Evaluation of Micronutrients and Pro-Inflammatory Cytokines Levels in Nutritionally Deprived Children—A Tertiary Care Hospital-Based Study
Abstract
1. Introduction
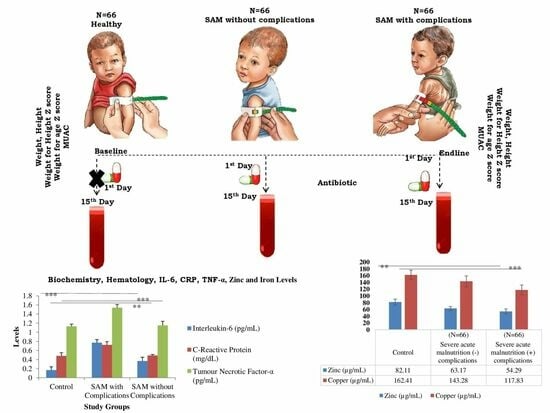
2. Materials and Methods
2.1. Sample Size
2.2. Inclusion Criteria for Subject Recruitment
- Weight for height below 3 standard deviations (SD or Z scores)
- Malnutrition with bilateral pedal edema or visible severe wasting
- Mid-upper arm circumference (MUAC) < 115 mm
2.3. Exclusion Criteria
- i.
- Children aged <6 months and >5 years, because infants under 6 months rely solely on breast milk or formula for their nutritional needs. The introduction of complementary foods typically begins around 6 months of age. The majority of the research that was available at the time of the WHO guidelines was geared toward children 6 months and older, or
- ii.
- Congenital malformation, which was assessed through a physical examination conducted by pediatricians; chronic diseases other than HIV and TB, confirmed through the identification of signs and symptoms; and children who underwent an assessment to determine the presence of palmar pallor. Those who exhibited extremely pale palms, appearing white, were identified as having severe anemia and were subsequently excluded from the study or analysis.
- iii.
- For children with uncomplicated SAM, a history was taken to assess for symptoms suggesting that the child was not clinically well (cough, shortness of breath, diarrhea, fever, and anorexia); children assessed as being clinically unwell based on symptoms and any recent use of antibiotics within the past 14 days, determined through interviews with parents and cross-referencing with previous medical records if the baby had been admitted during that period, were excluded from the group of uncomplicated SAM. (Figure 1).
2.4. Baseline Information
2.5. Sample Collection and Processing
2.6. Quantification of Micronutrients Using Inductively Coupled Plasma Mass Spectrometry
2.7. Quantification of Serum IL-6, TNF-α, and CRP
2.8. Treatment Optimization
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Family Health Survey (NFHS-5) (2019–2021). Available online: https://main.mohfw.gov.in/sites/default/files/NFHS-5_Phase-II_0.pdf (accessed on 19 October 2023).
- WHO. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children; World Health Organization: Geneva, Switzerland, 2013; Available online: http://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infant and children/en/ (accessed on 19 October 2023).
- Abdollahi, M.; Ajami, M.; Abdollahi, Z.; Kalantari, N.; Houshiarrad, A.; Fozouni, F.; Fallahrokni, A.; Mazandarani, F.S. Zinc supplementation is an effective and feasible strategy to prevent growth retardation in 6 to 24 month children: A pragmatic double blind, randomized trial. Heliyon 2019, 5, e0851. [Google Scholar] [CrossRef] [PubMed]
- Baqui, A.H.; Black, R.E.; El Arifeen, S.; Yunus, M.; Zaman, K.; Begum, M.; Roess, A.A.; Santosham, M. Zinc therapy for diarrhoea increased the use of oral rehydration therapy and reduced the use of antibiotics in Bangladeshi children. J. Health Popul. Nutr. 2004, 22, 440–442. [Google Scholar]
