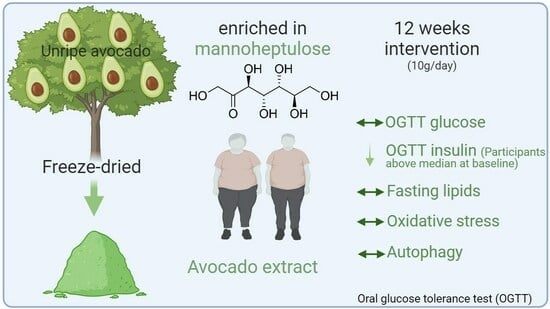
Effects of an Unripe Avocado Extract on Glycaemic Control in Individuals with Obesity: A Double-Blinded, Parallel, Randomised Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
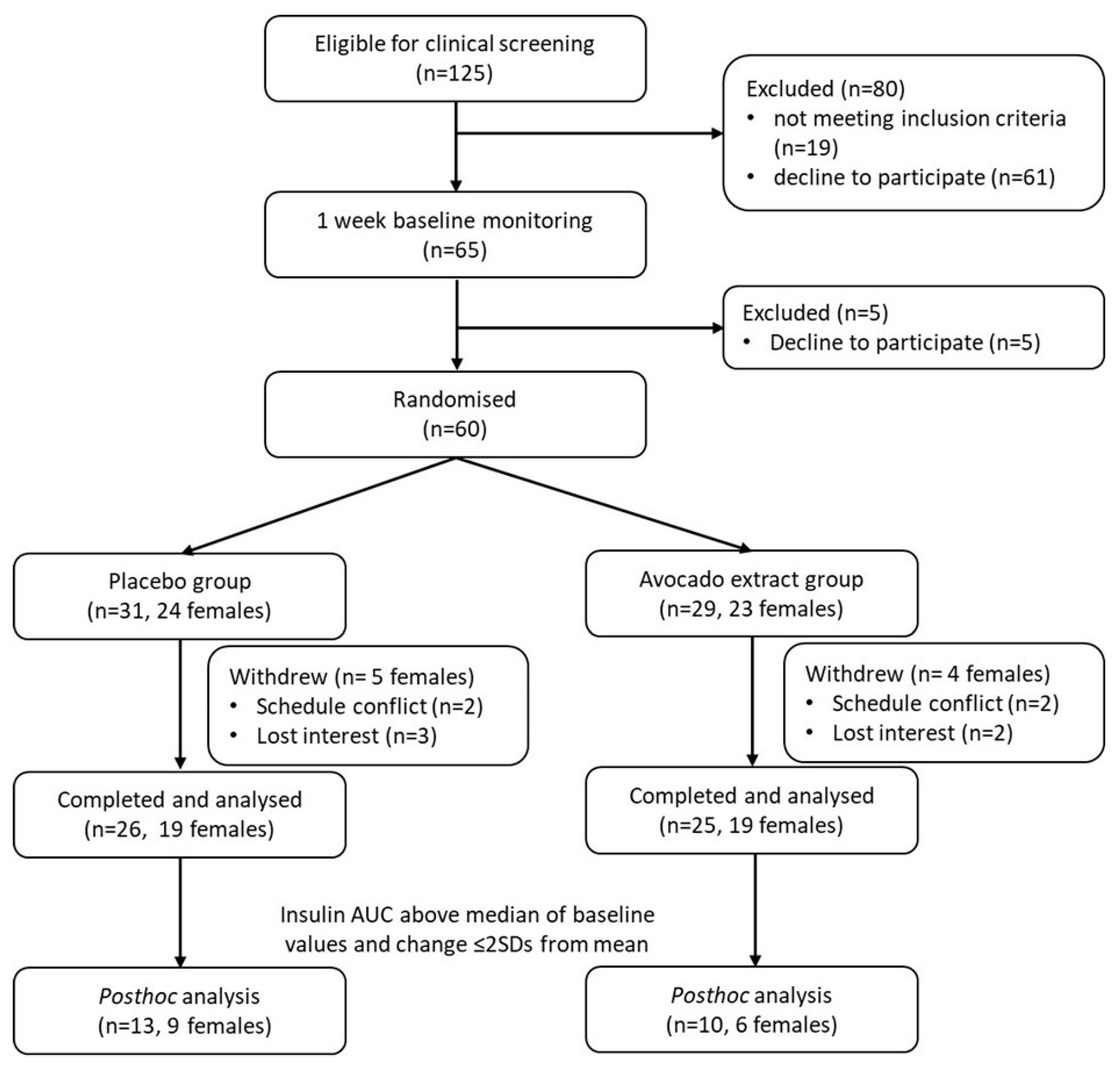
2.1. Participants
2.2. Study Design
2.3. Supplement Preparations
2.4. Dietary Intervention and Randomisation
2.5. Outcome Measures
2.6. Body Composition
2.7. Blood Pressure
2.8. Dietary Intake
2.9. Physical Activity
2.10. OGTTs
2.11. Blood Analysis
2.12. Autophagy Gene Expression
2.13. Calculations and Statistical Analysis
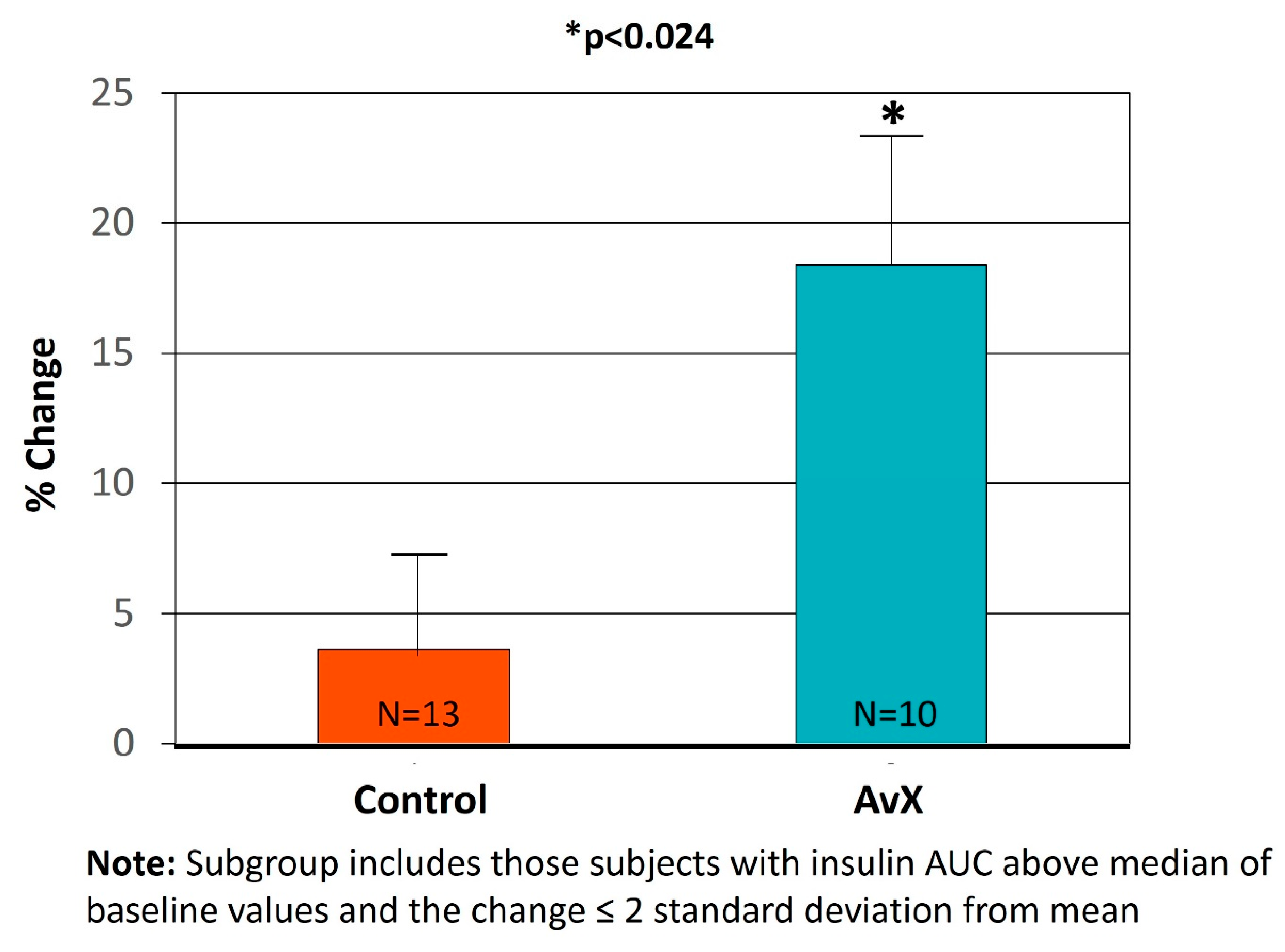
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Soare, A.; Weiss, E.P.; Pozzilli, P. Benefits of caloric restriction for cardiometabolic health, including type 2 diabetes mellitus risk. Diabetes Metab. Res. Rev. 2014, 30 (Suppl. S1), 41–47. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; de Jonge, L. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 2006, 295, 1539–1548. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef]
- Monnier, L.; Schlienger, J.L.; Colette, C.; Bonnet, F. The obesity treatment dilemma: Why dieting is both the answer and the problem? A mechanistic overview. Diabetes Metab. 2021, 47, 101192. [Google Scholar] [CrossRef]
- Ingram, D.K.; Roth, G.S. Calorie restriction mimetics: Can you have your cake and eat it, too? Ageing Res. Rev. 2015, 20, 46–62. [Google Scholar] [CrossRef]
- Ingram, D.K.; Roth, G.S. Glycolytic inhibition: An effective strategy for developing calorie restriction mimetics. Geroscience 2021, 43, 1159–1169. [Google Scholar] [CrossRef]
- Roth, G.; Hayek, M.; Massimino, S.; Davenport, G.; Arking, R.; Bartke, A.; Bonkowski, M.; Ingram, D. Mannoheptulose: Glycolytic inhibitor and novel caloric restriction mimetic. FASEB J. 2009, 23, 553.1. [Google Scholar] [CrossRef]
