The Real-Life Use of a Protein-Sparing Modified Fast Diet by Nasogastric Tube (ProMoFasT) in Adults with Obesity: An Open-Label Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Nutritional Protocol
2.3. Naso-Gastric Tube Placement and Home’s Enteral Nutrition Management
2.4. Statistical Analysis
3. Results
3.1. Study Population
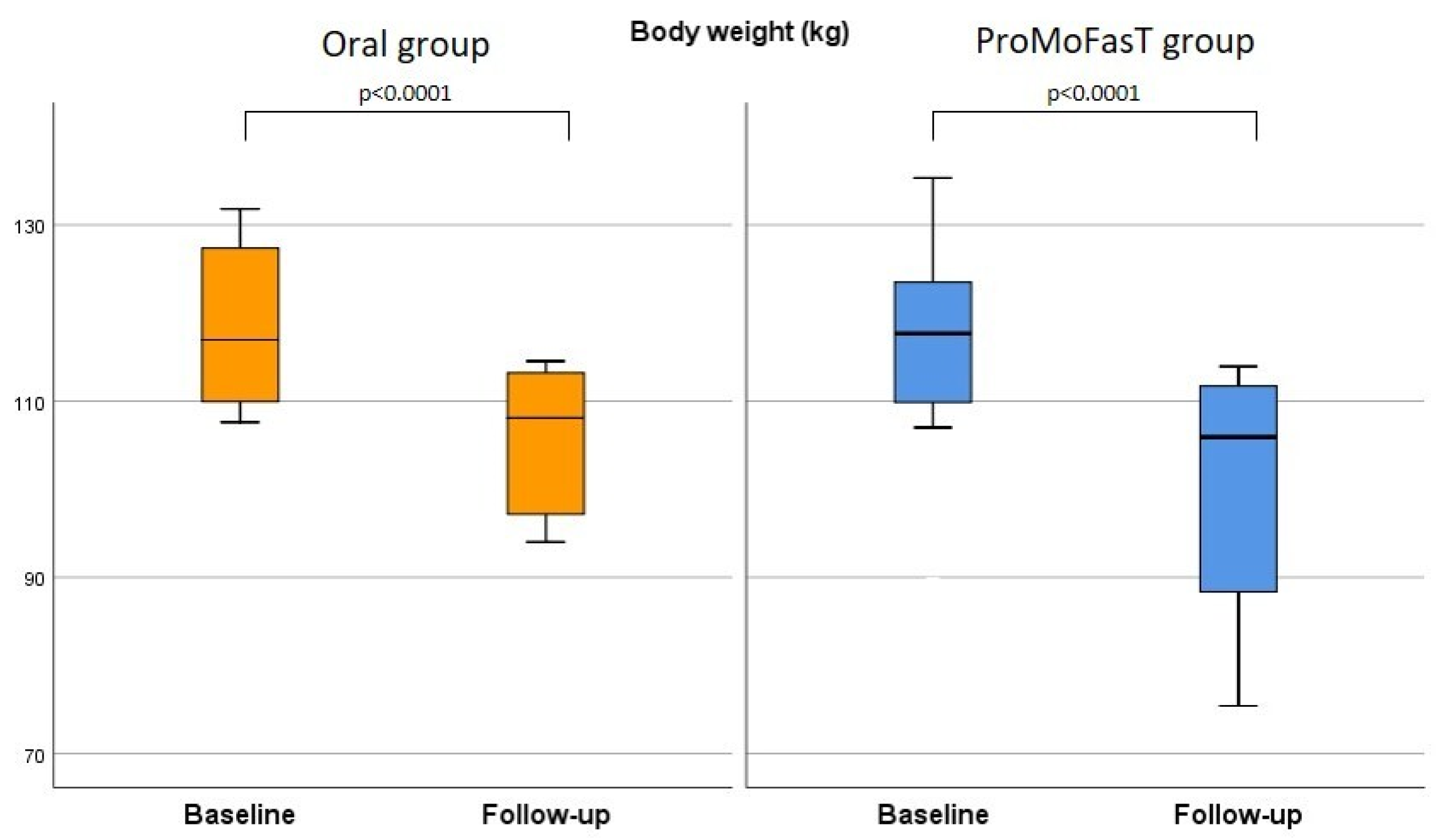
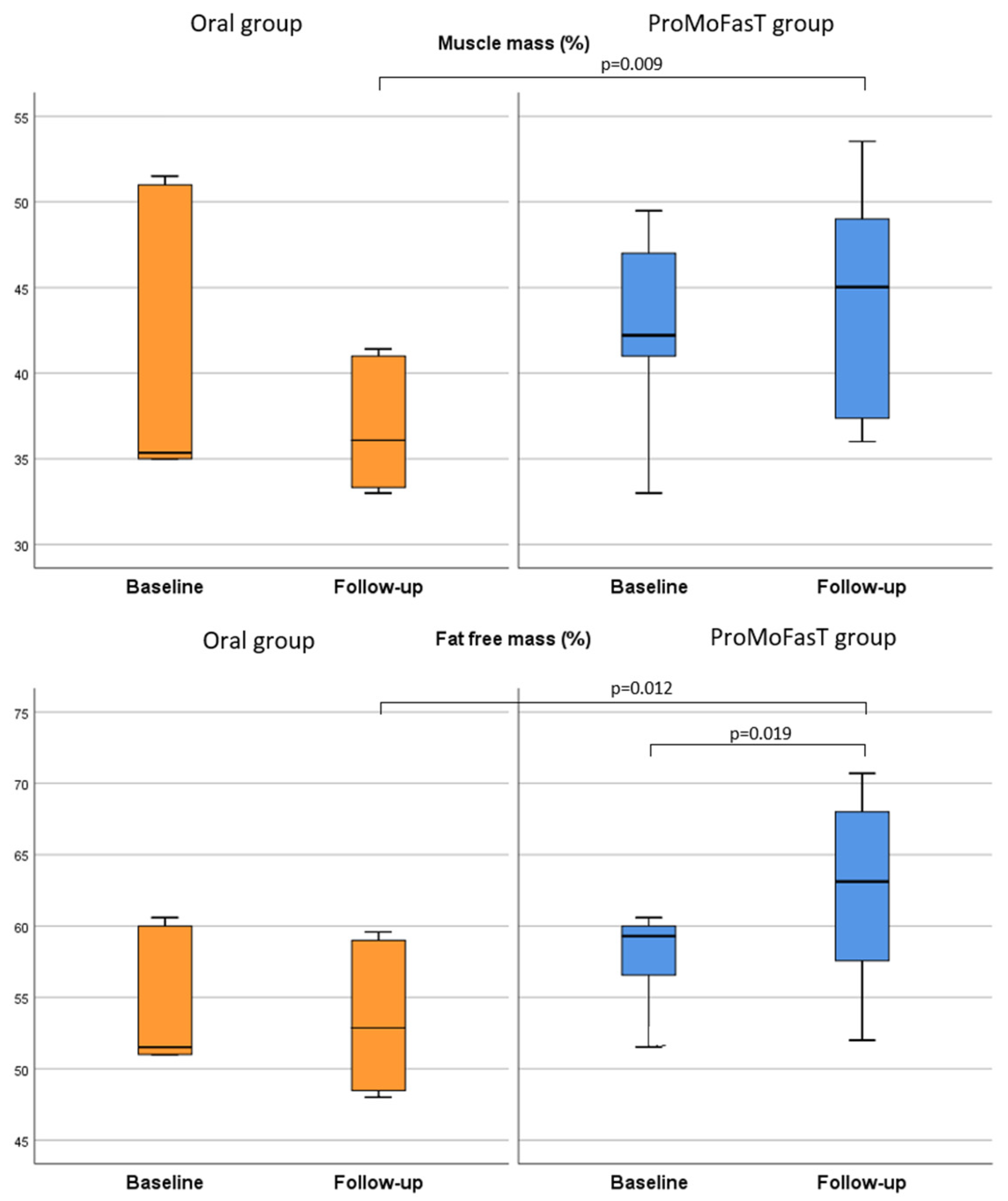
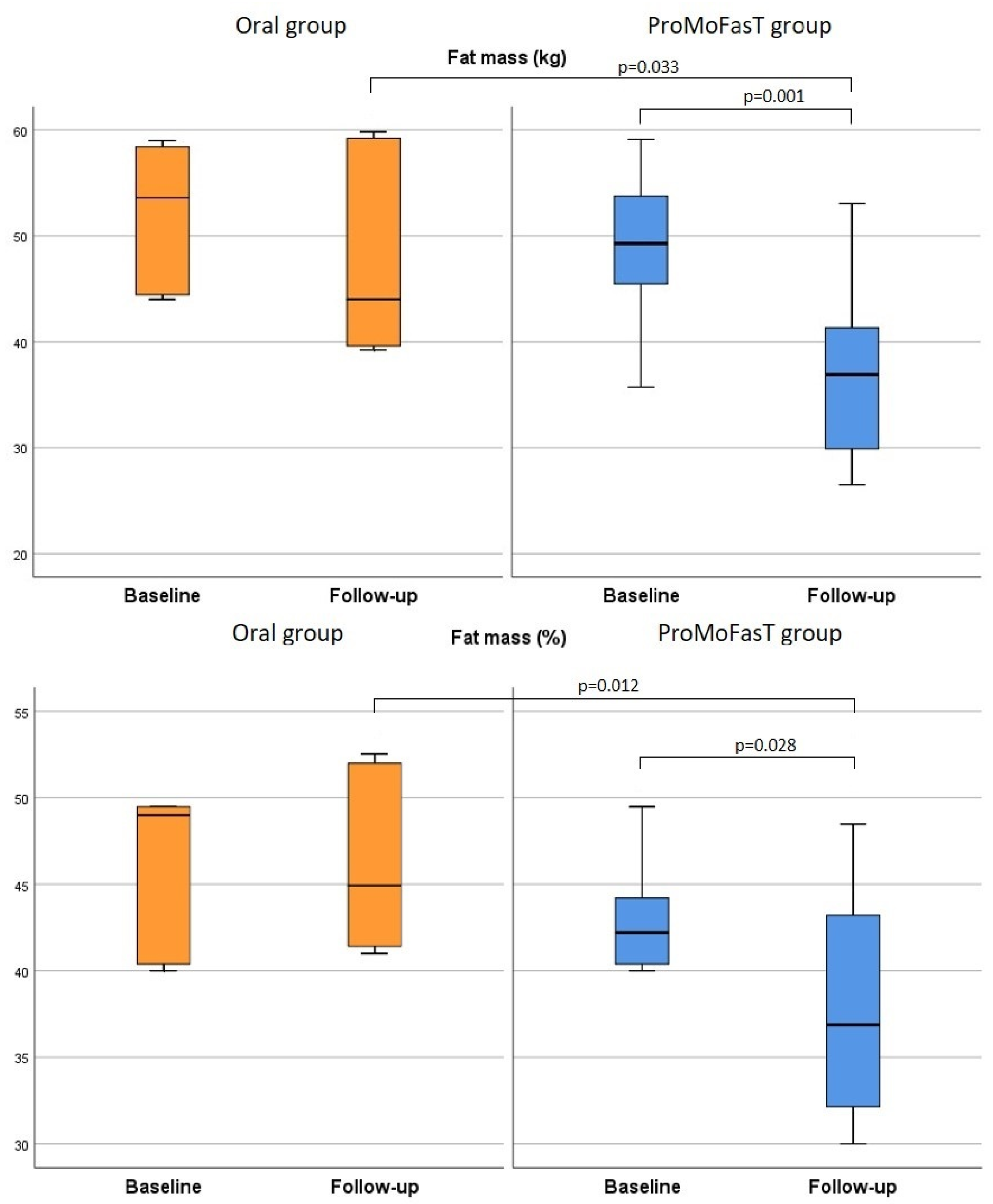
3.2. Anthropometric Parameters and Body Composition Analysis
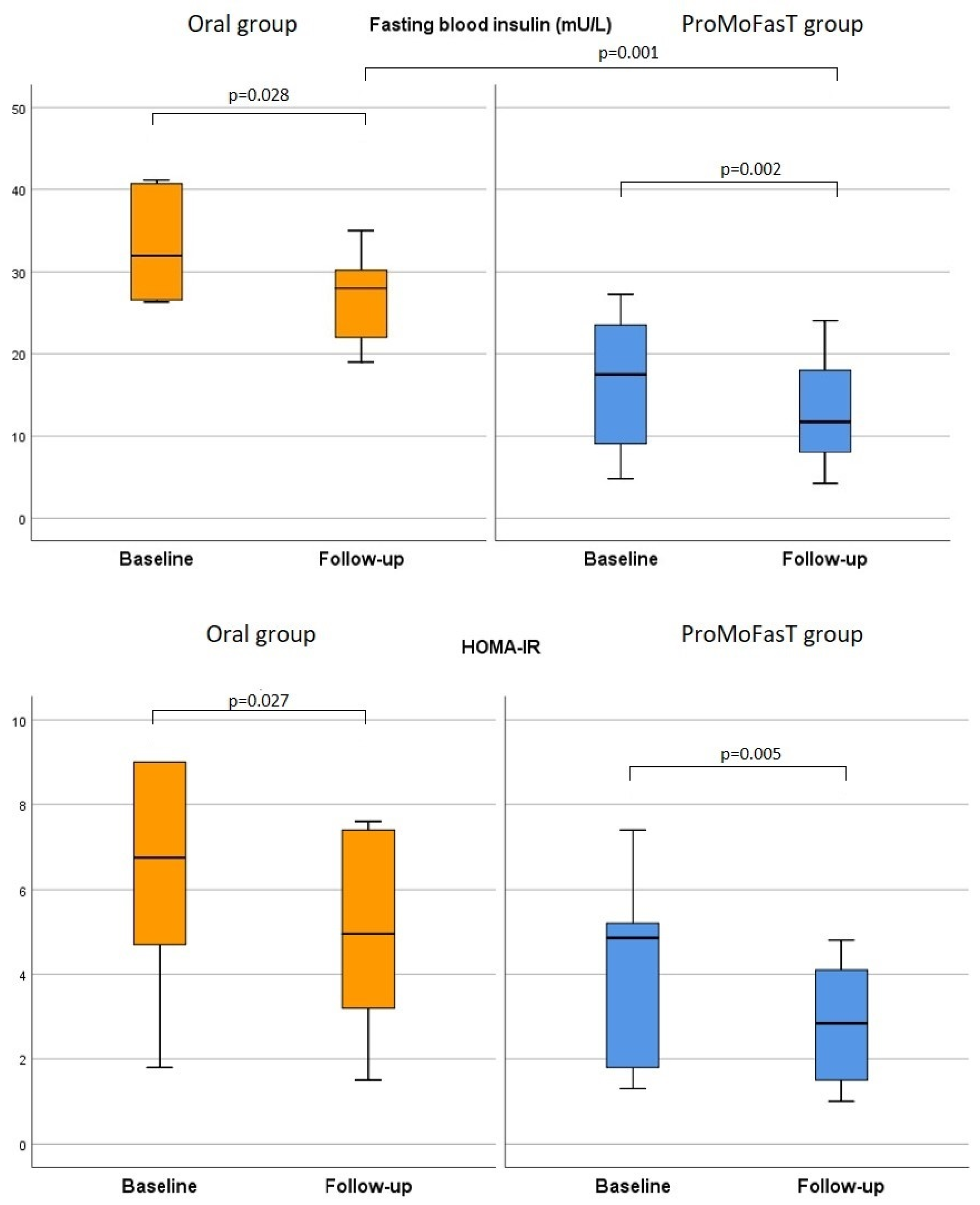
3.3. Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 June 2023).
- Loos, R.J.F.; Yeo, G.S.H. The Genetics of Obesity: From Discovery to Biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Brownell, K.D.; Foster, G.D. Obesity: Responding to the Global Epidemic. J. Consult. Clin. Psychol. 2002, 70, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and Mortality Associated with Obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Pi-Sunyer, X. The Medical Risks of Obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Lemstra, M.; Bird, Y.; Nwankwo, C.; Rogers, M.; Moraros, J. Weight Loss Intervention Adherence and Factors Promoting Adherence: A Meta-Analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, P.; Kowalski, J.; Ekblom, Ö.; Marcus, C. Response of Severely Obese Children and Adolescents to Behavioral Treatment. Arch. Pediatr. Adolesc. Med. 2012, 166, 1103–1108. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Varela, J.E. Bariatric Surgery for Obesity and Metabolic Disorders: State of the Art. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 160–169. [Google Scholar] [CrossRef]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarçon, I.; Azran, C.; et al. Clinical Practice Guidelines of the European Association for Endoscopic Surgery (EAES) on Bariatric Surgery: Update 2020 Endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Felsenreich, D.M.; Langer, F.B.; Prager, G. Weight Loss and Resolution of Comorbidities After Sleeve Gastrectomy: A Review of Long-Term Results. Scand. J. Surg. 2019, 108, 3–9. [Google Scholar] [CrossRef]