- Lazzerini, M.; Wanzira, H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst. Rev. 2016, 12, CD005436. [Google Scholar] [CrossRef]
- Prada, J.A.; Sánchez, A.; Hernández, G. Cytokine and nitric oxide levels in patients with sepsis—Severe systemic inflammatory response syndrome. Pediatr. Crit. Care Med. 2002, 3, 31–36. [Google Scholar]
- Reddy, P.; Shekhawat, N.S.; Mishra, V.K.; Jajoo, M.; Reddy, R. Comparative study of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) as indicators of systemic inflammatory response in severe sepsis and trauma cases. J. Forensic. Sci. 2013, 58, 1468–1472. [Google Scholar]
- Munthali, R.J.; Kagura, J.; Lombard, Z.; Norris, S.A. Childhood undernutrition and systemic inflammation are associated with stunting in early adolescence. J. Nutr. 2016, 146, 2437–2443. [Google Scholar]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Bhattacharjee, J.; Yadav, D.K. Proinflammatory cytokines predict clinical outcome in severely acute malnourished children. Eur. J. Clin. Nutr. 2018, 72, 1530–1537. [Google Scholar]
- Ahmed, T.; Ali, M.; Ullah, M.M. Effects of micronutrients (including zinc) on diarrhoea duration and clinical features in malnourished children. J. Health Popul. Nutr. 2005, 23, 206–213. [Google Scholar]
- Siyah, B.B.; Gökçay, G.; Peker, E.; Aksu, T. Zinc supplementation in children with cholera in Bangladesh: Randomised controlled trial. BMJ 2001, 323, 333–336. [Google Scholar]
- Prohaska, J.R.; Erdman, J.W.; Macdonald, I.A.; Zeisel, S.H. Present Knowledge in Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 496–511. [Google Scholar]
- Deng, H.; Zhu, S.; Yang, H. The Dysregulation of Inflammatory Pathways Triggered by Copper Exposure. Biol. Trace Elem. Res. 2023, 201, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Liu, M.J.; Lee, B.; Besecker, B.; Lai, J.P.; Guttridge, D.C. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-κB. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L744–L754. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, U.; Aryal, B.K.; Gupta, A.K.; Sapkota, S. Severe acute malnutrition and its associated factors among children under five years: A facility-based cross-sectional study. BMC Pediatr. 2020, 20, 249. [Google Scholar] [CrossRef]
- Sharghi, A.; Kamran, A.; Faridan, M. Evaluating risk factors for protein-energy malnutrition in children under the age of six years: A case-control study from Iran. Int. J. Gen. Med. 2011, 4, 607–611. [Google Scholar] [PubMed]
- Mukuku, O.; Mutombo, A.M.; Kamona, L.K.; Lubala, T.K.; Mawaw, P.M.; Aloni, M.N.; Wembonyama, S.O.; Luboya, O.N. Predictive Model for the Risk of Severe Acute Malnutrition in Children. J. Nutr. Metab. 2019, 2019, 4740825. [Google Scholar] [CrossRef]
- Gebremaryam, T.; Amare, D.; Ayalew, T. Determinants of severe acute malnutrition among children aged 6–23 months in bahir dar city public hospitals, Northwest Ethiopia, 2020: A case control study. BMC Pediatr. 2022, 22, 296. [Google Scholar] [CrossRef]