- Ingram, D.K.; Pistell, P.J.; Wang, Z.Q.; Yu, Y.; Massimino, S.; Davenport, G.M.; Hayek, M.; Roth, G.S. Characterization and Mechanisms of Action of Avocado Extract Enriched in Mannoheptulose as a Candidate Calorie Restriction Mimetic. J. Agric. Food Chem. 2021, 69, 7367–7376. [Google Scholar] [CrossRef]
- Pistell, P.J.; Utsuki, T.; Francis, J.; Ebenezer, P.J.; Terrebonne, J.; Roth, G.S.; Ingram, D.K. An Avocado Extract Enriched in Mannoheptulose Prevents the Negative Effects of a High-Fat Diet in Mice. Nutrients 2021, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Davenport, G.; Massimino, S.; Hayek, M.; Ceddia, M.; Burr, J.; Yeh, C.; Li, L.; Roth, G.; Ingram, D. Bioavailability of avocado-derived mannoheptulose in dogs. FASEB J. 2010, 24, 725.3. [Google Scholar] [CrossRef]
- McKnight, L.L.; France, J.; Wright, D.; Davenport, G.; Shoveller, A.K. Dietary mannoheptulose does not alter glucose or lipid metabolism in adult Labrador Retrievers. J. Anim. Physiol. Anim. Nutr. 2018, 102, e122–e131. [Google Scholar] [CrossRef] [PubMed]
- McKnight, L.L.; Root-McCaig, J.; Wright, D.; Davenport, G.M.; France, J.; Shoveller, A.K. Dietary Mannoheptulose Does Not Significantly Alter Daily Energy Expenditure in Adult Labrador Retrievers. PLoS ONE 2015, 10, e0143324. [Google Scholar] [CrossRef] [PubMed]
- Viktora, J.K.; Johnson, B.F.; Penhos, J.C.; Rosenberg, C.A.; Wolff, F.W. Effect of ingested mannoheptulose in animals and man. Metabolism 1969, 18, 87–102. [Google Scholar] [CrossRef]
- Kappler-Tanudyaya, N.; Schmitt, H.; Tippkötter, N.; Meyer, L.; Lenzen, S.; Ulber, R. Combination of biotransformation and chromatography for the isolation and purification of mannoheptulose. Biotechnol. J. 2007, 2, 692–699. [Google Scholar] [CrossRef]
