Micronutrient Status in Adult Crohn’s Disease during Clinical Remission: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Exclusion Criteria
2.4. Search Strategy
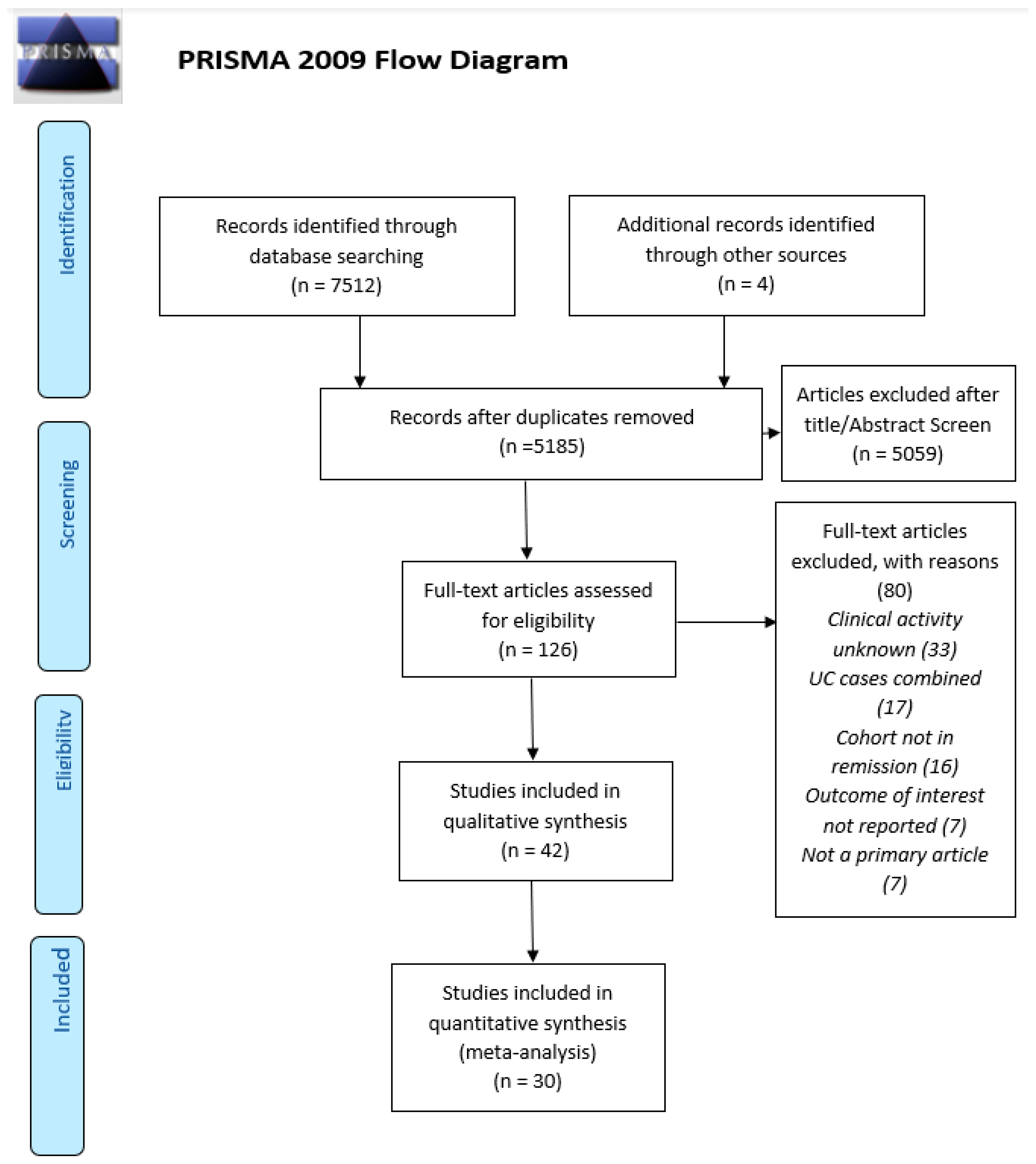
2.5. Study Selection
2.6. Data Extraction
2.7. Quality/Risk of Bias
2.8. Data Synthesis
3. Results
| Micronutrient | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liposoluble Vitamins | Hydrosoluble Vitamins | Minerals | ||||||||||||||||||||||
| Author | Year | n CD | % Low in CD | CD vs. HC | A | βc | D | E | K | B1 | B2 | B3 | B6 | B9 | B12 | HcY | C | Ca | Cu | Mn | Mg | PO4 | Se | Zn |
| Schoelmerich [21] | 1985 | 54 | X | X | X | X | ||||||||||||||||||
| Imes [22] | 1986 | 137 | X | X | ||||||||||||||||||||
| Imes [23] | 1987 | 137 | X | X | X | |||||||||||||||||||
| Geerling [16] | 1998 | 32 | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Geerling [17] | 1999 | 62 | X | X | X | X | X | X | X | X | ||||||||||||||
| Genser [24] | 1999 | 24 | X | X | X | X | ||||||||||||||||||
| Geerling [18] | 2000 | 23 | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Koutroubakis [25] | 2000 | 55 | X | X | X | X | ||||||||||||||||||
| D’Odorico [26] | 2001 | 37 | X | X | X | X | ||||||||||||||||||
| Schoon [27] | 2001 | 32 | X | X | ||||||||||||||||||||
| Wendland [28] | 2001 | 37 | X | X | X | X | X | X | ||||||||||||||||
| Duggan [29] | 2004 | 44 | X | X | X | |||||||||||||||||||
| Tajika [30] | 2004 | 33 | X | X | X | X | X | X | ||||||||||||||||
| McCarthy [31] | 2005 | 44 | X | X | X | |||||||||||||||||||
| Filippi [19] | 2006 | 54 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Gilman [32] | 2006 | 58 | X | X | ||||||||||||||||||||
| Roblin [33] | 2007 | 92 | X | X | X | X | ||||||||||||||||||
| Vagianos [34] | 2007 | 84 | X | X | X | X | X | X | X | X | X | |||||||||||||
| Valentini [35] | 2008 | 91 | X | X | X | X | X | X | X | |||||||||||||||
| Kallel [36] | 2011 | 89 | X | X | X | X | X | |||||||||||||||||
| Nakajima [37] | 2011 | 47 | X | X | X | |||||||||||||||||||
| Nic Suibhne [38] | 2013 | 81 | X | X | X | |||||||||||||||||||
| Vagianos [39] | 2012 | 70 | X | X | X | X | X | |||||||||||||||||
| Bermejo [40] | 2013 | 180 | X | X | X | |||||||||||||||||||
| Garg [41] | 2013 | 40 | X | X | X | |||||||||||||||||||
| Grunbaum [42] | 2013 | 34 | X | X | X | |||||||||||||||||||
| Jorgensen [43] | 2013 | 182 | X | X | ||||||||||||||||||||
| Kini [44] | 2014 | 32 | X | X | ||||||||||||||||||||
| Lupu [45] | 2015 | 115 | X | X | X | |||||||||||||||||||
| Soares-Mota [46] | 2015 | 38 | X | X | ||||||||||||||||||||
| Ward [47] | 2015 | 381 | X | X | ||||||||||||||||||||
| Basson [48] | 2016 | 44 | X | X | ||||||||||||||||||||
| Battat [49] | 2017 | 66 | X | X | ||||||||||||||||||||
| Frigstad [50] | 2017 | 230 | X | X | ||||||||||||||||||||
| Caviezel [51] | 2018 | 99 | X | X | ||||||||||||||||||||
| Branco [52] | 2019 | 106 | X | X | ||||||||||||||||||||
| De Castro [53] | 2019 | 31 | X | X | X | X | X | |||||||||||||||||
| Marcil [54] | 2019 | 274 | X | X | X | |||||||||||||||||||
| Olmedo-Martin [55] | 2019 | 150 | X | X | ||||||||||||||||||||
| Zhao [56] | 2019 | 21 | X | X | ||||||||||||||||||||
| Domislovic [57] | 2020 | 123 | X | X | ||||||||||||||||||||