- Kaul, A.; Sharma, J. Impact of Bariatric Surgery on Comorbidities. Surg. Clin. N. Am. 2011, 91, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.; Harrison, T.D.; McGraw, S.L. Treatment of Adult Obesity with Bariatric Surgery. Am. Fam. Phys. 2016, 93, 31–37. [Google Scholar] [PubMed]
- Inoue, K.; Yoshiuchi, S.; Yoshida, M.; Nakamura, N.; Nakajima, S.; Kitamura, A.; Mouri, K.; Michiura, T.; Mukaide, H.; Ozaki, T.; et al. Preoperative Weight Loss Program Involving a 20-Day Very Low-Calorie Diet for Obesity before Laparoscopic Gastrectomy for Gastric Cancer. Asian J. Endosc. Surg. 2019, 12, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, S.G.; Muscaritoli, M. A Clinical Perspective of Low Carbohydrate Ketogenic Diets: A Narrative Review. Front. Nutr. 2021, 8, 642628. [Google Scholar] [CrossRef] [PubMed]
- Alabdali, F.; Rueda-Clausen, C.F.; Robbins, S.; Sharma, A.M. Efficacy and Safety of Long-Term Low-Calorie Diet in Severely Obese Patients Non-Eligible for Surgery. Clin. Obes. 2013, 3, 90–94. [Google Scholar] [CrossRef]
- Blackburn, G.L.; Flatt, J.P.; Clowes, G.H.; O’Donnell, T.F.; Hensle, T.E. Protein Sparing Therapy during Periods of Starvation with Sepsis or Trauma. Ann. Surg. 1973, 177, 588–594. [Google Scholar] [CrossRef]
- Cappello, G.; Franceschelli, A.; Cappello, A.; De Luca, P. Ketogenic Enteral Nutrition as a Treatment for Obesity: Short Term and Long Term Results from 19,000 Patients. Nutr. Metab. 2012, 9, 96. [Google Scholar] [CrossRef]
- Beale, E.O.; Lee, W.; Lee, A.; Lee, C.; Soffer, E.; Crookes, P.F.; Eagilen, K.; Chen, R.; Mack, W.J.; Tong, H. Effect of Bolus Enteral Tube Feeding on Body Weight in Ambulatory Adults with Obesity and Type 2 Diabetes: A Feasibility Pilot Randomized Trial. Nutr. Diabetes 2020, 10, 22. [Google Scholar] [CrossRef]
- Kumar, N.K.; Merrill, J.D.; Carlson, S.; German, J.; Yancy, W.S. Adherence to Low-Carbohydrate Diets in Patients with Diabetes: A Narrative Review. Diabetes Metab. Syndr. Obes. 2022, 15, 477–498. [Google Scholar] [CrossRef]
