Sleeve Gastrectomy Provides Cardioprotection from Oxidative Stress In Vitro Due to Reduction of Circulating Myeloperoxidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Study Designs
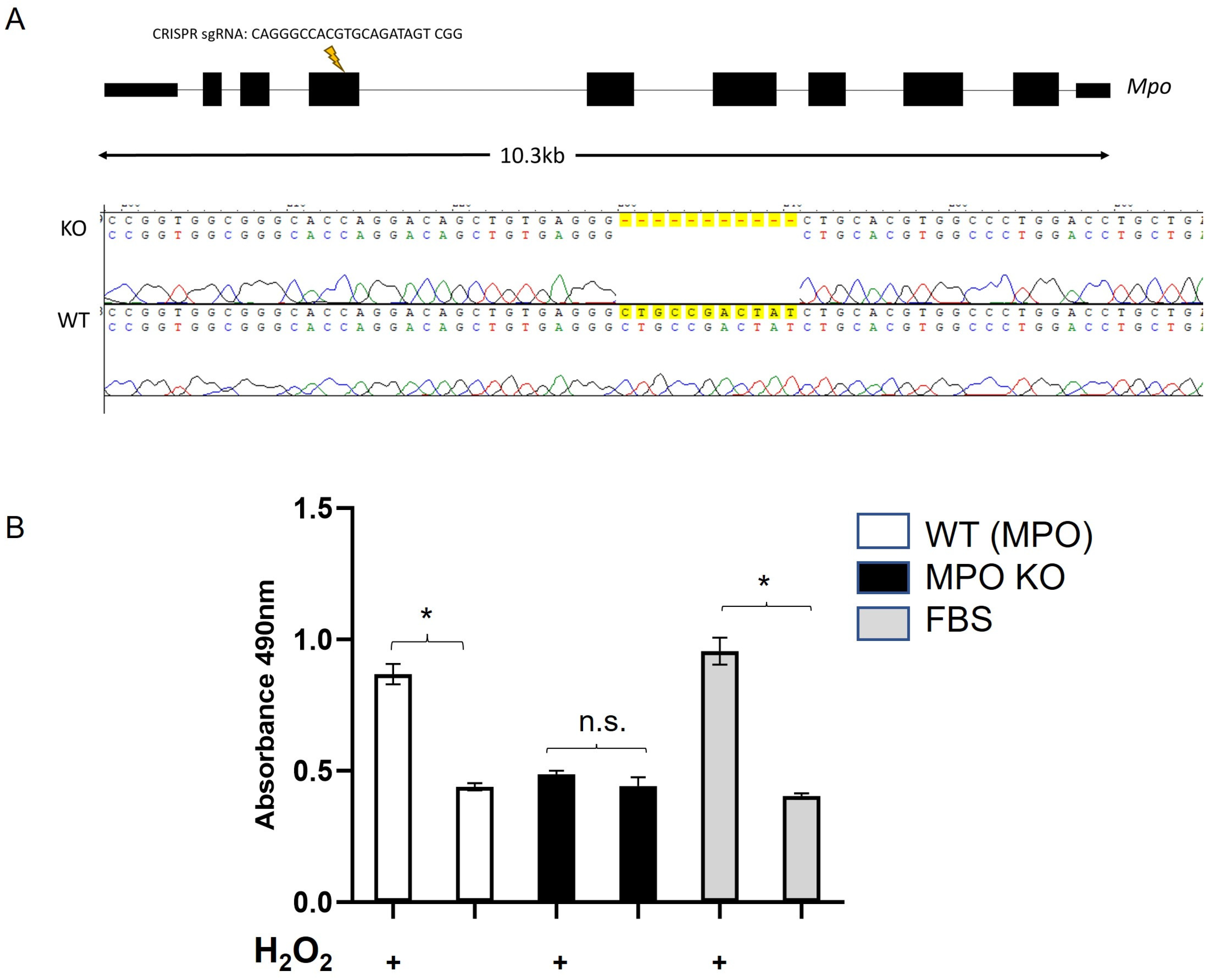
2.2. Construction of Myeloperoxidase (MPO) Knockout (KO) Rat Model
2.3. In Vitro Oxidative Stress Culture Assay
2.4. Redox Measurements Using Fluorescence Plate Read
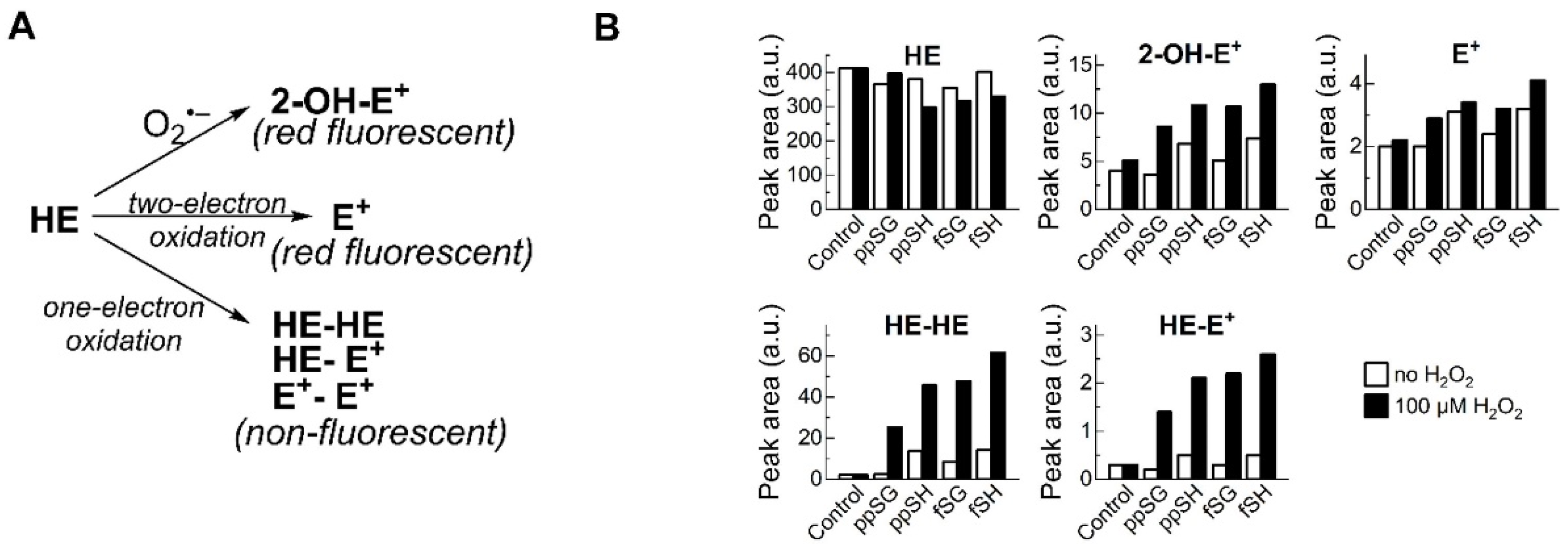
2.5. Hydroethidine (HE) Oxidation Assay
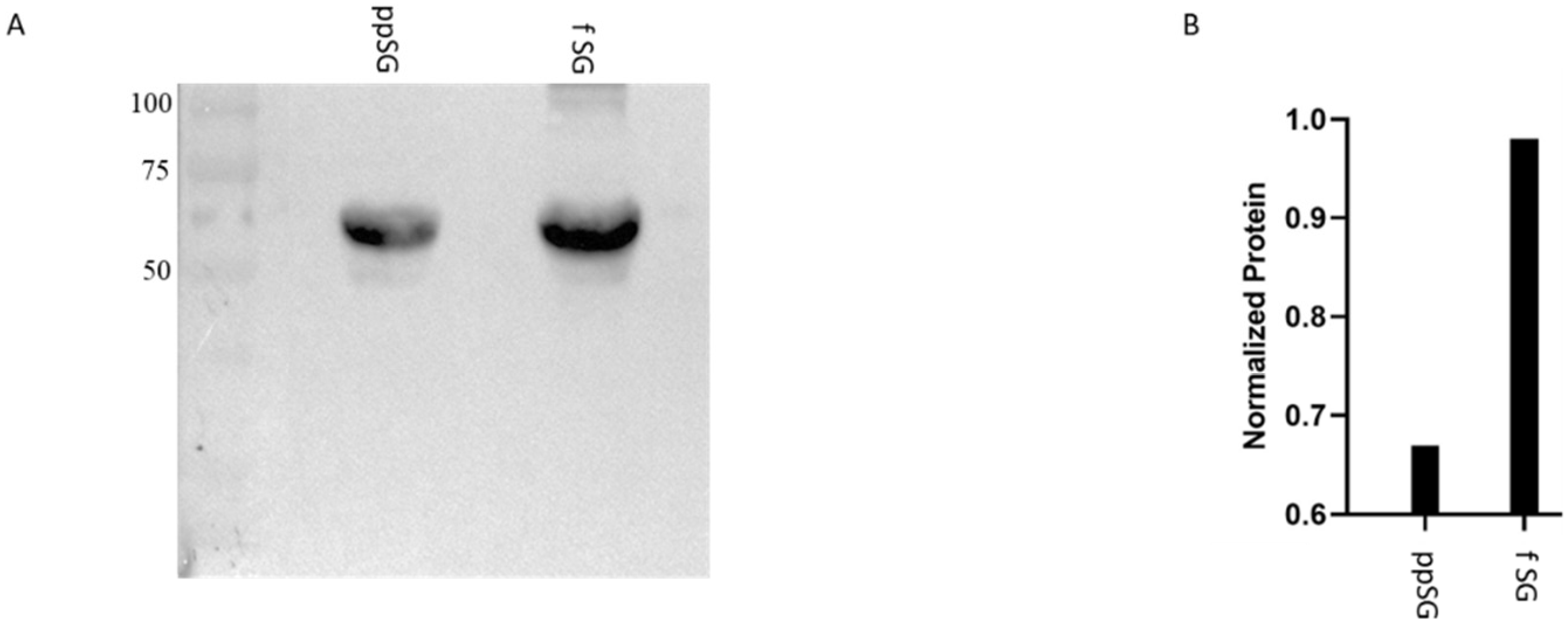
2.6. Western Blot
2.7. Statistical Analysis
3. Results
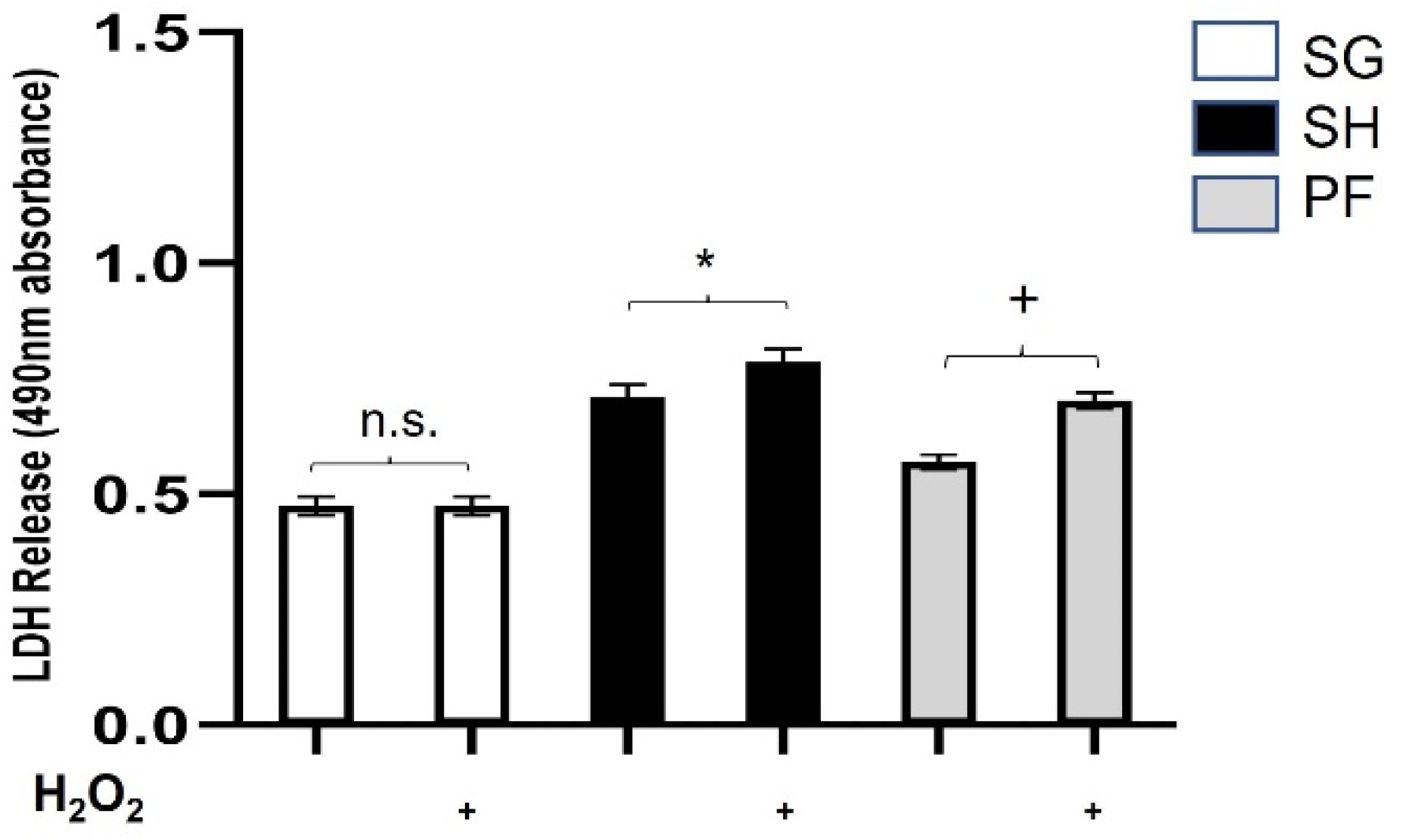
3.1. Plasma from Zucker Rats Undergoing SG Is Protective against Oxidative/Metabolic Stress and Weight Loss-Independent
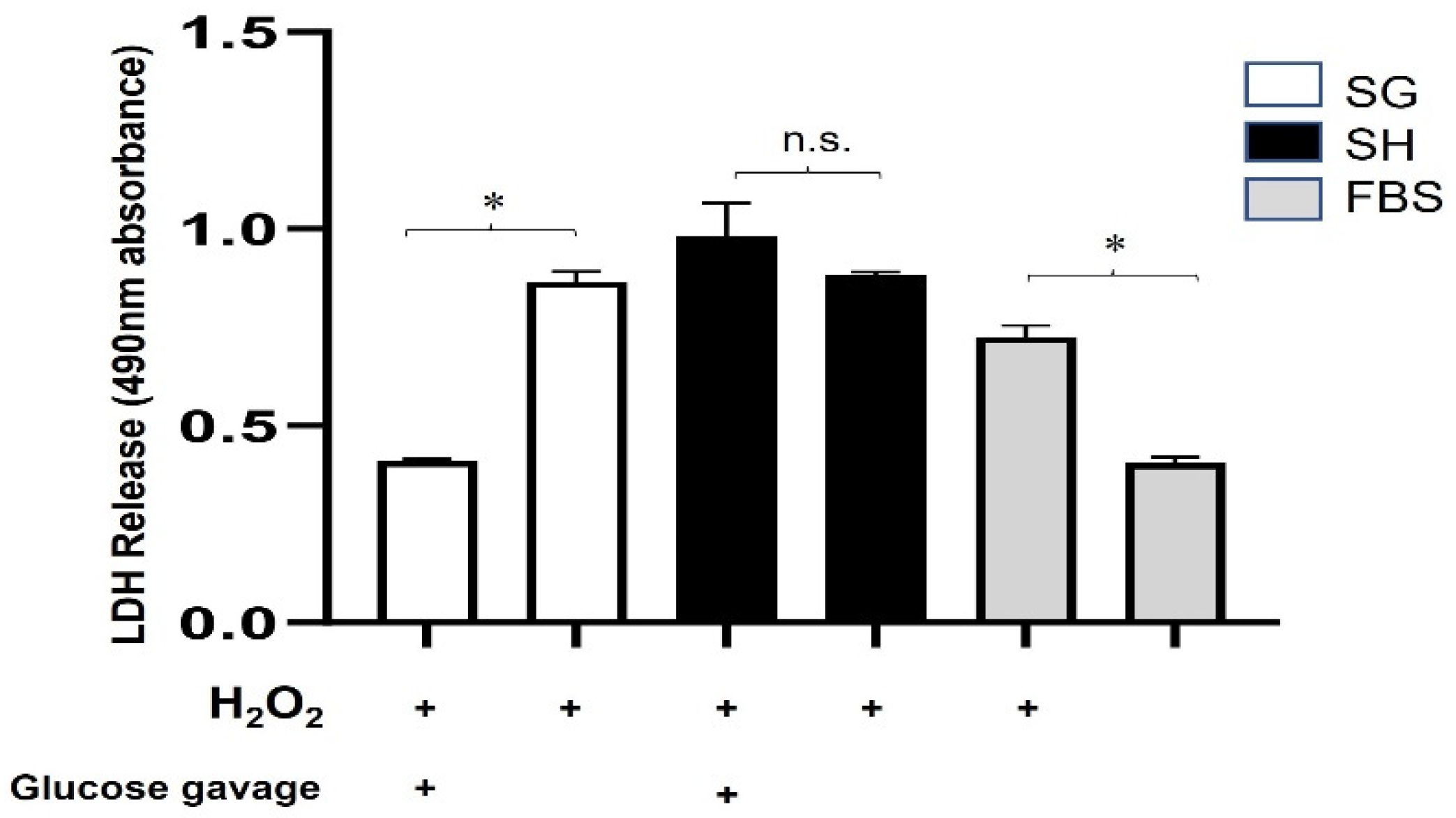
3.2. Protection against Oxidative/Metabolic Stress by SG Plasma Is Acute and Nutrient-Dependent
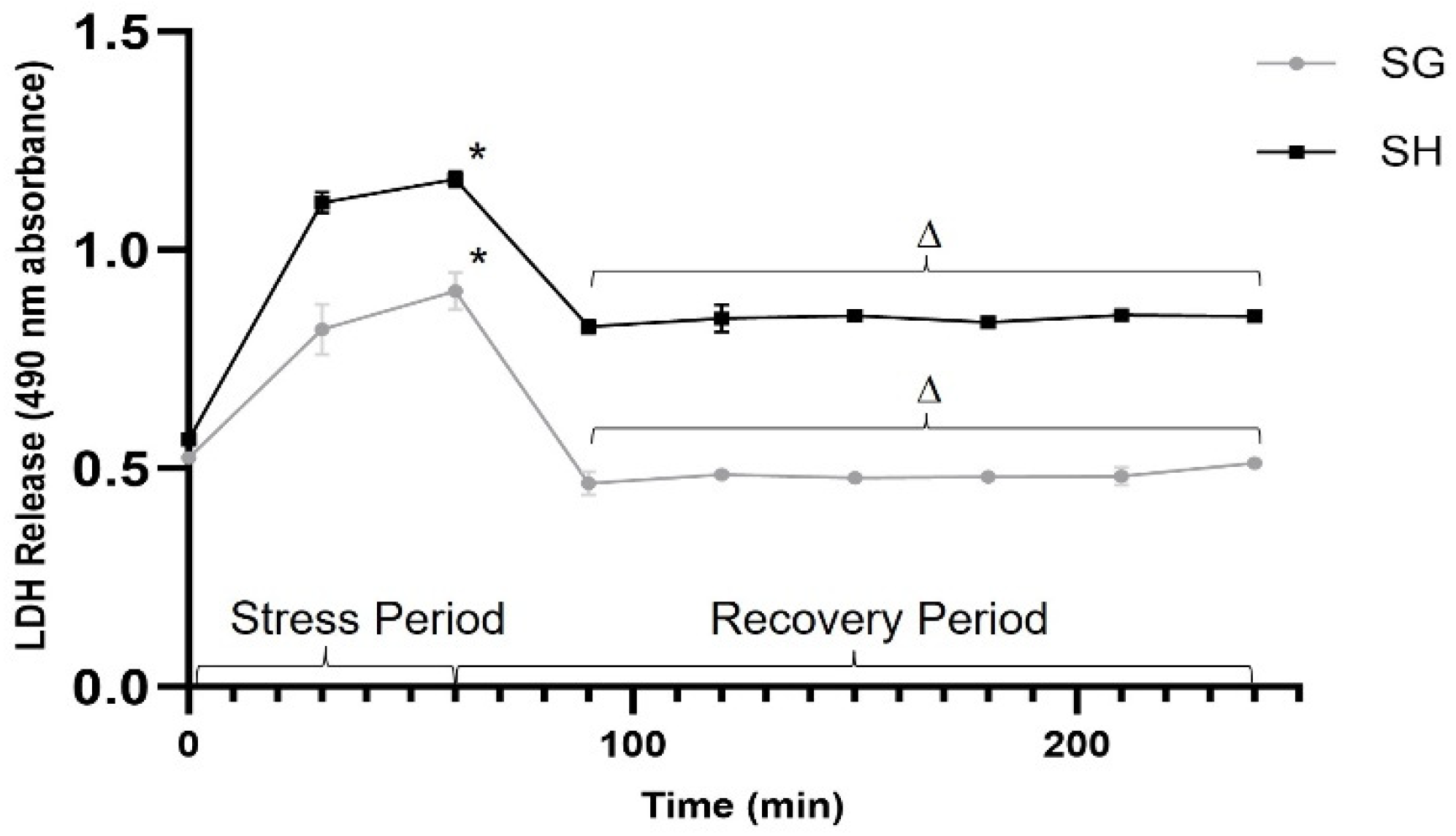
3.3. Plasma-Induced Reduction in Oxidative/Metabolic Cellular Stress after SG Is Not Transient
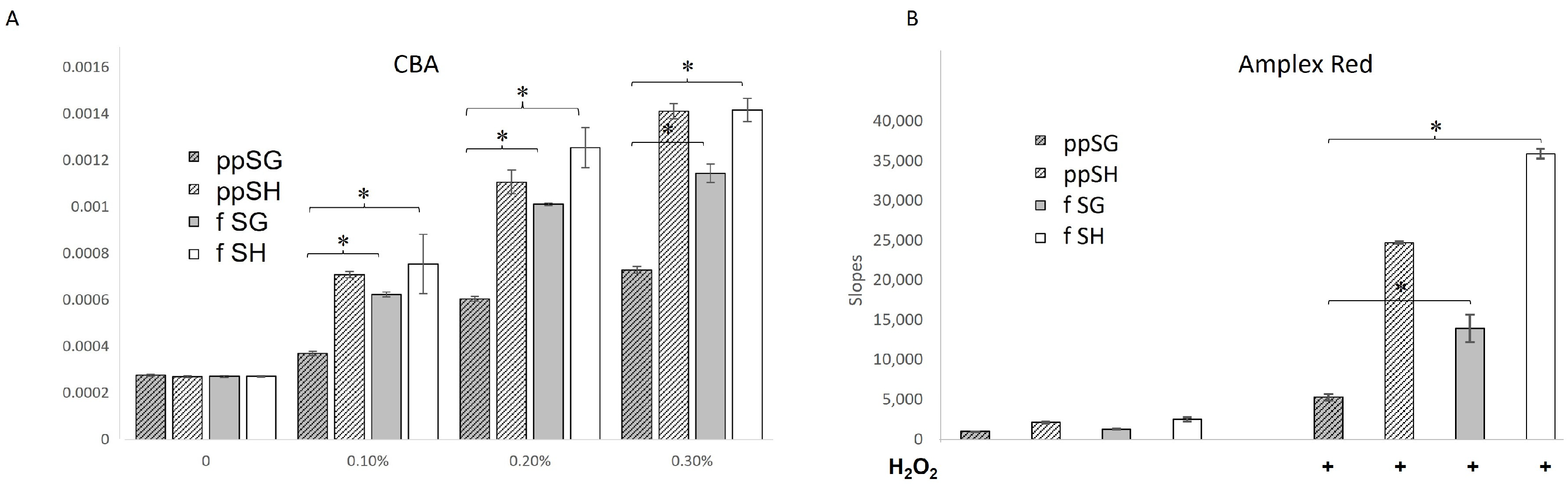
3.4. Post-Prandial SG Plasma Has a Slow Rate of Hydrogen Peroxide Consumption
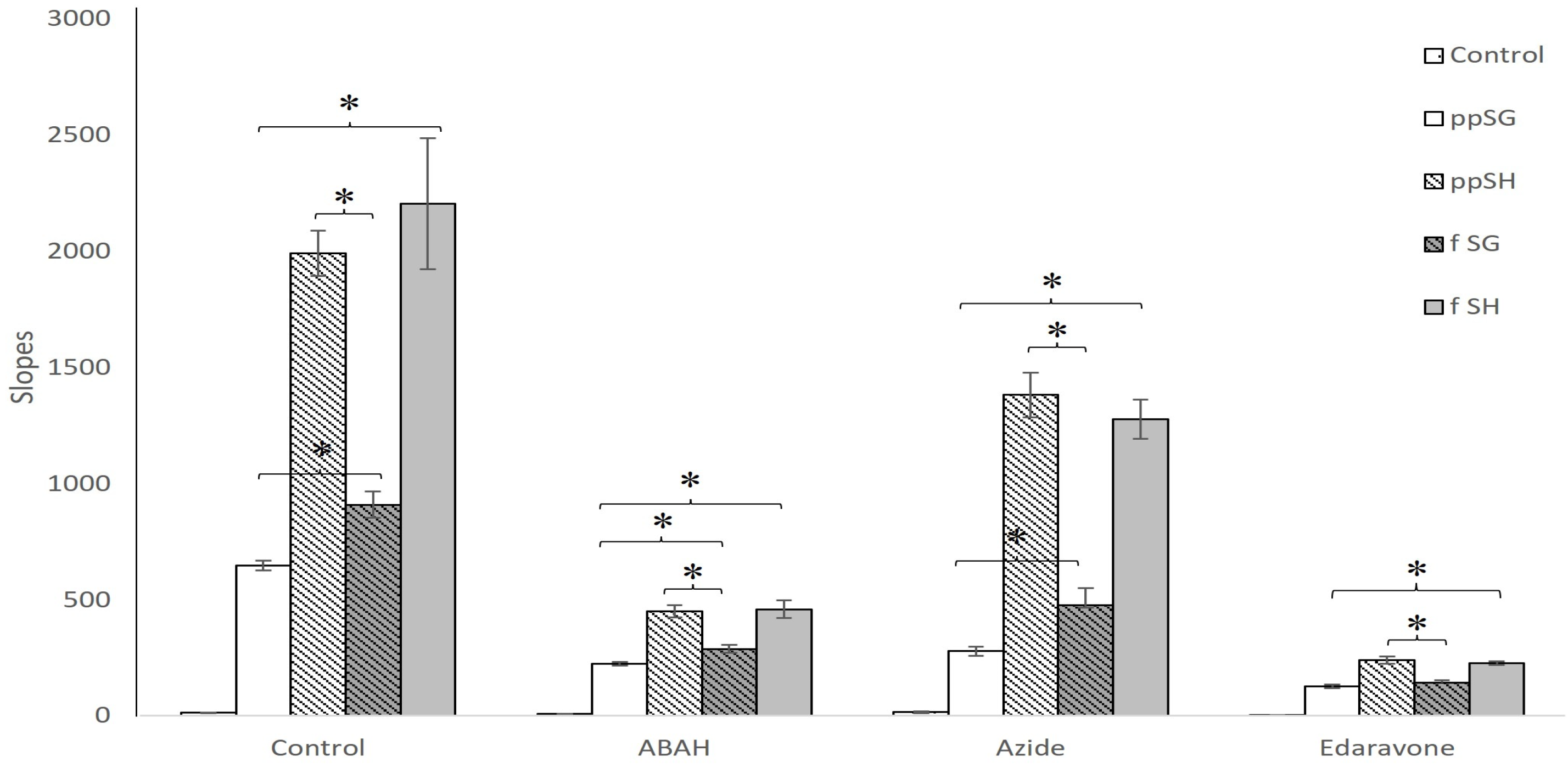
3.5. Peroxidase Activity Is Uniquely Decreased in Post-Prandial SG Plasma
3.6. Products of HE Oxidation by Plasma Suggest MPO Involvement
3.7. Myeloperoxidase Protein Levels Are Decreased in Post-Prandial SG Plasma