| MacMaster [20] | 2021 | 59 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Reported in CD | Representative of Current IBD Cohorts | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Primary | CRP | Resect | Supp | CRP | Resect | Supp | Recruitment | Prosp | Clear Data | Sep Rem | % Rem Desc | Overall Quality |
| Schoelmerich [21] | 1985 | Yes | No | Yes | Yes | ? | High | Yes | Yes | Yes | Yes | Yes | n/a | Medium |
| Imes [22] | 1986 | No | No | Yes | Yes | ? | Yes | Yes | ? | Yes | Yes | No | Yes | Medium |
| Imes [23] | 1987 | No | No | Yes | Yes | ? | Yes | Yes | ? | Yes | No | No | Yes | Low |
| Geerling [16] | 1998 | Yes | Yes | Yes | Yes | Yes | High | Yes | Yes | Yes | No | No | Yes | Medium |
| Geerling [17] | 1999 | No | Yes | Yes | No | Yes | High | ? | Yes | Yes | No | Yes | n/a | Medium |
| Genser [24] | 1999 | Yes | Yes | Yes | ? | High | High | ? | ? | Yes | Yes | No | Yes | Medium |
| Geerling [18] | 2000 | No | Yes | Yes | ? | High | Yes | ? | Yes | Yes | Yes | Yes | n/a | High |
| Koutroubakis [25] | 2000 | Yes | No | No | No | ? | ? | ? | ? | Yes | No | No | Yes | Low |
| Wendland [28] | 2001 | Yes | No | Yes | Yes | ? | Yes | Excluded | ? | Yes | Yes | Yes | Yes | Medium |
| D’Odorico [26] | 2001 | Yes | No | No | No | ? | ? | ? | ? | ? | Yes | Yes | Yes | Low |
| Schoon [27] | 2001 | No | No | Yes | Yes | ? | High | Yes | Unclear | Yes | Yes | Yes | n/a | Medium |
| Duggan [29] | 2004 | No | No | Yes | Yes | ? | Yes | Yes | Unclear | Yes | No | Yes | n/a | Low |
| Tajika [30] | 2004 | Yes | Yes | Yes | Yes | Yes | High | Excluded | No | Yes | Yes | No | Yes | High |
| McCarthy [31] | 2005 | Yes | Yes | Yes | Yes | Yes | Yes | Excluded | Yes | Yes | Yes | Yes | n/a | High |
| Filippi [19] | 2006 | Yes | Yes | Yes | No | Yes | No | ? | Yes | Yes | No | Yes | n/a | Low |
| Gilman [32] | 2006 | No | Yes | Yes | Yes | Yes | High | Yes | Yes | Yes | Yes | No | Yes | High |
| Roblin [33] | 2007 | No | No | No | Yes | ? | ? | Excluded | Yes | Yes | Yes | No | Yes | Medium |
| Valentini [35] | 2008 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | n/a | High |
| Vagianos [34] | 2007 | Yes | No | Yes | Yes | ? | Yes | Yes | Yes | Yes | Yes | No | Yes | Medium |
| Kallel [36] | 2011 | Yes | Yes | Yes | Yes | Yes | Excluded | ? | Yes | Yes | No | Yes | High | |
| Nakajima [37] | 2011 | No | No | No | Yes | ? | ? | Yes | Yes | Yes | No | No | Yes | Low |
| Nic Suibhne [38] | 2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | High |
| Vagianos [39] | 2012 | Yes | Yes | Yes | Yes | Yes | High | Yes | Yes | Yes | Yes | No | Yes | High |
| Bermejo [40] | 2013 | Yes | No | Yes | Yes | ? | Yes | Excluded | Yes | Yes | Yes | No | Yes | Medium |
| Garg [41] | 2013 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Medium |
| Grunbaum [42] | 2013 | No | No | Yes | Yes | ? | Yes | Yes | Yes | Yes | No | No | Yes | Low |
| Kini [44] | 2013 | No | No | No | Yes | ? | ? | Yes | Yes | Yes | No | Yes | Yes | Low |
| Lupu [45] | 2014 | No | No | Yes | Yes | ? | Yes | No | Yes | Yes | Yes | No | Yes | Medium |
| Soares-Mota [46] | 2015 | Yes | No | Yes | No | ? | Yes | ? | Yes | Yes | No | No | No | Low |
| Ward [47] | 2015 | Yes | No | Yes | No | ? | Yes | ? | Yes | Yes | Yes | No | Yes | Medium |
| Basson [48] | 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Excluded | Yes | No | Yes | Yes | n/a | High |