- Castaldo, G.; Schiavo, L.; Pagano, I.; Molettieri, P.; Conte, A.; Sarno, G.; Pilone, V.; Rastrelli, L. Clinical Impact of Enteral Protein Nutritional Therapy on Patients with Obesity Scheduled for Bariatric Surgery: A Focus on Safety, Efficacy, and Pathophysiological Changes. Nutrients 2023, 15, 1492. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Signori, A.; Borrini, C.; Barisione, G.; Ivaldi, C.; Romeo, C.; Gradaschi, R.; Machello, N.; Nanetti, E.; Vaccaro, A.L. Feasibility of Protein-Sparing Modified Fast by Tube (ProMoFasT) in Obesity Treatment: A Phase II Pilot Trial on Clinical Safety and Efficacy (Appetite Control, Body Composition, Muscular Strength, Metabolic Pattern, Pulmonary Function Test). Med. J. Nutr. Metab. 2013, 6, 165–176. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and Whey Exert Different Effects on Plasma Amino Acid Profiles, Gastrointestinal Hormone Secretion and Appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, S.G.; Vaccaro, A.; Ravera, G.B.; Borrini, C.; Gradaschi, R.; Massa Sacchi-Nemours, A.; Cordera, R.; Andraghetti, G. Appetite Control and Gastrointestinal Hormonal Behavior (CCK, GLP-1, PYY 1–36) Following Low Doses of a Whey Protein-Rich Nutraceutic. Med. J. Nutr. Metab. 2013, 6, 259–266. [Google Scholar] [CrossRef]
- Poppitt, S.D.; Proctor, J.; McGill, A.-T.; Wiessing, K.R.; Falk, S.; Xin, L.; Budgett, S.C.; Darragh, A.; Hall, R.S. Low-Dose Whey Protein-Enriched Water Beverages Alter Satiety in a Study of Overweight Women. Appetite 2011, 56, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Bakhach, M.; Shah, V.; Harwood, T.; Lappe, S.; Bhesania, N.; Mansoor, S.; Alkhouri, N. The Protein-Sparing Modified Fast Diet: An Effective and Safe Approach to Induce Rapid Weight Loss in Severely Obese Adolescents. Glob. Pediatr. Health 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Cincione, R.I.; Losavio, F.; Cibelli, G.; Messina, G.; Polito, R.; Casula, E.; Cincione, P.P.; Amatruda, M.; Limone, P. Revised Protein Sparing Diet in Obesity and Type 2 Diabetes Mellitus. Nutrients 2022, 14, 5325. [Google Scholar] [CrossRef]