3.8. Plasma from MPO Knockout Rats Is Similarly Protective against In Vitro Oxidative/Metabolic Stress
3.9. Plasma from MPO KO Rats Has a Rate of Hydrogen Peroxide Oxidation Similar to ppSG Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABAH | aminobenzoic acid hydrazide |
| CBA | coumarin-7-boronic acid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| HFpEF | heart failure with preserved ejection fraction |
| HE | hydroethidine |
| H2O2 | hydrogen peroxide |
| KO | knockout |
| LDH | lactate dehydrogenase |
| MPO | myeloperoxidase |
| PF | pair-fed |
| PP | post-prandial |
| SH | sham |
| SG | sleeve gastrectomy |
References
- Stafeev, I.S.; Vorotnikov, A.V.; Ratner, E.I.; Menshikov, M.Y.; Parfyonova, Y.V. Latent Inflammation and Insulin Resistance in Adipose Tissue. Int. J. Endocrinol. 2017, 2017, 5076732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Y.; Xu, X.; Li, X.C. Cardiovascular diseases: Oxidative damage and antioxidant protection. Eur. Rev. Med. Pharm. Sci. 2014, 18, 3091–3096. [Google Scholar]
- Straface, E.; Marchesi, A.; Gambardella, L.; Metere, A.; Tarissi de Jacobis, I.; Viora, M.; Giordani, L.; Villani, A.; Del Principe, D.; Malorni, W.; et al. Does oxidative stress play a critical role in cardiovascular complications of Kawasaki disease? Antioxid. Redox Signal. 2012, 17, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Zakeri, R.; Chamberlain, A.M.; Roger, V.L.; Redfield, M.M. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: A community-based study. Circulation 2013, 128, 1085–1093. [Google Scholar] [CrossRef]