| Battat [49] | 2016 | Yes | No | Yes | Yes | ? | No | Yes | High | Yes | Yes | Yes | n/a | Medium |
| Frigstad [50] | 2017 | Yes | Yes | Yes | No | Yes | Yes | ? | Yes | Yes | Yes | No | Yes | Medium |
| Caviezel [51] | 2017 | Yes | Yes | Yes | Yes | Yes | High | Yes | Yes | Yes | Yes | No | Yes | High |
| Branco [52] | 2018 | Yes | Yes | No | Yes | Yes | ? | Yes | Yes | Yes | Yes | No | Yes | Medium |
| De Castro [53] | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Excluded | Yes | Yes | Yes | No | Yes | High |
| Marcil [54] | 2019 | Yes | Yes | Yes | No | Yes | High | ? | Yes | Yes | Yes | Yes | n/a | Medium |
| Olmedo-Martin [55] | 2019 | No | Yes | No | No | High | ? | ? | Low | Yes | Yes | No | Yes | Low |
| Zhao [56] | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | n/a | High |
| Domislovic [57] | 2019 | No | No | No | No | ? | ? | ? | ? | Y | No | Yes | n/a | Low |
| MacMaster [20] | 2020 | Yes | No | Yes | Yes | ? | Yes | Yes | Yes | Yes | No | No | Yes | Low |
3.1. Micronutrient Status in CD by Nutrient as Prevalence Compared against Reference Ranges or Compared with HC
3.1.1. Vitamin A (10 Studies)
3.1.2. β-Carotene (8 Studies)
3.1.3. Vitamin D, Specifically, 25-OH Vitamin D (20 Studies)
3.1.4. Vitamin E (7 Studies)
3.1.5. Vitamin K (4 Studies)
3.1.6. Thiamine (B1) (4 Studies)
3.1.7. Riboflavin (B2) (1 Study)
3.1.8. Niacin (B3) (1 Study)
3.1.9. Pantothenic Acid (B5)
3.1.10. Pyridoxic Acid (B6) (4 Studies)
3.1.11. Biotin (B7)
3.1.12. Folic Acid (B9) (15 Studies)
3.1.13. Cobalamin (B12) (18 Studies)
3.1.14. Homocysteine (HcY) (3 Studies)
3.1.15. Vitamin C (7 Studies)
3.1.16. Calcium (2 Studies)
3.1.17. Copper (4 Studies)
3.1.18. Manganese (1 Study)
3.1.19. Magnesium (7 Studies)
3.1.20. Phosphorous (2 Studies)
3.1.21. Selenium (6 Studies)
3.1.22. Zinc (9 Studies)
3.2. Summary Statement for Micronutrient Status in CD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, N.; Dotan, I.; Halack, A.; Niv, E. Malabsorption is a major contributor to underweight in Crohn’s disease patients in remission. Nutrition 2006, 22, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.Q.; Strauss, B.J.; Moore, G.T. Patients with inflammatory bowel disease and their treating clinicians have different views regarding diet. J. Hum. Nutr. Diet 2017, 30, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Duerksen, D.R.; Fallows, G.; Bernstein, C.N. Vitamin B12 malabsorption in patients with limited ileal resection. Nutrition 2006, 22, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Burden of infection on growth failure. J. Nutr. 1999, 129, 534S–538S. [Google Scholar] [CrossRef]
- Shenkin, A. Micronutrients and outcome. Nutrition 1997, 13, 825–828. [Google Scholar] [CrossRef]
- Black, R. Micronutrient deficiency: An underlying cause of morbidity and mortality. SciELO Public Health 2003, 81, 79. [Google Scholar]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef]