- Shaw, P.E.; Wilson, C.W., 3rd; Knight, R.J., Jr. High-performance liquid chromatographic analysis of D-manno-heptulose, perseitol, glucose, and fructose in avocado cultivars. J. Agric. Food Chem. 1980, 28, 379–382. [Google Scholar] [CrossRef]
- Roberts, W.C. The Friedewald-Levy-Fredrickson formula for calculating low-density lipoprotein cholesterol, the basis for lipid-lowering therapy. Am. J. Cardiol. 1988, 62, 345–346. [Google Scholar] [CrossRef]
- Mori, T.A.; Croft, K.D.; Puddey, I.B.; Beilin, L.J. An improved method for the measurement of urinary and plasma F2-isoprostanes using gas chromatography-mass spectrometry. Anal. Biochem. 1999, 268, 117–125. [Google Scholar] [CrossRef]
- Liu, B.; Hutchison, A.T.; Thompson, C.H.; Lange, K.; Heilbronn, L.K. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes. Res. Clin. Pract. 2019, 13, 408–415. [Google Scholar] [CrossRef]
- Chaudhary, R.; Liu, B.; Bensalem, J.; Sargeant, T.J.; Page, A.J.; Wittert, G.A.; Hutchison, A.T.; Heilbronn, L.K. Intermittent fasting activates markers of autophagy in mouse liver, but not muscle from mouse or humans. Nutrition 2022, 101, 111662. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Mitrakou, A.; Pimenta, W.; Jenssen, T.; Yki-Järvinen, H.; Van Haeften, T.; Renn, W.; Gerich, J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Chauvenet, W. A Manual of Spherical and Practical Astronomy, 5th ed.; Kessinger Publishing: Whitefish, MT, USA, 1960; Volume 2, pp. 474–566. [Google Scholar]
- The Jamovi Project Jamovi. Version 2.3. Available online: https://www.jamovi.org (accessed on 14 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.1; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- McKnight, L.L.; Flickinger, E.A.; France, J.; Davenport, G.M.; Shoveller, A.K. Mannoheptulose has differential effects on fasting and postprandial energy expenditure and respiratory quotient in adult Beagle dogs fed diets of different macronutrient contents. J. Nutr. Sci. 2014, 3, e17. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Motta, J.R.; Jung, I.E.D.C.; Azzolin, V.F.; Teixeira, C.F.; Braun, L.E.; De Oliveira Nerys, D.A.; Motano, M.A.E.; Duarte, M.M.M.F.; Maia-Ribeiro, E.A.; da Cruz, I.B.M.; et al. Avocado oil (Persea americana) protects SH-SY5Y cells against cytotoxicity triggered by cortisol by the modulation of BDNF, oxidative stress, and apoptosis molecules. J. Food Biochem. 2021, 45, e13596. [Google Scholar] [CrossRef]
- James-Martin, G.; Brooker, P.G.; Hendrie, G.A.; Stonehouse, W. Avocado Consumption and Cardiometabolic Health: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2022. [Google Scholar] [CrossRef]
- Li, Z.; Wong, A.; Henning, S.M.; Zhang, Y.; Jones, A.; Zerlin, A.; Thames, G.; Bowerman, S.; Tseng, C.H.; Heber, D. Hass avocado modulates postprandial vascular reactivity and postprandial inflammatory responses to a hamburger meal in healthy volunteers. Food Funct. 2013, 4, 384–391. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, D.; Guzman, G.; Edirisinghe, I.; Burton-Freeman, B. Avocado Consumption for 12 Weeks and Cardiometabolic Risk Factors: A Randomized Controlled Trial in Adults with Overweight or Obesity and Insulin Resistance. J. Nutr. 2022, 152, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tao, L.; Hao, L.; Stanley, T.H.; Huang, K.H.; Lambert, J.D.; Kris-Etherton, P.M. A Moderate-Fat Diet with One Avocado per Day Increases Plasma Antioxidants and Decreases the Oxidation of Small, Dense LDL in Adults with Overweight and Obesity: A Randomized Controlled Trial. J. Nutr. 2020, 150, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Edirisinghe, I.; Burton-Freeman, B. Avocado Fruit on Postprandial Markers of Cardio-Metabolic Risk: A Randomized