- Pfoh, E.R.; Lowenthal, G.; Jeffers, L.; Burguera, B.; Cetin, D.; Hu, B.; Gupta, N.M.; Cantave, M.; Rothberg, M.B. The Effect of Starting the Protein-Sparing Modified Fast on Weight Change over 5 Years. J. Gen. Intern. Med. 2020, 35, 704–710. [Google Scholar] [CrossRef]
- Prodam, F.; Me, E.; Riganti, F.; Gramaglia, E.; Bellone, S.; Baldelli, R.; Rapa, A.; van der Lely, A.J.; Bona, G.; Ghigo, E.; et al. The Nutritional Control of Ghrelin Secretion in Humans: The Effects of Enteral vs. Parenteral Nutrition. Eur. J. Nutr. 2006, 45, 399–405. [Google Scholar] [CrossRef]
- Marsset-Baglieri, A.; Fromentin, G.; Tomé, D.; Bensaid, A.; Makkarios, L.; Even, P.C. Increasing the Protein Content in a Carbohydrate-Free Diet Enhances Fat Loss during 35% but Not 75% Energy Restriction in Rats. J. Nutr. 2004, 134, 2646–2652. [Google Scholar] [CrossRef]
- Di Girolamo, F.G.; Situlin, R.; Fiotti, N.; Biolo, G. Intermittent vs. Continuous Enteral Feeding to Prevent Catabolism in Acutely Ill Adult and Pediatric Patients. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 390–395. [Google Scholar] [CrossRef]
- Gazzaneo, M.C.; Suryawan, A.; Orellana, R.A.; Torrazza, R.M.; El-Kadi, S.W.; Wilson, F.A.; Kimball, S.R.; Srivastava, N.; Nguyen, H.V.; Fiorotto, M.L.; et al. Intermittent Bolus Feeding Has a Greater Stimulatory Effect on Protein Synthesis in Skeletal Muscle than Continuous Feeding in Neonatal Pigs. J. Nutr. 2011, 141, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Rudar, M.; Naberhuis, J.K.; Suryawan, A.; Nguyen, H.V.; Stoll, B.; Style, C.C.; Verla, M.A.; Olutoye, O.O.; Burrin, D.G.; Fiorotto, M.L.; et al. Intermittent Bolus Feeding Does Not Enhance Protein Synthesis, Myonuclear Accretion, or Lean Growth More than Continuous Feeding in a Premature Piglet Model. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E737–E752. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; McGregor, R.A.; D’Souza, R.F.; Thorstensen, E.B.; Markworth, J.F.; Fanning, A.C.; Poppitt, S.D.; Cameron-Smith, D. Consumption of Milk Protein or Whey Protein Results in a Similar Increase in Muscle Protein Synthesis in Middle Aged Men. Nutrients 2015, 7, 8685–8699. [Google Scholar] [CrossRef] [PubMed]