- Graham, I.M.; Daly, L.E.; Refsum, H.M.; Robinson, K.; Brattström, L.E.; Ueland, P.M.; Palma-Reis, R.J.; Boers, G.H.; Sheahan, R.G.; Israelsson, B.; et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA 1997, 277, 1775–1781. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European Consensus on the Diagnosis and Management of Iron Deficiency and Anaemia in Inflammatory Bowel Diseases. J. Crohn Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn Colitis 2019, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Geerling, B.J.; Badart-Smook, A.; Stockbrugger, R.W.; Brummer, R.J. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am. J. Clin. Nutr. 1998, 67, 919–926. [Google Scholar] [CrossRef]
- Geerling, B.J.; v Houwelingen, A.C.; Badart-Smook, A.; Stockbrugger, R.W.; Brummer, R.J. The relation between antioxidant status and alterations in fatty acid profile in patients with Crohn disease and controls. Scand. J. Gastroenterol. 1999, 34, 1108–1116. [Google Scholar] [CrossRef]
- Geerling, B.J.; Badart-Smook, A.; Stockbrugger, R.W.; Brummer, R.J. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur. J. Clin. Nutr. 2000, 54, 514–521. [Google Scholar] [CrossRef]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.B.; Hebuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef]
- MacMaster, M.J.; Damianopoulou, S.; Thomson, C.; Talwar, D.; Stefanowicz, F.; Catchpole, A.; Gerasimidis, K.; Gaya, D.R. A prospective analysis of micronutrient status in quiescent inflammatory bowel disease. Clin. Nutr. 2021, 40, 327–331. [Google Scholar] [CrossRef]
- Schoelmerich, J.; Becher, M.S.; Hoppe-Seyler, P.; Matern, S.; Haeussinger, D.; Loehle, E.; Koettgen, E.; Gerok, W. Zinc and vitamin A deficiency in patients with Crohn’s disease is correlated with activity butlocalisationcalization or extent of the disease. Hepato-Gastroenterol. 1985, 32, 34–38. [Google Scholar]
- Imes, S.; Dinwoodie, A.; Walker, K. Vitamin C status in 137 outpatients with Crohn’s disease. Effect of diet counseling. J. Clin. Gastroenterol. 1986, 8, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Imes, S.; Pinchbeck, B.R.; Dinwoodie, A.; Walker, K.; Thomson, A.B. Iron, folate, vitamin B-12, zinc, and copper status in outpatients with Crohn’s disease: Effect of diet counseling. J. Am. Diet. Assoc. 1987, 87, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Genser, D.; Kang, M.H.; Vogelsang, H.; Elmadfa, I. Status of lipidsoluble antioxidants and TRAP in patients with Crohn’s disease and healthy controls. Eur. J. Clin. Nutr. 1999, 53, 675–679. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Dilaveraki, E.; Vlachonikolis, I.G.; Vardas, E.; Vrentzos, G.; Ganotakis, E.; Mouzas, I.A.; Gravanis, A.; Emmanouel, D.; Kouroumalis, E.A. Hyperhomocysteinemia in Greek patients with inflammatory bowel disease. Dig. Dis. Sci. 2000, 45, 2347–2351. [Google Scholar] [CrossRef]
- D’Odorico, A.; Bortolan, S.; Cardin, R.; D’Inca, R.; Martines, D.; Ferronato, A.; Sturniolo, G.C. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand. J. Gastroenterol. 2001, 36, 1289–1294. [Google Scholar]
- Schoon, E.J.; Muller, M.C.A.; Vermeer, C.; Schurgers, L.J.; Brummer, R.J.M.; Stockbrugger, R.W. Low serum and bone vitamin K status in patients with longstanding Crohn’s disease: Another pathogenetic factor of osteoporosis in Crohn’s disease? Gut 2001, 48, 473–477. [Google Scholar] [CrossRef]
- Wendland, B.E.; Aghdassi, E.; Tam, C.; Carrrier, J.; Steinhart, A.H.; Wolman, S.L.; Baron, D.; Allard, J.P. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am. J. Clin. Nutr. 2001, 74, 259–264. [Google Scholar] [CrossRef]
- Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Vitamin K status in patients with Crohn’s disease and relationship to bone turnover. Am. J. Gastroenterol. 2004, 99, 2178–2185. [Google Scholar] [CrossRef]
- Tajika, M.; Matsuura, A.; Nakamura, T.; Suzuki, T.; Sawaki, A.; Kato, T.; Hara, K.; Ookubo, K.; Yamao, K.; Kato, M.; et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J. Gastroenterol. 2004, 39, 527–533. [Google Scholar] [CrossRef]
- McCarthy, D.; Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Seasonality of vitamin D status and bone turnover in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2005, 21, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.; Shanahan, F.; Cashman, K.D. Determinants of vitamin D status in adult Crohn’s disease patients, with particular emphasis on supplemental vitamin D use. Eur. J. Clin. Nutr. 2006, 60, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Phelip, J.M.; Genevois, M.; Ducros, V.; Bonaz, B. Hyperhomocysteinaemia is associated with osteoporosis in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2007, 25, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Vagianos, K.; Bector, S.; McConnell, J.; Bernstein, C.N.; Vagianos, K.; Bector, S.; McConnell, J.; Bernstein, C.N. Nutrition assessment of patients with inflammatory bowel disease. J. Parenter. Enter. Nutr. 2007, 31, 311–319. [Google Scholar] [CrossRef]
- Valentini, L.; Schaper, L.; Buning, C.; Hengstermann, S.; Koernicke, T.; Tillinger, W.; Guglielmi, F.W.; Norman, K.; Buhner, S.; Ockenga, J.; et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition 2008, 24, 694–702. [Google Scholar] [CrossRef]
- Kallel, L.; Feki, M.; Sekri, W.; Segheir, L.; Fekih, M.; Boubaker, J.; Kaabachi, N.; Filali, A. Prevalence and risk factors of hyperhomocysteinemia in Tunisian patients with Crohn’s disease. J. Crohn Colitis 2011, 5, 110–114. [Google Scholar] [CrossRef]
- Nakajima, S.; Iijima, H.; Egawa, S.; Shinzaki, S.; Kondo, J.; Inoue, T.; Hayashi, Y.; Ying, J.; Mukai, A.; Akasaka, T.; et al. Association of vitamin K deficiency with bone metabolism and clinical disease activity in inflammatory bowel disease. Nutrition 2011, 27, 1023–1028. [Google Scholar] [CrossRef]
- Suibhne, T.N.; Cox, G.; Healy, M.; O’Morain, C.; O’Sullivan, M. Vitamin D deficiency in Crohn’s disease: Prevalence, risk factors and supplement use in an outpatient setting. J. Crohn Colitis 2012, 6, 182–188. [Google Scholar] [CrossRef]
- Vagianos, K.; Bernstein, C.N. Homocysteinemia and B vitamin status among adult patients with inflammatory bowel disease: A one-year prospective follow-up study. Inflamm. Bowel Dis. 2012, 18, 718–724. [Google Scholar] [CrossRef]
- Bermejo, F.; Algaba, A.; Guerra, I.; Chaparro, M.; De-La-Poza, G.; Valer, P.; Piqueras, B.; Bermejo, A.; Garcia-Alonso, J.; Perez, M.J.; et al. Should we monitor vitamin B12 and folate levels in Crohn’s disease patients? Scand. J. Gastroenterol. 2013, 48, 1272–1277. [Google Scholar] [CrossRef]
- Garg, M.; Rosella, O.; Lubel, J.S.; Gibson, P.R. Association of Circulating Vitamin D Concentrations with Intestinal but Not Systemic Inflammation in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 2634–2643. [Google Scholar] [CrossRef]