Controlled Dose Response Trial in Overweight and Obese Men and Women. Nutrients 2018, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Edwards, C.G.; Thompson, S.V.; A Hannon, B.; Burke, S.K.; Walk, A.D.M.; A Mackenzie, R.W.; E Reeser, G.; Fiese, B.H.; A Burd, N.; et al. Avocado Consumption, Abdominal Adiposity, and Oral Glucose Tolerance Among Persons with Overweight and Obesity. J. Nutr. 2021, 151, 2513–2521. [Google Scholar] [CrossRef]
- Lerman-Garber, I.; Ichazo-Cerro, S.; Zamora-González, J.; Cardoso-Saldaña, G.; Posadas-Romero, C. Effect of a high-monounsaturated fat diet enriched with avocado in NIDDM patients. Diabetes Care 1994, 17, 311–315. [Google Scholar] [CrossRef]
| AvX (n = 29) | Placebo (n = 31) | |
|---|---|---|
| Female/male, n | 23/6 | 24/7 |
| Age (Years) | 51 ± 12 | 46 ± 13 |
| Body weight (kg) | 93.6 ± 10.0 | 94.5 ± 9.3 |
| BMI (kg/m2) | 33.8 ± 2.7 | 34.2 ± 2.5 |
| Waist circumference (cm) | 105 ± 10 | 106.0 ± 10.0 |
| Systolic blood pressure (mmHg) a | 120 ± 15 | 114 ± 9 |
| Diastolic blood pressure (mmHg) a | 77 ± 7 | 75 ± 6 |
| Fasting plasma glucose (mmol/L) b | 5.3 ± 0.6 | 5.1 ± 0.6 |
| Fasting insulin (mU/L) b | 10.7 ± 9.6 | 10.9 ± 7.5 |
| HOMA-IR b | 2.6 ± 2.6 | 2.6 ± 2.4 |
| Avocado Extract Group (N = 25) | Placebo Group (N = 26) | Δ Between-Group Estimates (AvX–Placebo) | p Value (Between Group) | |||||
|---|---|---|---|---|---|---|---|---|
| W0 | W12 | Δ (W12–W0) | W0 | W12 | Δ (W12–W0) | |||
| Anthropometrics | ||||||||
| Body weight (kg) | 93.5 ± 2.1 | 94.1 ± 2.1 | 0.6 ± 0.3 * | 95.0 ± 2.0 | 94.8 ± 2.1 | −0.3 ±0.6 | 0.9 ± 0.6 | 0.176 |
| BMI (kg/m2) | 33.70 ± 0.55 | 33.90 ± 0.59 | 0.24 ± 0.09 * | 34.10 ± 0.48 | 34.00 ± 0.55 | −0.10 ± 0.21 | 0.36 ± 0.23 | 0.149 |
| Waist circumference (cm) | 105.0 ± 2.0 | 104.0 ± 1.9 | −0.8 ± 1.2 | 107.0 ± 1.9 | 107.0 ± 2.3 | 0.92 ± 1.67 | −2.0 ± 2.0 | 0.337 |