- Paulus, W.J.; Zile, M.R. From systemic inflammation to myocardial fibrosis: The heart failure with preserved ejection fraction paradigm revisited. Circ. Res. 2021, 128, 1451–1467. [Google Scholar] [CrossRef]
- Kindel, T.L.; Foster, T.; Harmann, L.; Strande, J. Sleeve Gastrectomy in Obese Zucker Rats Restores Cardiac Function and Geometry Toward a Lean Phenotype Independent of Weight Loss. J. Card. Fail. 2019, 25, 372–379. [Google Scholar] [CrossRef]
- Mikhalkova, D.; Holman, S.R.; Jiang, H.; Saghir, M.; Novak, E.; Coggan, A.R.; O’Connor, R.; Bashir, A.; Jamal, A.; Ory, D.S.; et al. Bariatric Surgery-Induced Cardiac and Lipidomic Changes in Obesity-Related Heart Failure with Preserved Ejection Fraction. Obesity 2018, 26, 284–290. [Google Scholar] [CrossRef]
- Hayes, H.; Patz, J.; Corbett, J.; Afzal, M.Z.; Strande, J.; Kindel, T.L. Sleeve gastrectomy in obese Wistar rats improves diastolic function and promotes cardiac recovery independent of weight loss. Surg. Obes. Relat. Dis. 2019, 15, 837–842. [Google Scholar] [CrossRef]
- Liou, A.P.; Paziuk, M.; Luevano, J.M., Jr.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Flynn, C.R.; Tamboli, R.A.; Abumrad, N.N. Recent advances in metabolic and bariatric surgery. F1000Research 2016, 5, F1000. [Google Scholar] [CrossRef] [PubMed]