- Grunbaum, A.; Holcroft, C.; Heilpern, D.; Gladman, S.; Burstein, B.; Menard, M.; Al-Abbad, J.; Cassoff, J.; Macnamara, E.; Gordon, P.H.; et al. Dynamics of vitamin D in patients with mild or inactive inflammatory bowel disease and their families. Nutr. J. 2013, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.P.; Hvas, C.L.; Agnholt, J.; Christensen, L.A.; Heickendorff, L.; Dahlerup, J.F. Active Crohn’s disease is associated with low vitamin D levels. J. Crohn Colitis 2013, 7, e407–e413. [Google Scholar] [CrossRef] [PubMed]
- Kini, G.P.; Young, B.; Herbison, P.; Schultz, M. Does seasonal level of serum 25-OH vitamin D correlate with the activity of Crohn’s disease? N. Z. Med. J. 2014, 127, 51–59. [Google Scholar] [PubMed]
- Lupu, A.; Diculescu, M.; Diaconescu, R.; Tantau, M.; Tantau, A.; Visovan, I.; Gheorghe, C.; Lupei, C.; Gheorghe, L.; Cerban, R.; et al. Prevalence of anemia and iron deficiency in Romanian patients with inflammatory bowel disease: A prospective multicenter study. J. Gastrointest. Liver Dis. 2015, 24, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Soares-Mota, M.; Silva, T.A.; Gomes, L.M.; Pinto, M.A.; Mendonca, L.M.; Farias, M.L.; Nunes, T.; Ramalho, A.; Zaltman, C. High prevalence of vitamin A deficiency in Crohn’s disease patients according to serum retinol levels and the relative dose-response test. World J. Gastroenterol. 2015, 21, 1614–1620. [Google Scholar] [CrossRef]
- Ward, M.G.; Kariyawasam, V.C.; Mogan, S.B.; Patel, K.V.; Pantelidou, M.; Sobczynska-Malefora, A.; Porte, F.; Griffin, N.; Anderson, S.H.; Sanderson, J.D.; et al. Prevalence and Risk Factors for Functional Vitamin B12 Deficiency in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 2839–2847. [Google Scholar] [CrossRef]
- Raffner Basson, A.; Swart, R.; Jordaan, E.; Mazinu, M.; Watermeyer, G. Vitamin D Deficiency Increases the Risk for Moderate to Severe Disease Activity in Crohn’s Disease Patients in South Africa, Measured by the Harvey Bradshaw Index. J. Am. Coll. Nutr. 2016, 35, 163–174. [Google Scholar] [CrossRef]
- Battat, R.; Kopylov, U.; Byer, J.; Sewitch, M.J.; Rahme, E.; Nedjar, H.; Zelikovic, E.; Dionne, S.; Bessissow, T.; Afif, W.; et al. Vitamin B-12 deficiency in inflammatory bowel disease: A prospective observational pilot study. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1361–1367. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Hoivik, M.; Jahnsen, J.; Dahl, S.R.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, O.; Torp, R.; et al. Vitamin D deficiency in inflammatory bowel disease: Prevalence and predictors in a Norwegian outpatient population. Scand. J. Gastroenterol. 2017, 52, 100–106. [Google Scholar] [CrossRef]
- Caviezel, D.; Maissen, S.; Niess, J.H.; Kiss, C.; Hruz, P. High Prevalence of Vitamin D Deficiency among Patients with Inflammatory Bowel Disease. Inflamm. Intest. Dis. 2018, 2, 200–210. [Google Scholar] [CrossRef]