- Hong, R.; Banta, J.E.; Betancourt, J.A. Relationship between household wealth inequality and chronic childhood under-nutrition in Bangladesh. Int. J. Equity Health 2006, 5, 15. [Google Scholar] [CrossRef]
- Sinha, R.K.; Kumar, P.; Daniel, A.; Shah, H.; Sriswan, R.; Kokane, A.; Mohapatra, A.; Kashyap, V.; Goel, A.K.; Kumar, V.; et al. Association between anthropometric criteria and body composition among children aged 6–59 months with severe acute malnutrition: A cross-sectional assessment from India. BMC Nutr. 2020, 8, 56. [Google Scholar]
- Dailey, C.T.; Freemark, M.; Muehlbauer, M.; Roberfroid, D.; Kemokai, I.A.; Mostak, M.R.; Alim, M.A.; Khan, M.M.S.T.; Khan, M.A.H.; Bawo, L.; et al. Clinical and Biochemical Markers of Risk in Uncomplicated Severe Acute Malnutrition. Pediatrics 2021, 147, e2020027003. [Google Scholar] [CrossRef]
- Attia, S.; Versloot, C.J.; Voskuijl, W.; Van Vliet, S.J.; Di Giovanni, V.; Zhang, L.; Richardson, S.; Bourdon, C.; Netea, M.G.; Berkley, J.A.; et al. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: An observational cohort study. Am. J. Clin. Nutr. 2016, 104, 1441–1449. [Google Scholar] [CrossRef]
- Santetti, D.; De Albuquerque Wilasco, M.I.; Dornelles, C.T.; Werlang, I.C.; Fontella, F.U.; Kieling, C.O.; Dos Santos, J.L.; Vieira, S.M.; Goldani, H.A. Serum proinflammatory cytokines and nutritional status in pediatric chronic liver disease. World J. Gastroenterol. 2015, 21, 8927–8934. [Google Scholar] [CrossRef]
- Black, M.M. Micronutrient deficiencies and cognitive functioning. J. Nutr. 2003, 133, 3927S–3931S. [Google Scholar] [CrossRef]
- Nikièma, V.; Kangas, S.T.; Salpéteur, C.; Ouédraogo, A.; Lachat, C.; Bassolé, N.H.I.; Fogny, N.F. Adequacy of Nutrient Intakes of Severely and Acutely Malnourished Children Treated with Different Doses of Ready-To-Use Therapeutic Food in Burkina Faso. J. Nutr. 2021, 151, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.K.; Tebha, S.S.; Sangi, R.; Kamran, A.; Zaidi, Z.A.; Haque, T.; Ali, H.M.S. Zinc Micronutrient Deficiency and Its Prevalence in Malnourished Pediatric Children as Compared to Well-Nourished Children: A Nutritional Emergency. Glob. Pediatr. Health 2021, 8, 2333794X211050316. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Wilson, E.; Junior, J.A.; Imdad, A.; Dean, S.; Chan, X.H.S.; Chan, E.S.; Jaswal, A.; Bhutta, Z.A. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst. Rev. 2014, 5, CD009384. [Google Scholar] [CrossRef]
- Awatif, M.; Abd, E.; Maksoud, S.A.; Khairy, H.M.; Sharada, M.S.; Abdalla, N.F. Evaluation of pro-inflammatory cytokines in nutritionally stunted Egyptian children. Egypt. Pediatr. Assoc. Gaz. 2017, 65, 80–84. [Google Scholar]
- Lindenmayer, G.W.; Stoltzfus, R.J.; Prendergast, A.J. Interactions between zinc deficiency and environmental enteropathy in developing countries. Adv. Nutr. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.C.M.; Berkley, J.A. Guidelines for the treatment of severe acute malnutrition: A systematic review of the evidence for antimicrobial therapy. Paediatr. Int. Child Health 2018, 38, S32–S49. [Google Scholar] [CrossRef] [PubMed]