- Nemati, R.; Lu, J.; Dokpuang, D.; Booth, M.; Plank, L.D.; Murphy, R. Increased bile acids and FGF19 after sleeve gastrectomy and Rouxen-Y gastric bypass correlate with improvement in type 2 diabetes in a randomized trial. Obes. Surg. 2018, 28, 2672–2686. [Google Scholar] [CrossRef]
- Kindel, T.L.; Foster, T.; Goldspink, P.; Kindel, S.J.; Corbett, J.; Widlanksy, M.; Strande, J. Early Weight Loss Independent Effects of Sleeve Gastrectomy on Diet-Induced Cardiac Dysfunction in Obese, Wistar Rats. Obes. Surg. 2017, 27, 2370–2377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patz, J.J.; Helm, M.C.; Higgins, R.M.; Goldblatt, M.I.; Gould, J.C.; Kindel, T.L. Peri-operative, intravenous clindamycin may improve the resolution rate of hypertension after Roux-en-Y gastric bypass in morbidly obese patients. Surg. Endosc. 2019, 33, 3984–3989. [Google Scholar] [CrossRef]
- Kindel, T.L.; Strande, J.L. Bariatric surgery as a treatment for heart failure: Review of the literature and potential mechanisms. Surg. Obes. Relat. Dis. 2018, 14, 117–122. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Reiter, M.; Gastonguay, C.; McGivern, J.V.; Guan, X.; Ge, Z.D.; Mack, D.L.; Childers, M.K.; Ebert, A.D.; Strande, J.L. Nicorandil, a Nitric Oxide Donor and ATP-Sensitive Potassium Channel Opener, Protects Against Dystrophin-Deficient Cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Sikora, A.; Joseph, J.; Kalyanaraman, B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: Direct reaction with boronate-based fluorescent probe. J. Biol. Chem. 2010, 285, 14210–14216. [Google Scholar] [CrossRef]