- Branco, J.C.; Cardoso, M.F.; Anapaz, V.; Lourenco, L.C.; Oliveira, A.M.; Rodrigues, C.G.; Santos, L.; Reis, J.A. Vitamin D Deficiency in a Portuguese Cohort of Patients with Inflammatory Bowel Disease: Prevalence and Relation to Disease Activity. Ge Port. J. Gastroenterol. 2019, 26, 155–162. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.M.; Corona, L.P.; Pascoal, L.B.; Rodrigues, B.L.; de Lourdes Setsuko Ayrizono, M.; Rodrigues Coy, C.S.; Leal, R.F.; Milanski, M. Impaired nutritional status in outpatients in remission or with active Crohn’s disease—Classified by objective endoscopic and imaging assessments. Clin. Nutr. ESPEN 2019, 33, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Marcil, V.; Levy, E.; Amre, D.; Bitton, A.; Sant’Anna, A.; Szilagy, A.; Sinnett, D.; Seidman, E.G. A Cross-Sectional Study on Malnutrition in Inflammatory Bowel Disease: Is There a Difference Based on Pediatric or Adult Age Grouping? Inflamm. Bowel Dis. 2019, 25, 1428–1441. [Google Scholar] [CrossRef]
- Olmedo-Martin, R.V.; Gonzalez-Molero, I.; Olveira-Fuster, G.; Amo-Trillo, V.; Jimenez-Perez, M. Vitamin D deficiency in outpatients with inflammatory bowel disease: Prevalence and association with clinical-biological activity. Rev. Esp. Enfermedades Dig. 2019, 111, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Gu, Q.; Du, Z.; Chen, W. The association between serum Vitamin D and inflammatory bowel disease. Medicine 2019, 98, e15233. [Google Scholar] [CrossRef]
- Domislovic, V.; Vranesic Bender, D.; Barisic, A.; Brinar, M.; Ljubas Kelecic, D.; Rotim, C.; Novosel, M.; Matasin, M.; Krznaric, Z. High Prevalence of Untreated and Undertreated Vitamin D Deficiency and Insufficiency in Patients with Inflammatory Bowel Disease. Acta Clin. Croat. 2020, 59, 109–118. [Google Scholar]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Hengstermann, S.; Valentini, L.; Schaper, L.; Buning, C.; Koernicke, T.; Maritschnegg, M.; Buhner, S.; Tillinger, W.; Regano, N.; Guglielmi, F.; et al. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin. Nutr. 2008, 27, 571–578. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- Ham, M.; Longhi, M.S.; Lahiff, C.; Cheifetz, A.; Robson, S.; Moss, A.C. Vitamin D Levels in Adults with Crohn’s Disease Are Responsive to Disease Activity and Treatment. Inflamm. Bowel Dis. 2014, 20, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, D.; Whiting, S.J.; Vatanparast, H.; Jones, J.; El-Matary, W.; Aljebreen, A.; Mirhosseini, N. The Association of Vitamin D Status with Disease Activity in a Cohort of Crohn’s Disease Patients in Canada. Nutrients 2017, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Baert, F.; van Assche, G.; Caenepeel, P.; Vergauwe, P.; Tuynman, H.; De Vos, M.; van Deventer, S.; Stitt, L.; Donner, A.; et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s diseaserandomisedrandomised trial. Lancet 2008, 371, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V.; Feagan, B.G.; Colombel, J.F.; Rubin, D.T.; Wu, E.Q.; Yu, A.P.; Pollack, P.F.; Chao, J.; Mulani, P. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: Patient-reported outcomes of the CHARM trial. Am. J. Gastroenterol. 2008, 103, 3132–3141. [Google Scholar] [CrossRef]
- Galloway, S.P.; McMillan, D.C.; Sattar, N. Effect of the inflammatory response on trace element and vitamin status. Ann. Clin. Biochem. 2000, 37, 289–297. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Edwards, C.; Stefanowicz, F.; Galloway, P.; McGrogan, P.; Duncan, A.; Talwar, D. Micronutrient Status in Children With IBD: True Deficiencies or Epiphenomenon of the Systemic Inflammatory Response. J. Pediatr. Gastroenterol. Nutr. 2013, 56, e50–e51. [Google Scholar] [CrossRef]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Quasim, T.; McMillan, D.C.; Talwar, D.; Vasilaki, A.; Denis, S.J.O.R.; Kinsella, J. The relationship between plasma and red cell B-vitamin concentrations in critically-ill patients. Clin. Nutr. 2005, 24, 956–960. [Google Scholar] [CrossRef]