- Enn, E. Routine amoxicillin use in treatment of uncomplicated SAM in children. Field Exch. 2016, 52, 15. [Google Scholar]





| Variables | Characters | Control (n = 66) | Uncomplicated SAM (n = 66) | Complicated SAM (n = 66) | χ2/p-Value |
|---|---|---|---|---|---|
| Sex | Male | 27 (41%) | 25(37.8%) | 34(51.5%) | 2.75/0.25 |
| Female | 39 (59%) | 41(62.1%) | 32(48.4%) | ||
| Socioeconomic status | High-Income Group (HIG) | 0 | 0 | 0 | - |
| Middle Income Group (MIG) | 4 (6.0%) | 3 (4.5%) | 3(4.5%) | ||
| Low Income Group (LIG) | 46 (69.6%) | 49 (74.2%) | 35 (53.0%) | ||
| Slum | 16 (24.2%) | 14 (21.2%) | 28 (42.4%) | ||
| Family type | Nuclear | 36 (54.5%) | 35 (53.0%) | 41(62.1%) | 1.27/0.52 |
| Joint | 30 (45.4%) | 31 (46.9%) | 25 (37.8%) | ||
| Household income (monthly) | <5000 | 37 (56.0%) | 33 (50%) | 17 (25.7%) | 13.77/0.00 |
| >5000 | 29 (43.9%) | 33 (50%) | 49 (74.2%) | ||
| Toilet facility | Open field | 3 (4.5%) | 4 (6.0%) | 2 (3.0%) | 1.26/0.86 |
| Community | 14 (21.2%) | 15 (22.7%) | 12 (18.1%) | ||
| Private toilet | 49 (74.2%) | 47 (71.2%) | 52 (78.7%) | ||
| Economic background | Above Poverty Line (APL) | 30 (45.4%) | 41 (62.1%) | 15 (22.7%) | 1.29/0.59 |
| Below Poverty Line (BPL) | 36 (54.5%) | 25 (37.8%) | 51 (77.2%) | ||
| Source of drinking water | Draw well | 0 | 0 | 0 | - |
| Tube | 0 | 0 | 0 | ||
| Community tap | 0 | 11(16.6%) | 11 (16.6%) | ||
| Individual tap | 19 (28.7%) | 14 (21.2%) | 13 (19.6%) | ||
| Filter/packed water | 0 | 0 | 0 | ||
| Reverse Osmosis plant | 6 (9.0%) | 1 (1.5%) | 2 (3.0%) | ||
| Hand pump | 41 (62.1%) | 38 (57.5%) | 39 (59.0%) | ||
| Other | 0 | 2 (3.0%) | 1 (1.5%) |
| Variables | Characters | Control (n = 66) | Uncomplicated SAM (n = 66) | Complicated SAM (n = 66) | χ2/p-Value |
|---|---|---|---|---|---|
| Mother age at birth (years) | <19 | 5 (7.5%) | 3 (4.5%) | 2 (3.0%) | 3.74/0.44 |
| 20–30 | 51 (77.2%) | 54 (81.8%) | 59 (89.3%) | ||
| >30 | 10 (15.1%) | 9 (13.6%) | 5 (7.5%) | ||
| Birth Interval (years) | First born | 44 (66.6%) | 46 (69.6%) | 49 (74.2%) | - |
| <1 | 13 (19.6%) | 14 (21.2%) | 9 (13.6%) | ||
| 1–2 | 8 (12.1%) | 3 (4.5%) | 6 (9.0%) | ||
| 2–3 | 1(1.5%) | 2 (3.0%) | 1 (1.5%) | ||
| >3 | 0 | 1 (1.5%) | 1 (1.5%) | ||
| Don’t know | 0 | 0 | 0 | ||
| Gravidity | <5 | 64 (96.9%) | 61 (92.4%) | 62 (93.9%) | 1.34/0.50 |
| >5 | 2 (3.0%) | 5 (7.5%) | 4 (6.0%) | ||
| Parity | <5 | 61 (92.4%) | 64 (96.9%) | 63 (95.4%) | 1.28/0.47 |
| >5 | 3 (4.5%) | 1 (1.5%) | 1 (1.5%) | ||
| Don’t know | 2 (3.0%) | 1 (1.5%) | 2 (3.0%) | ||
| ** Any other chronic disease | Yes | 0 | 0 | 1(1.51%) | - |
| No | 66 (100%) | 66 (100%) | 65 (98.4%) | ||
| Any special meal taken during pregnancy | Yes | 64 (96.9%) | 61 (92.4%) | 59 (89.3%) | 2.92/0.23 |
| No | 2 (3.0%) | 5 (7.5%) | 7 (10.6%) | ||
| Are you aware that your child is malnourished | Yes | 61 (92.4%) | 60 (90.9%) | 49 (74.2%) | 2.48/0.45 |
| No | 5 (7.5%) | 6 (9.0%) | 17 (25.7%) | ||
| Did you receive any antenatal care during this pregnancy | Yes | 65 (98.4%) | 65 (98.4%) | 59 (89.3%) | 8.38/0.15 |
| No | 1 (1.5%) | 1 (1.5%) | 7 (10.6%) | ||