- Zielonka, J.; Cheng, G.; Zielonka, M.; Ganesh, T.; Sun, A.; Joseph, J.; Michalski, R.; O’Brien, W.J.; Lambeth, J.D.; Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide: Design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014, 289, 16176–16189. [Google Scholar] [CrossRef]
- Zielonka, J.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection of 2-hydroxyethidium in cellular systems: A unique marker product of superoxide and hydroethidine. Nat. Protoc. 2008, 3, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Dębski, D.; Smulik, R.; Zielonka, J.; Michałowski, B.; Jakubowska, M.; Dębowska, K.; Adamus, J.; Marcinek, A.; Kalyanaraman, B.; Sikora, A. Mechanism of oxidative conversion of Amplex® Red to resorufin: Pulse radiolysis and enzymatic studies. Free Radic. Biol. Med. 2016, 95, 323–332. [Google Scholar] [CrossRef]
- Zielonka, J.; Srinivasan, S.; Hardy, M.; Ouari, O.; Lopez, M.; Vasquez-Vivar, J.; Avadhani, N.G.; Kalyanaraman, B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: Identification of homo- and heterodimers. Free Radic. Biol. Med. 2008, 44, 835–846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zielonka, J.; Kalyanaraman, B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic. Biol. Med. 2010, 48, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Jakopitsch, C.; Furtmüller, P.G.; Dunand, C.; Obinger, C. The peroxidase-cyclooxygenase superfamily: Reconstructed evolution of critical enzymes of the innate immune system. Proteins 2008, 72, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Gomez Garcia, A.; Rivera Rodriguez, M.; Gomez Alonso, C.; Rodriguez Ochoa, D.Y.; Alvarez Aguilar, C. Myeloperoxidase is associated with insulin resistance and inflammation in overweight subjects with first-degree relatives with type 2 diabetes mellitus. Diabetes Metab. J. 2015, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): An oxidative mechanism for restraining proteolytic activity during inflammation. J. Biol. Chem. 2003, 278, 28403–28409. [Google Scholar] [CrossRef]
- Tang, W.W.; Brennan, M.L.; Philip, K.; Tong, W.; Mann, S.; Van Lente, F.; Hazen, S.L. Plasma myeloperoxidase levels in patients with chronic heart failure. Am. J. Cardiol. 2006, 98, 796–799. [Google Scholar] [CrossRef]
- Michowitz, Y.; Kisil, S.; Guzner-Gur, H.; Rubinstein, A.; Wexler, D.; Sheps, D.; Keren, G.; George, J. Usefulness of serum myeloperoxidase in prediction of mortality in patients with severe heart failure. Isr. Med. Assoc. J. 2008, 10, 884–888. [Google Scholar]
- Rudolph, V.; Andrié, R.P.; Rudolph, T.K.; Friedrichs, K.; Klinke, A.; Hirsch-Hoffmann, B.; Schwoerer, A.P.; Lau, D.; Fu, X.; Klingel, K.; et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010, 16, 470–474. [Google Scholar] [CrossRef]