- Gleason, C.E.; Lu, D.; Witters, L.A.; Newgard, C.B.; Birnbaum, M.J. The Role of AMPK and mTOR in Nutrient Sensing in Pancreatic Beta-Cells. J. Biol. Chem. 2007, 282, 10341–10351. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Layman, D.K.; Moulton, C.J.; Norton, L.E.; Anthony, T.G.; Proud, C.G.; Rupassara, S.I.; Garlick, P.J. Leucine or Carbohydrate Supplementation Reduces AMPK and eEF2 Phosphorylation and Extends Postprandial Muscle Protein Synthesis in Rats. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1236–E1242. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Traverso, N.; Furfaro, A.L.; Tasso, B.; Marengo, B.; Domenicotti, C.; Pisciotta, L.; Pasta, A.; Marinari, U.M.; Pronzato, M.A.; et al. Whey Proteins Inhibit Food Intake and Tend to Improve Oxidative Balance in Obese Zucker Rats. Eat. Weight. Disord. 2021, 26, 2453–2461. [Google Scholar] [CrossRef]
- Mirabelli, M.; Russo, D.; Brunetti, A. The Role of Diet on Insulin Sensitivity. Nutrients 2020, 12, 3042. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular Pathways of Nonalcoholic Fatty Liver Disease Development and Progression. Cell Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Gosmanov, A.R.; Umpierrez, G.E. Management of Hyperglycemia during Enteral and Parenteral Nutrition Therapy. Curr. Diab Rep. 2013, 13, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. Nutrient Excess in AMPK Downregulation and Insulin Resistance. J. Endocrinol. Diabetes Obes. 2013, 1, 1008. [Google Scholar] [PubMed]
- Xiao, X.; Luo, Y.; Peng, D. Updated Understanding of the Crosstalk Between Glucose/Insulin and Cholesterol Metabolism. Front. Cardiovasc. Med. 2022, 9, 879355. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Sahni, S.; Kerstetter, J.E. Dietary Protein Is Beneficial to Bone Health under Conditions of Adequate Calcium Intake: An Update on Clinical Research. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 69–74. [Google Scholar] [CrossRef] [PubMed]

| Nutrients | 100 g |
|---|---|
| Proteins [g] | 88 |