| Were you given iron and folic acid tablets | Yes | 66 (100%) | 65 (98.4%) | 62 (93.9%) | - |
| No | 0 | 1 (1.5%) | 4 (6.0%) | ||
| How many iron and folic acid tablets have you consumed | <30 | 62 (93.9%) | 52 (78.7%) | 48 (72.7%) | - |
| 30–60 | 4 (6.0%) | 9 (13.6%) | 7 (10.6%) | ||
| 60–90 | 0 | 4 (6.0%) | 6 (9.0%) | ||
| >90 | 0 | 1 (1.5%) | 4 (6.0%) | ||
| Not consumed | 0 | 0 | (1.5%) |
| Variables | Characters | Control (n= 66) | Uncomplicated SAM (n = 66) | Complicated SAM (n = 66) | χ2 | p-Value |
|---|---|---|---|---|---|---|
| Child age (months) | 6–20 | 2 (3.0%) | 10 (15.1) | 49 (74.2%) | 95.11 | <0.001 |
| 21–40 | 59 (89.3%) | 51 (77.2%) | 11 (16.6%) | |||
| 41–60 | 5 (7.5%) | 5 (7.5%) | 6 (9.0%) | |||
| Child birth history | Preterm | 2 (3.0%) | 4 (6.0%) | 7 (10.6%) | 2.65 | 0.58 |
| Full term | 64 (96.9%) | 62 (93.9%) | 59 (89.3%) | |||
| Birth weight (g) | <2000 | 6 (9.0%) | 8 (12.1%) | 13 (19.6%) | - | |
| 2000–25,000 | 58 (87.8%) | 51 (77.2%) | 51 (77.2%) | |||
| >25,000 | 2 (3.0%) | 7 (10.6%) | 1 (1.5%) | |||
| Don’t know | 0 | 0 | 1 (1.5%) | |||
| Child illness history within two weeks before assessment (n = 66 for each group) | ||||||
| Diarrhea | Yes | 0 | 7 (10.6%) | 13(19.6%) | - | |
| No | 66 (100%) | 59 (89.3%) | 53 (80.3%) | |||
| History of recurrent/chronic diarrhea | Yes | 0 | 1 (1.5%) | 5 (7.5%) | - | |
| No | 66 (100%) | 65 (98.4%) | 61 (92.4%) | |||
| Fever | Yes | 0 | 0 | 65(98.4%) | - | |
| No | 66 (100%) | 66 (100%) | 1(1.5%) | |||
| Worm infection (Ascaris lumbricoids) | Yes | 1 (1.5%) | 2 (3.0%) | 7 (10.6%) | 1.28 | 0.54 |
| No | 65 (98.4%) | 64 (96.9%) | 59 (89.3%) | |||
| Cold and Cough | Yes | 0 | 0 | 1 (1.5%) | - | |
| No | 66 (100%) | 66 (100%) | 65 (98.4%) | |||
| Initiation of breastfeeding | After 1 h | 65 (98.4%) | 56 (84.8%) | 51 (77.2%) | 13.37 | <0.001 |
| Within 1 h | 1 (1.5%) | 10 (15.1%) | 15 (22.7%) | |||
| Bottle feeding | Yes | 56 (84.8%) | 52 (78.7%) | 24 (36.3%) | 41.45 | <0.001 |
| No | 10 (15.1%) | 14 (21.2%) | 42 (63.6%) | |||
| Pre-lactral feed | Yes | 61 (92.4%) | 48 (72.7%) | 40 (60.6%) | 14.67 | <0.001 |
| No | 5 (7.5%) | 18 (27.2%) | 26 (39.3%) | |||
| Number of daily meals | <3 | 62 (93.9%) | 61(92.4%) | 59 (89.3%) | 25.64 | <0.001 |
| >3 | 4 (6.0%) | 5 (7.5%) | 7 (10.6%) | |||
| Water consumption in a day (mL) | <500 | 62 (93.9%) | 64(96.9%) | 60 (90.9%) | 12.78 | <0.001 |
| >500 | 4 (6.0%) | 2 (3.0%) | 6 (9.0%) | |||
| Urination (n = 66 for each group) | ||||||
| Day | <3 times | 17 (25.7%) | 18 (27.2%) | 29 (43.9%) | 38.76 | <0.001 |
| >3 times | 49 (74.2%) | 48 (72.7%) | 37 (56.0%) | |||
| Night | <3 times | 24 (36.3%) | 26 (39.3%) | 47 (71.2%) | 8.77 | 0.69 |
| >3 times | 42 (63.6%) | 40 (60.6%) | 19 (28.7%) | |||
| Time of eating initiation (months) | <12 | 66 (100%) | 65 (98.4%) | 65 (98.4%) | - | |
| >12 | 0 | 1 (1.5%) | 1(1.5%) | |||
| Defecation (per day) | <2 | 62 (93.9%) | 53 (80.3%) | 56 (84.8%) | 13.88 | 0.87 |
| >2 | 4 (6.0%) | 13 (19.6%) | 10 (15.1%) | |||
| Family history of malnutrition | Yes | 0 | 1 (1.5%) | 2 (3.0%) | - | |
