380. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Crisma, A.R.; Nakajima, K.; Rogero, M.M.; Fock, R.A.; Borelli, P. Malnourished Mice Display an Impaired Hematologic Response to Granulocyte Colony-Stimulating Factor Administration. Nutr. Res. 2008, 28, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Markus, G.M. Dendritic Cell Homeostasis. Blood 2009, 113, 3418–3427. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.S.; Burke, E.J.; Marten, N.W. Induction of Insulin-Like Growth Factor Binding Protein-1 Gene Expression in Liver of Protein-Restricted Rats and in Rat Hepatoma Cells Limited for a Single Amino Acid. Endocrinology 1993, 132, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.S.; Marten, N.W.; Hayden, J.M.; Burke, E.J. Protein Restriction Specifically Decreases the Abundance of Serum Albumin and Transthyretin Nuclear Transcripts in Rat Liver. J. Nutr. 1994, 124, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Kang, K.W.; Kim, Y.G.; Cho, M.K.; Lee, M.G.; Shim, C.-K.; Chung, S.J.; Kim, S.G. Identification of Genes Enhanced by Protein-Calorie Malnutrition by Differential Display Polymerase Chain Reaction (Expression of Fibrinogen B β Chain, B Cell Translocation Gene 1 and Thyroid Hormone Responsive Protein Genes). Mol. Cell. Biochem. 2002, 231, 163–171. [Google Scholar] [CrossRef]
- Mayatepek, E.; Becker, K.; Gana, L.; Hoffmann, G.F.; Leichsenring, M. Leukotrienes in the Pathophysiology of Kwashiorkor. Lancet 1993, 342, 958–960. [Google Scholar] [CrossRef]
- Chaveroux, C.; Lambert-Langlais, S.; Cherasse, Y.; Averous, J.; Parry, L.; Carraro, V.; Jousse, C.; Maurin, A.-C.; Bruhat, A.; Fafournoux, P. Molecular Mechanisms Involved in the Adaptation to Amino Acid Limitation in Mammals. Biochimie 2010, 92, 736–745. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Adams, C.M. ATF4 Is a Fundamental Regulator of Nutrient Sensing and Protein Turnover. J. Nutr. 2020, 150, 979–980. [Google Scholar] [CrossRef]
- Jahoor, F.; Badaloo, A.; Reid, M.; Forrester, T. Protein Metabolism in Severe Childhood Malnutrition. Annals Trop. Paed. 2008, 28, 87–101. [Google Scholar] [CrossRef]
- Devlin, M.J. Why Does Starvation Make Bones Fat? Am. J. Human Biol. 2011, 23, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone Marrow Adipose Tissue Is an Endocrine Organ That Contributes to Increased Circulating Adiponectin During Caloric Restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.J.; Cloutier, A.M.; Thomas, N.A.; Panus, D.A.; Lotinun, S.; Pinz, I.; Baron, R.; Rosen, C.J.; Bouxsein, M.L. Caloric Restriction Leads to High Marrow Adiposity and Low Bone Mass in Growing Mice. J. Bone Min. Res. 2010, 25, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; Parlee, S.D.; Pham, H.A.; Learman, B.S.; Redshaw, C.M.H.; Sulston, R.J.; Burr, A.A.; Das, A.K.; Simon, B.R.; et al. Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated with Increased Circulating Glucocorticoids and Not with Hypoleptinemia. Endocrinology 2016, 157, 508–521. [Google Scholar] [CrossRef]
- Cunha, M.C.R.; da Silva Lima, F.; Vinolo, M.A.R.; Hastreiter, A.; Curi, R.; Borelli, P.; Fock, R.A. Protein Malnutrition Induces Bone Marrow Mesenchymal Stem Cells Commitment to Adipogenic Differentiation Leading to Hematopoietic Failure. PLoS ONE 2013, 8, e58872. [Google Scholar] [CrossRef] [PubMed]
- Osorio, E.Y.; Gugala, Z.; Patterson, G.T.; Palacios, G.; Cordova, E.; Uscanga-Palomeque, A.; Travi, B.L.; Melby, P.C. Inflammatory Stimuli Alter Bone Marrow Composition and Compromise Bone Health in the Malnourished Host. Front. Immunol. 2022, 13, 846246. [Google Scholar] [CrossRef]
- Procaccini, C.; de Candia, P.; Russo, C.; de Rosa, G.; Lepore, M.T.; Colamatteo, A.; Matarese, G. Caloric Restriction for the Immunometabolic Control of Human Health. Cardiovasc. Res. 2023. [Google Scholar] [CrossRef]
- Attia, S.; Versloot, C.J.; Voskuijl, W.; van Vliet, S.J.; Di Giovanni, V.; Zhang, L.; Richardson, S.; Bourdon, C.; Netea, M.G.; Berkley, J.A.; et al. Mortality in Children with Complicated Severe Acute Malnutrition Is Related to Intestinal and Systemic Inflammation: An Observational Cohort Study. Am. J. Clin. Nutr. 2016, 104, 1441–1449. [Google Scholar] [CrossRef]
- Esquifino, A.I.; Cano, P.; Jimenez-Ortega, V.; Fernandez-Mateos, M.P.; Cardinali, D.P. Immune Response after Experimental Allergic Encephalomyelitis in Rats Subjected to calorie Restriction. J. Neuroinflammation. 2007, 4, 6. [Google Scholar] [CrossRef]