| Lipids [g] | 1 |
| Carbohydrates [g] | 0.3 |
| Fiber [g] | 0 |
| Sodium [mg] | 550 |
| Potassium [mg] | 1170 |
| Calcium [mg] | 55 |
| Phosphorus [mg] | 220 |
| Oral Group (n = 24) | ProMoFasT Group (n = 20) | p-Value | |
|---|---|---|---|
| Female gender [n, %] | 14 (58.3) | 15 (75.0) | 0.246 |
| Age (years: median; IQR) | 49.0 (39.0–56.5) | 49.0 (41.0–53.0) | 1.000 |
| Parameters | Group | Baseline | Follow-Up (150 Days) | p-Value (Baseline vs. Follow-Up) | p-Value (Oral vs. ProMoFasT) |
|---|---|---|---|---|---|
| Body weight (kg) | Oral | 117.5 (109.9–127.9) | 108.1 (97.2–113.2) | <0.0001 | 0.210 |
| ProMoFasT | 113.9 (102.2–121.6) | 105.9 (88.4–111.7) | <0.0001 | ||
| BMI (kg/m2) | Oral | 40.8 (40.0–49.4) | 37.8 (35.7–44.8) | <0.0001 | 0.564 |
| ProMoFasT | 43.0 (38.3–47.5) | 39.3 (32.4–42.9) | <0.0001 | ||
| Waist circumference (cm) | Oral | 129.6 (121.0–132.3) | 111.0 (107.5–116.5) | 0.002 | 0.527 |
| ProMoFasT | 131.7 (117.0–141.9) | 112.5 (103.0–130.0) | 0.001 | ||
| Phase angle (°) | Oral | 6.8 (6.4–7.4) | 6.5 (6.5–6.6) | 0.463 | 0.153 |
| ProMoFasT | 7.0 (6.4–7.8) | 7.1 (6.3–7.5) | 0.308 | ||
| BCM (kg) | Oral | 39.1 (33.1–52.0) | 30.5 (27.5–32.4) | 0.028 | 0.130 |
| ProMoFasT | 36.3 (31.8–46.0) | 34.0 (27.8–41.1) | 0.096 | ||
| BCM (%) | Oral | 57.8 (55.3–60.3) | 56.5 (56.0–57.0) | 0.465 | 0.207 |
| ProMoFasT | 58.3 (55.0–61.6) | 59.1 (55.6–60.6) | 0.060 | ||
| FFM (kg) | Oral | 67.1 (59.7–86.4) | 54.5 (49.1–57.1) | 0.028 | 0.207 |
| ProMoFasT | 60.7 (56.8–67.1) | 57.4 (53.4–69.9) | 0.035 | ||
| FFM (%) | Oral | 56.8 (51.8–64.8) | 52.9 (48.5–59.0) | 0.463 | 0.012 |
| ProMoFasT | 59.0 (55.6–60.6) | 63.1 (57.6–68.0 | 0.019 | ||
| MM (kg) | Oral | 47.5 (40.8–60.7) | 37.2 (33.5–39.5) | 0.028 | 0.130 |
| ProMoFasT | 43.8 (38.9–54.7) | 41.2 (34.3–49.8) | 0.064 | ||
| MM (%) | Oral | 37.7 (35.7–49.7) | 36.1 (33.3–41.0) | 0.116 | 0.009 |
| ProMoFasT | 41.4 (38.4–43.4) | 45.0 (37.4–49.0) | 0.272 | ||
| FM (kg) | Oral | 50.4 (44.2–56.7) | 44.0 (39.6–59.2) | 0.116 | 0.033 |
| ProMoFasT | 47.6 (39.2–50.7) | 36.9 (29.9–41.3) | 0.001 | ||