| No | 66 (100%) | 65 (98.4%) | 64(96.9%) | |||
| Variables | Characters | Control (n = 66) | Uncomplicated SAM (n = 66) | Complicated SAM (n = 66) | ANOVA with p-Values * |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Complete blood count | Hemoglobin (Hb) (g/dL) | 10.69 ± 1.18 | 10.11 ± 1.74 | 7.75 ± 1.02 | 0.001 * |
| Total Leukocyte Cell (TLC) (cells/mm3) | 11,632.54 ± 2115.41 | 13,478.33 ± 3540.91 | 15,632.18 ± 2896.11 | 0.001 * | |
| Platelet count (lac cells/mm3) | 3.89 ± 1.65 | 3.49 ± 1.15 | 3.36 ± 0.96 | 0.001 * | |
| Total RBCs (m cells/mm3) | 4.39 ± 0.69 | 4.21 ± 0.42 | 4.07 ± 0.69 | 0.001 * | |
| Mean platelet volume (MPV)(fl.) | 8.88 ± 2.01 | 8.91 ± 1.19 | 8.06 ± 0.96 | 0.001 * | |
| Kidney function test | Serum Urea(mg/dL) | 26.22 ± 1.89 | 28.01 ± 4.96 | 29.06 ± 2.11 | 0.001 * |
| Serum Creatinine (mg/dL) | 0.56 ± 0.18 | 0.81 ± 0.12 | 0.84 ± 0.19 | 0.001 * | |
| Liver function test | Serum Bilrubin Total | 0.36 ± 0.09 | 0.48 ± 0.12 | 0.55 ± 0.17 | 0.001 * |
| Serum Bilrubin direct | 0.18 ± 0.11 | 0.21 ± 0.07 | 0.32 ± 0.09 | 0.001 * | |
| Serum Bilirubin Indirect | 0.19 ± 0.05 | 0.26 ± 0.08 | 0.29 ± 0.03 | 0.001 * | |
| Serum Protein | 6.02 ± 0.08 | 7.1 ± 0.67 | 7.26 ± 0.05 | 0.001 * | |
| Serum Albumin | 3.19 ± 0.14 | 4.52 ± 0.28 | 4.89 ± 0.33 | 0.001 * | |
| SGOT(IU/L) | 39.56 ± 0.56 | 46.95 ± 0.52 | 47.88 ± 0.23 | 0.001 * | |
| SGPT(IU/L) | 29.44 ± 14.26 | 32.16 ± 16.11 | 35.89 ± 14.32 | 0.001 * |
| Study Variable | Baseline (n = 66) | SAM Children on the 15th Day of Antibiotic Interventions | ||
|---|---|---|---|---|
| Received Antibiotics (Mean ± SD) (n = 33) | Did not Receive Antibiotics (Mean ± SD) (n = 33) | p-Value | ||
| Weight (kg) | 7.31 ± 1.6 | 7.9 ± 2.0 | 7.6 ± 1.7 7 | <0.05 |
| Height (cm) | 74.73 ± 9.8 | 75.03 ± 9.7 | 74.73 ± 9.8 | - |
| Weight-for-Height Z score | −2.8 ± 1.4 | −1.93 ± 1.2 | −2.29 ± 1.7 | <0.05 |
| Weight-for-age Z score | −4.4 ± 1.7 | −3.50 ± 1.88 | −4.0 ± 2.4 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, M.; Raghav, A.; Tripathi, P.; Rao, Y.K.; Singh, D.D. Evaluation of Micronutrients and Pro-Inflammatory Cytokines Levels in Nutritionally Deprived Children—A Tertiary Care Hospital-Based Study. Nutrients 2023, 15, 4865. https://doi.org/10.3390/nu15234865
Mishra M, Raghav A, Tripathi P, Rao YK, Singh DD. Evaluation of Micronutrients and Pro-Inflammatory Cytokines Levels in Nutritionally Deprived Children—A Tertiary Care Hospital-Based Study. Nutrients. 2023; 15(23):4865. https://doi.org/10.3390/nu15234865
Chicago/Turabian StyleMishra, Malvika, Alok Raghav, Prashant Tripathi, Yashwant Kumar Rao, and Desh Deepak Singh. 2023. "Evaluation of Micronutrients and Pro-Inflammatory Cytokines Levels in Nutritionally Deprived Children—A Tertiary Care Hospital-Based Study" Nutrients 15, no. 23: 4865. https://doi.org/10.3390/nu15234865
APA StyleMishra, M., Raghav, A., Tripathi, P., Rao, Y. K., & Singh, D. D. (2023). Evaluation of Micronutrients and Pro-Inflammatory Cytokines Levels in Nutritionally Deprived Children—A Tertiary Care Hospital-Based Study. Nutrients, 15(23), 4865. https://doi.org/10.3390/nu15234865