- Mollenhauer, M.; Friedrichs, K.; Lange, M.; Gesenberg, J.; Remane, L.; Kerkenpaß, C.; Krause, J.; Schneider, J.; Ravekes, T.; Maass, M.; et al. Myeloperoxidase Mediates Postischemic Arrhythmogenic Ventricular Remodeling. Circ. Res. 2017, 121, 56–70. [Google Scholar] [CrossRef]
- Askari, A.T.; Brennan, M.L.; Zhou, X.; Drinko, J.; Morehead, A.; Thomas, J.D.; Topol, E.J.; Hazen, S.L.; Penn, M.S. Myeloperoxidase and plasminogen activator inhibitor one play a central role in ventricular remodeling after myocardial infarction. J. Exp. Med. 2003, 197, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Pulli, B.; Courties, G.; Tricot, B.; Sebas, M.; Iwamoto, Y.; Hilgendorf, I.; Schob, S.; Dong, A.; Zheng, W.; et al. Myeloperoxidase Inhibition Improves Ventricular Function and Remodeling After Experimental Myocardial Infarction. JACC Basic Transl. Sci. 2016, 1, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Vanhamme, L.; Zouaoui Boudjeltia, K.; Van Antwerpen, P.; Delporte, C. The other myeloperoxidase: Emerging functions. Arch. Biochem. Biophys. 2018, 649, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, J.; Rensen, S.S.; Slaats, Y.; Van Dielen, F.M.; Buurman, W.A.; Greve, J.W.M. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity 2009, 17, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Galijasevic, S. The development of myeloperoxidase inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 1–7. [Google Scholar] [CrossRef]