- Conway, F.J.S.; Talwar, D.; McMillan, D.C. The relationship between acute changes in the systemic inflammatory response and plasma ascorbic acid, alpha-tocopherol and lipid peroxidation after elective hip arthroplasty. Clin. Nutr. 2015, 34, 642–646. [Google Scholar] [CrossRef]
| Prevalence Studies | Healthy Control (HC) Studies | ||||||
|---|---|---|---|---|---|---|---|
| Micronutrient | Number of Studies (Total Number of Subjects) | Range of Reported Lower Cut-Offs | Reported Prevalence (%) | Evidence of Deficiency in CD Population | Number of Studies vs. HC (n) Year | Number of Studies in Which CD < HC | Evidence Supports CD < HC |
| Retinol (Vitamin A) | 3 (154) | 0.9–1.2 µmol/L | 1–29 | Yes | 5 (178) | 2/5 | Uncertain |
| β-carotene | 2 (116) | 0.4–1 µmol/L | 29–100 | Yes | 5 (178) | 4/5 | Yes |
| Vitamin D | 11 (968) | 25–50 nmol/L | 14–76 | Yes | 4 (198) | 1/4 | Uncertain |
| Vitamin E | 1 (32) | 14 µmol/L | 45 | Yes | 5 (178) | 3/5 | Uncertain |
| Vitamin K | None | - | - | Unknown | 1 (32) | 1/1 | Yes |
| B2/B3/B5 | 0 | - | - | Unknown | 0 | - | Unknown |
| Vitamin B6 | 2 (154) | 5 µg/L | 30–46 | Yes | 0 | - | Unknown |
| Vitamin B9 | 6 (551) | 6.8–11.8 nmol/L RBC 210–725 nmol/L | 0–35 | Yes | 4 (238) | 0/4 | No |
| Vitamin B12 | 8 (998) | Low holoTC or high MMA or B12 < 197 pg/ml | 4–33 | Yes | 4 (238) | 2/4 | Yes |
| Homocysteine | 3 (251) | 13–15 µmol/L | 14–60 | Yes | 1 (89) | 1/1 | Yes |
| Vitamin C | 2 (169) | 11–30 µmol/L | 15–50 | Yes | 4 (154) | 2/2 | Yes |
| Calcium | 0 | Unknown | 1 (33) | 0/1 | No | ||
| Copper | 1 (32) | 12 µmol/L | 0 | Unknown | 3 (117) | 0/3 | No |
| Manganese | 0 | - | - | Unknown | 0 | - | Unknown |
| Magnesium | 3 (157) | 0.75 mmol/L | 15–50 | Yes | 4 (182) | 2/4 | Uncertain |
| Phosphorous | 0 | Unknown | 1 (33) | 1/1 | Yes | ||
| Selenium | 2 (157) | 0.59–0.91 µmol/L | 50–61 | Yes | 5 (248) | 2/5 | Uncertain |
| Zinc | 2 (129) | 10–10.3 µmol/L | 50 | Yes | 4 (211) | 2/4 | Uncertain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonnell, M.; Sartain, S.; Westoby, C.; Katarachia, V.; Wootton, S.A.; Cummings, J.R.F. Micronutrient Status in Adult Crohn’s Disease during Clinical Remission: A Systematic Review. Nutrients 2023, 15, 4777. https://doi.org/10.3390/nu15224777
McDonnell M, Sartain S, Westoby C, Katarachia V, Wootton SA, Cummings JRF. Micronutrient Status in Adult Crohn’s Disease during Clinical Remission: A Systematic Review. Nutrients. 2023; 15(22):4777. https://doi.org/10.3390/nu15224777
Chicago/Turabian StyleMcDonnell, Martin, Stephanie Sartain, Catherine Westoby, Vasiliki Katarachia, Stephen A. Wootton, and J. R. Fraser Cummings. 2023. "Micronutrient Status in Adult Crohn’s Disease during Clinical Remission: A Systematic Review" Nutrients 15, no. 22: 4777. https://doi.org/10.3390/nu15224777
APA StyleMcDonnell, M., Sartain, S., Westoby, C., Katarachia, V., Wootton, S. A., & Cummings, J. R. F. (2023). Micronutrient Status in Adult Crohn’s Disease during Clinical Remission: A Systematic Review. Nutrients, 15(22), 4777. https://doi.org/10.3390/nu15224777