| FM (%) | Oral | 43.7 (35.7–48.7) | 44.9 (41.4–52.0) | 0.917 | 0.012 |
| ProMoFasT | 41.2 (40.0–44.4) | 37.4 (32.3–43.0) | 0.028 | ||
| TBW (L) | Oral | 49.2 (46.1–63.3) | 39.9 (35.9–41.8) | 0.028 | 0.207 |
| ProMoFasT | 44.8 (41.5–49.3) | 42.0 (39.1–51.2) | 0.060 | ||
| TBW (%) | Oral | 42.9 (38.6–47.2) | 38.7 (35.4–43.4) | 0.463 | 0.012 |
| ProMoFasT | 43.0 (40.4–44.1) | 46.2 (42.2–49.9) | 0.028 | ||
| ICW (L) | Oral | 29.7 (25.6–38.0) | 21.3 (10.0–23.2) | 0.012 | 0.020 |
| ProMoFasT | 26.5 (23.4–32.9) | 25.0 (20.6–30.2) | 0.331 | ||
| ICW (%) | Oral | 58.8 (56.5–60.9) | 56.8 (56.3–56.9) | 0.463 | 0.179 |
| ProMoFasT | 58.6 (55.6–61.5) | 59.2 (55.8–60.2) | 0.826 |
| Parameters | Group | Baseline | Follow-Up (150 Days) | p-Value (Baseline vs. Follow-Up) | p-Value (Oral vs. ProMoFasT) |
|---|---|---|---|---|---|
| Fasting blood glucose | Oral | 100.5 (92.5–114.6) | 94.0 (90.5–97.5) | 0.002 | 0.631 |
| ProMoFasT | 109.5 (91.0–112.1) | 97.0 (84.0–102.0) | 0.001 | ||
| HbA1c | Oral | 5.8 (5.5–6.1) | 5.4 (5.3–5.7) | 0.005 | 0.009 |
| ProMoFasT | 6.2 (5.7–6.7) | 6.2 (5.7–6.4) | 0.015 | ||
| HOMA-IR | Oral | 6.2 (4.7–9) | 3.6 (1.5–7.4) | 0.027 | 0.364 |
| ProMoFasT | 5.0 (2.9–6.6) | 2.8 (1.5–4.1) | 0.005 | ||
| Fasting blood insulin | Oral | 26.4 (14.6–32.1) | 28.0 (22.0–30.2) | 0.028 | 0.001 |
| ProMoFasT | 19.4 (9.8–25.0) | 11.8 (8.0–18.0) | 0.002 | ||
| Creatinine | Oral | 0.8 (0.7–1.0) | 0.8 (0.6–1.1) | 0.218 | 0.631 |
| ProMoFasT | 0.8 (0.6–0.8) | 0.8 (0.7–0.8) | 0.550 | ||
| GGT | Oral | 29.1 (21.6–51.2) | 26.0 (18.0–36.0) | 0.444 | 0.286 |
| ProMoFasT | 19.2 (16.0–35.4) | 19.0 (17.0–29.5) | 0.513 | ||
| ALP | Oral | 124.6 (67.0–190.9) | 128.0 (78.0–199.0) | 0.018 | 0.569 |
| ProMoFasT | 100.5 (66.0–183.8) | 101.5 (84.0–168.0 | 0.016 | ||
| AST | Oral | 20.1 (15.6–26.6) | 21.0 (16.6–40.0) | 0.153 | 0.201 |
| ProMoFasT | 17.1 (16.0–22.1) | 17.0 (13.5–20.0) | 0.055 | ||
| ALT | Oral | 29.1 (17.1–41.7) | 20.0 (14.0–35.0) | 0.016 | 0.841 |
| ProMoFasT | 19.6 (17.6–46.7) | 18.5 (17.0–29.0) | 0.011 | ||
| TC | Oral | 194.0 (177.3–238.6) | 218.1 (184.0–237.5) | 0.004 | 0.314 |