- Mei, Y.; Li, Y.; Cheng, Y.; Gao, L. The effect of gastric bypass surgery on cognitive function of Alzheimer’s disease and the role of GLP1-SGLT1 pathway. Exp. Neurol. 2023, 363, 114377. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barron, M.; Hayes, H.; Bice, Z.; Pritchard, K.; Kindel, T.L. Sleeve Gastrectomy Provides Cardioprotection from Oxidative Stress In Vitro Due to Reduction of Circulating Myeloperoxidase. Nutrients 2023, 15, 4776. https://doi.org/10.3390/nu15224776
Barron M, Hayes H, Bice Z, Pritchard K, Kindel TL. Sleeve Gastrectomy Provides Cardioprotection from Oxidative Stress In Vitro Due to Reduction of Circulating Myeloperoxidase. Nutrients. 2023; 15(22):4776. https://doi.org/10.3390/nu15224776
Chicago/Turabian StyleBarron, Matthew, Hailey Hayes, Zachary Bice, Kirkwood Pritchard, and Tammy Lyn Kindel. 2023. "Sleeve Gastrectomy Provides Cardioprotection from Oxidative Stress In Vitro Due to Reduction of Circulating Myeloperoxidase" Nutrients 15, no. 22: 4776. https://doi.org/10.3390/nu15224776
APA StyleBarron, M., Hayes, H., Bice, Z., Pritchard, K., & Kindel, T. L. (2023). Sleeve Gastrectomy Provides Cardioprotection from Oxidative Stress In Vitro Due to Reduction of Circulating Myeloperoxidase. Nutrients, 15(22), 4776. https://doi.org/10.3390/nu15224776