| Body fat mass (%) | 44.90 ± 1.42 | 44.90 ± 1.47 | −0.1 ± 0.2 | 46.10 ± 1.33 | 46.30 ± 1.56 | −0.1 ± 0.4 | 0.08 ± 0.48 | 0.559 |
| SBP (mmHg) | 122 ± 3 | 118 ± 2 | −4 ± 2 * | 115.00 ± 1.77 | 113.00 ± 2.03 | −2 ± 1 | 0.5 ± 2.1 | 0.825 |
| DBP (mmHg) | 78 ± 1 | 77 ± 1 | −1 ± 1 | 75.80 ± 1.19 | 74.70 ± 1.26 | −1 ± 1 | 0.7 ± 1.0 | 0.482 |
| Fasting glycaemic markers | ||||||||
| Fasting insulin (mU/L) | 11.30 ± 1.99 | 10.60 ± 1.52 | −0.71 ± 0.98 | 10.40 ± 0.86 | 9.17 ± 0.80 | −1.2 ± 0.77 | 0.87 ± 1.0 | 0.883 |
| Fasting glucose (mmol/L) | 5.29 ± 0.12 | 5.13 ± 0.11 | −0.16 ± 0.10 | 5.07 ± 0.08 | 5.00 ± 0.08 | −0.07 ± 0.06 | −0.02 ± 0.10 | 0.864 |
| HOMA-beta | 124.0 ± 16.4 | 137.0 ± 16.7 | 12.9 ± 11.1 | 141.0 ± 13.3 | 128.0 ± 12.1 | −12.8 ± 10.7 | 21.4 ± 14 | 0.508 |
| HOMA-IR | 2.79 ± 0.54 | 2.49 ± 45.3 | −0.30 ± 0.27 | 2.34 ± 0.20 | 17.8 ± 17.1 | −0.28 ± 0.18 | 0.16 ± 0.24 | 0.506 |
| Oral glucose tolerance test | ||||||||
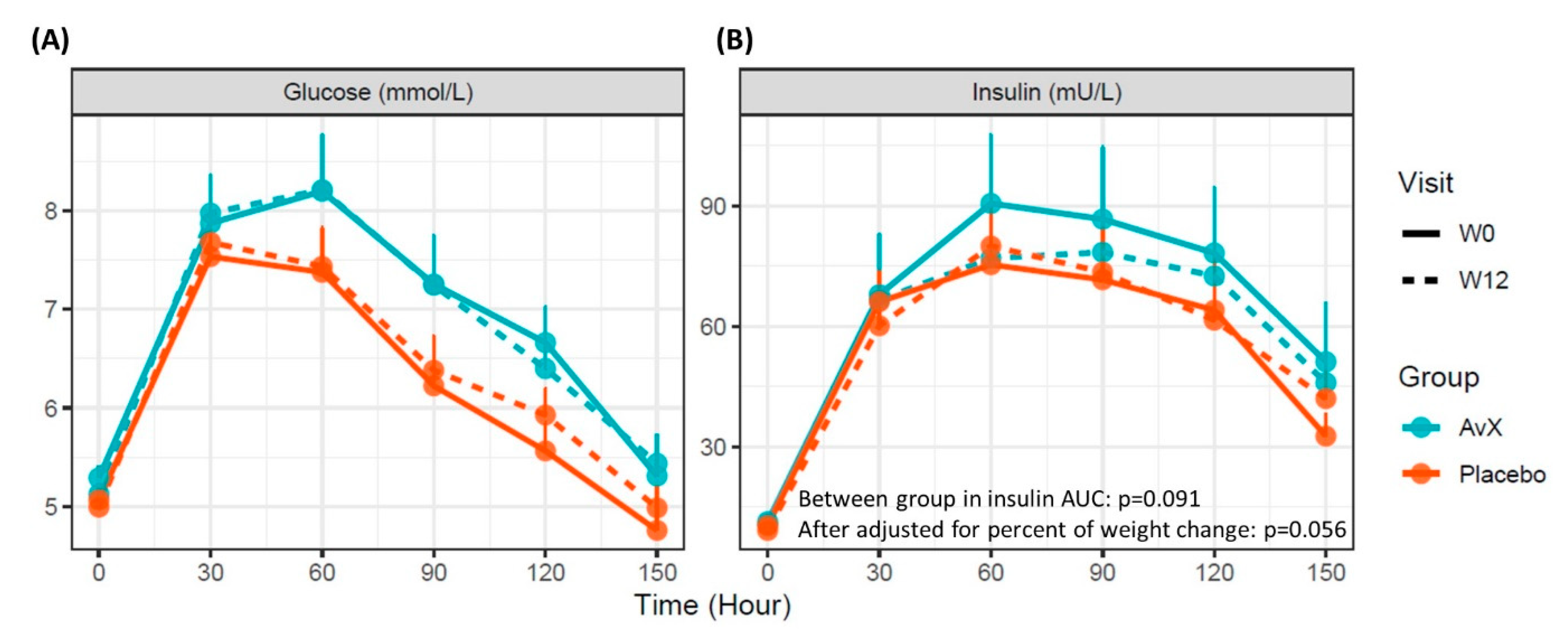
| Glucose AUC (mmol/L/hour) | 7.06 ± 0.36 | 7.02 ± 0.35 | −0.04 ± 0.20 | 6.32 ± 0.20 | 6.48 ± 0.25 | 0.16 ± 0.14 | −0.11 ± 0.25 | 0.678 |
| Insulin AUC (mU/L/hour) | 71.00 ± 14.30 | 64.60 ± 10.7 | −6.38 ± 6.12 | 59.70 ± 7.77 | 60.20 ± 6.01 | 0.43 ± 3.24 | −3.16 ± 4.82 | 0.091 |
| Matsuda insulin sensitivity index | 30.5 ± 74.2 | 24.7 ± 45.3 | −5.8 ± 6.1 | 14.9 ±12.2 | 17.8 ± 17.1 | 2.98 ± 2.1 | −2.78 ± 3.00 | 0.337 |
| Lipid profile | ||||||||
| HDL-C (mmol/L) | 1.43 ± 0.08 | 1.41 ± 0.07 | −0.03 ± 0.04 | 1.27 ± 0.06 | 1.24 ± 0.06 | −0.03 ± 0.03 | 0.03 ± 0.05 | 0.495 |
| LDL-C (mmol/L) | 3.38 ± 0.17 | 3.42 ± 0.18 | 0.04 ± 0.15 | 3.83 ± 0.19 | 3.70 ± 0.13 | −0.13 ± 0.09 | −0.02 ± 0.16 | 0.939 |
| Total cholesterol (mmol/L) | 5.11 ± 0.18 | 5.09 ± 0.19 | −0.02 ± 0.16 | 5.41 ± 0.21 | 5.23 ± 0.15 | −0.18 ± 0.10 | −0.05 ± 0.17 | 0.859 |