| ProMoFasT | 187.9 (171.7–241.0) | 181.4 (159.9–229.5) | 0.001 | ||
| HDL–C | Oral | 53.3 (47.5–56.0) | 55.9 (54.6–57.8) | 0.005 | 0.722 |
| ProMoFasT | 49.2 (44.4–57.0 | 53.0 (46.7–60.4) | 0.002 | ||
| LDL–C | Oral | 129.6 (99.5–167.8) | 143.7 (103.8–149.1) | 0.012 | 0.539 |
| ProMoFasT | 116.6 (94.9–158.0) | 107.7 (93.8–161.4) | 0.099 | ||
| TG | Oral | 127.1 (98.5–192.4) | 98.5 (67.0–165.0) | 0.005 | 0.821 |
| ProMoFasT | 119.6 (75.8–200.0) | 94.5 (52.3–151.0) | 0.002 | ||
| Calcium | Oral | 9.0 (8.9–9.7) | 9.3 89.2–10.0) | 0.005 | 0.310 |
| ProMoFasT | 9.4 (9.2–9.6) | 9.7 (9.4–9.9) | <0.0001 | ||
| Phosphorus | Oral | 3.1 (2.9–3.3) | 3.2 (3.1–3.4) | 0.005 | 0.643 |
| ProMoFasT | 3.4 (2.9–4.4) | 3.5 (2.6–4.2) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formisano, E.; Schiavetti, I.; Gradaschi, R.; Gardella, P.; Romeo, C.; Pisciotta, L.; Sukkar, S.G. The Real-Life Use of a Protein-Sparing Modified Fast Diet by Nasogastric Tube (ProMoFasT) in Adults with Obesity: An Open-Label Randomized Controlled Trial. Nutrients 2023, 15, 4822. https://doi.org/10.3390/nu15224822
Formisano E, Schiavetti I, Gradaschi R, Gardella P, Romeo C, Pisciotta L, Sukkar SG. The Real-Life Use of a Protein-Sparing Modified Fast Diet by Nasogastric Tube (ProMoFasT) in Adults with Obesity: An Open-Label Randomized Controlled Trial. Nutrients. 2023; 15(22):4822. https://doi.org/10.3390/nu15224822
Chicago/Turabian StyleFormisano, Elena, Irene Schiavetti, Raffaella Gradaschi, Paolo Gardella, Carlotta Romeo, Livia Pisciotta, and Samir Giuseppe Sukkar. 2023. "The Real-Life Use of a Protein-Sparing Modified Fast Diet by Nasogastric Tube (ProMoFasT) in Adults with Obesity: An Open-Label Randomized Controlled Trial" Nutrients 15, no. 22: 4822. https://doi.org/10.3390/nu15224822
APA StyleFormisano, E., Schiavetti, I., Gradaschi, R., Gardella, P., Romeo, C., Pisciotta, L., & Sukkar, S. G. (2023). The Real-Life Use of a Protein-Sparing Modified Fast Diet by Nasogastric Tube (ProMoFasT) in Adults with Obesity: An Open-Label Randomized Controlled Trial. Nutrients, 15(22), 4822. https://doi.org/10.3390/nu15224822