- Gerriets, V.A.; MacIver, N.J. Role of T Cells in Malnutrition and Obesity. Front. Immunol. 2014, 5, 379. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Ghezzi, L.; Cross, A.H.; Piccio, L. Effects of Dietary Restriction on Neuroinflammation in Neurodegenerative Diseases. J. Exp. Med. 2021, 218, E20190086. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A. Mechanisms of Tolerance. Immunol. Rev. 2011, 241, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Abanni, L.; Cali, G.; Porcellini, A.; Carbone, F.; Fontana, S.; Horvath, T.L.; La Cava, A.; et al. An Oscillatory Switch in mTOR Kinase Activity Sets Regulatory T Cell Responsiveness. Immunity 2010, 33, 929–941. [Google Scholar] [CrossRef]
- Hori, S. FOXP3 as a Master Regulator of Treg Cells. Nat. Rev. Immunol. 2021, 21, 618–619. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235. [Google Scholar] [CrossRef]
- Devaud, C.; Darcy, P.K.; Kershaw, M.H. Foxp3 Expression in T Regulatory Cells and Other Cell Lineages. Cancer Immunol. Immunother. 2014, 63, 869–876. [Google Scholar] [CrossRef]
- Soares, M.P.; Teixeira, L.; Moita, L.F. Disease Tolerance and Immunity in Host protection Against Infection. Nat. Rev. Immunol. 2017, 17, 83–96. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 27410. [Google Scholar] [CrossRef]
- Chandra, R.K.; Newberne, P.M. Infections in Undernourished Individuals. In Nutrition, Immunity, and Infection; Plenum Press: New York, NY, USA, 1977; pp. 41–46. [Google Scholar]
- Edelman, R. Cell-Mediated Immunity in Protein-Calorie Malnutrition. In Protein-Calorie Malnutrition; Olson, R.E., Ed.; Academic Press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1975; pp. 377–387. [Google Scholar]
- Bourke, C.D.; Jones, K.D.J.; Prendergast, A.J. Current Understanding of Innate Immune Cell Dysfunction in Childhood Malnutrition. Front. Immunol. 2019, 10, 1728. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Paim, F.C.; Kandasamy, S.; Alhamo, M.A.; Fischer, D.D.; Langel, S.N.; Deblais, L.; Kumar, A.; Chepngeno, J.; Shao, L.; et al. Intestinal Epithelial Cells and Human Rotavirus Infection in Neonatal Gnotobiotic Pigs. mSphere 2017, 2, e00046-17. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cao, S.; Zhou, Y.; Xiong, Y. Recent Advances in Endotoxin Tolerance. J. Cell. Biochem. 2019, 120, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Dejenie, T.A.; Ayele, T.M.; Baye, N.D.; Teshome, A.A.; Muche, Z.T. The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. J. Inflamm. Res. 2021, 14, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Yu, C.-R.; Bing, S.J.; Jittayasothorn, Y.; Mattapallil, M.J.; Kang, M.; Park, S.B.; Lee, H.-S.; Dong, L.; Shi, G.; et al. IL-27-Producing B-1a Cells Suppress Neuroinflammation and CNS Autoimmune Diseases. Proc. Nat. Acad. Sci. USA 2021, 118, e2109548118. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Chaib, M.; Rathmell, J.C. Immunometabolism: From Basic Mechanisms to Translation. Immunol. Rev. 2020, 295, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K.; Newberne, P.M. Biological Implications. In Nutrition, Immunity, and Infection; Plenum Press: New York, NY, USA, 1977; pp. 181–197. [Google Scholar]
- Olusi, S.O.; Thurman, G.B.; Goldstein, A.L. Effect of Thymosin on T-Lymphocyte Rosette Formation in Children with Kwashiorkor. Clin. Immunol. Immunopathol. 1980, 15, 687–691. [Google Scholar] [CrossRef]
- Ghoshal, D.; Barua, A.K.; Roy, S.R.; Bhattacharrya, H. Vaccine Failure in Malnourished Animals: Use of a Bioimmunomodulator to Improve Immunocompetence. Nutrition 1990, 6, 153–157. [Google Scholar]
- Filteau, S.M.; Woodward, B. Immune Stimulation in Nutritional Deficiency. Nutrition 1991, 7, 157–158. [Google Scholar]
- Nimmanwudipong, T.; Cheadle, W.G.; Appel, S.H.; Polk, H.C. Effect of Protein Malnutrition and Immunomodulation on Immune Cell Populations. J. Surg. Res. 1992, 52, 233–238. [Google Scholar] [CrossRef]
- Brundtland, G.H. Nutrition and Infection: Malnutrition and Mortality in Public Health. Nutr. Rev. 2000, 58, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodward, B.; Hillyer, L.M.; Monk, J.M. The Tolerance Model of Non-Inflammatory Immune Competence in Acute Pediatric Malnutrition: Origins, Evidence, Test of Fitness and Growth Potential. Nutrients 2023, 15, 4922. https://doi.org/10.3390/nu15234922
Woodward B, Hillyer LM, Monk JM. The Tolerance Model of Non-Inflammatory Immune Competence in Acute Pediatric Malnutrition: Origins, Evidence, Test of Fitness and Growth Potential. Nutrients. 2023; 15(23):4922. https://doi.org/10.3390/nu15234922
Chicago/Turabian StyleWoodward, Bill, Lyn M. Hillyer, and Jennifer M. Monk. 2023. "The Tolerance Model of Non-Inflammatory Immune Competence in Acute Pediatric Malnutrition: Origins, Evidence, Test of Fitness and Growth Potential" Nutrients 15, no. 23: 4922. https://doi.org/10.3390/nu15234922
APA StyleWoodward, B., Hillyer, L. M., & Monk, J. M. (2023). The Tolerance Model of Non-Inflammatory Immune Competence in Acute Pediatric Malnutrition: Origins, Evidence, Test of Fitness and Growth Potential. Nutrients, 15(23), 4922. https://doi.org/10.3390/nu15234922