| Triglyceride (mmol/L) | 1.48 ± 0.13 | 1.34 ± 0.11 | −0.15 ± 0.10 * | 1.55 ± 0.14 | 1.49 ± 0.12 | −0.06 ± 0.09 | −0.11 ± 0.11 | 0.532 |
| Inflammation and oxidative stress biomarker | ||||||||
| hsCRP (mg/L) | 2.10 ± 0.51 | 2.68 ± 0.65 | 0.58 ± 0.29 | 1.58 ± 0.27 | 1.94 ± 0.32 | 0.36 ± 0.20 | 0.18 ± 0.36 | 0.69 |
| F2-Isoprostane (pmol/L) | 582.0 ± 60.8 | 530.0 ± 53.8 | −52.8 ± 63.0 | 624.0 ± 62.5 | 627.0 ± 57.8 | 3.2 ± 23.9 | −72.6 ± 56.4 | 0.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Ingram, D.K.; Gumpricht, E.; De Paoli, T.; Teong, X.T.; Liu, B.; Mori, T.A.; Heilbronn, L.K.; Roth, G.S. Effects of an Unripe Avocado Extract on Glycaemic Control in Individuals with Obesity: A Double-Blinded, Parallel, Randomised Clinical Trial. Nutrients 2023, 15, 4812. https://doi.org/10.3390/nu15224812
Zhao L, Ingram DK, Gumpricht E, De Paoli T, Teong XT, Liu B, Mori TA, Heilbronn LK, Roth GS. Effects of an Unripe Avocado Extract on Glycaemic Control in Individuals with Obesity: A Double-Blinded, Parallel, Randomised Clinical Trial. Nutrients. 2023; 15(22):4812. https://doi.org/10.3390/nu15224812
Chicago/Turabian StyleZhao, Lijun, Donald K. Ingram, Eric Gumpricht, Trent De Paoli, Xiao Tong Teong, Bo Liu, Trevor A. Mori, Leonie K. Heilbronn, and George S. Roth. 2023. "Effects of an Unripe Avocado Extract on Glycaemic Control in Individuals with Obesity: A Double-Blinded, Parallel, Randomised Clinical Trial" Nutrients 15, no. 22: 4812. https://doi.org/10.3390/nu15224812
APA StyleZhao, L., Ingram, D. K., Gumpricht, E., De Paoli, T., Teong, X. T., Liu, B., Mori, T. A., Heilbronn, L. K., & Roth, G. S. (2023). Effects of an Unripe Avocado Extract on Glycaemic Control in Individuals with Obesity: A Double-Blinded, Parallel, Randomised Clinical Trial. Nutrients, 15(22), 4812. https://doi.org/10.3390/nu15224812