Randomized Controlled Trials to Treat Obesity in Military Populations: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- ➢
- Human studies
- ➢
- The studies included only individuals aged ≥ 18 years
- ➢
- The studies involved military populations: Army, Navy, or Air Force personnel, Active duty-military personnel, veterans
- ➢
- The topic of the studies focused on weight management interventions (any kind of treatment, e.g., pharmacological, psychotherapeutic, lifestyle, and nutritional) to treat obesity and overweight
- ➢
- The studies assessed randomized controlled trials (RCTs) to test the treatment
- ➢
- The studies are original articles
- ➢
- The studies are published in the English, German, Italian, Spanish or French language
- ➢
- Ongoing studies were eligible in order to maximize inclusion
- ➢
- Articles from literature that met the following criteria were eligible for exclusion:
- ➢
- The topic is not related to weight management interventions or obesity treatment
- ➢
- The sample is different from the military population
- ➢
- The study design is different from a randomized controlled trial
- ➢
- Articles not published in English, German, Italian, Spanish, or French.
- ➢
- Animal or pre-clinical studies
- ➢
- Article type other than original articles (i.e., systematic reviews, narrative reviews, meta-analyses, cross-sectional studies, perspective papers, letters (without data), masters or doctoral theses, case reports).
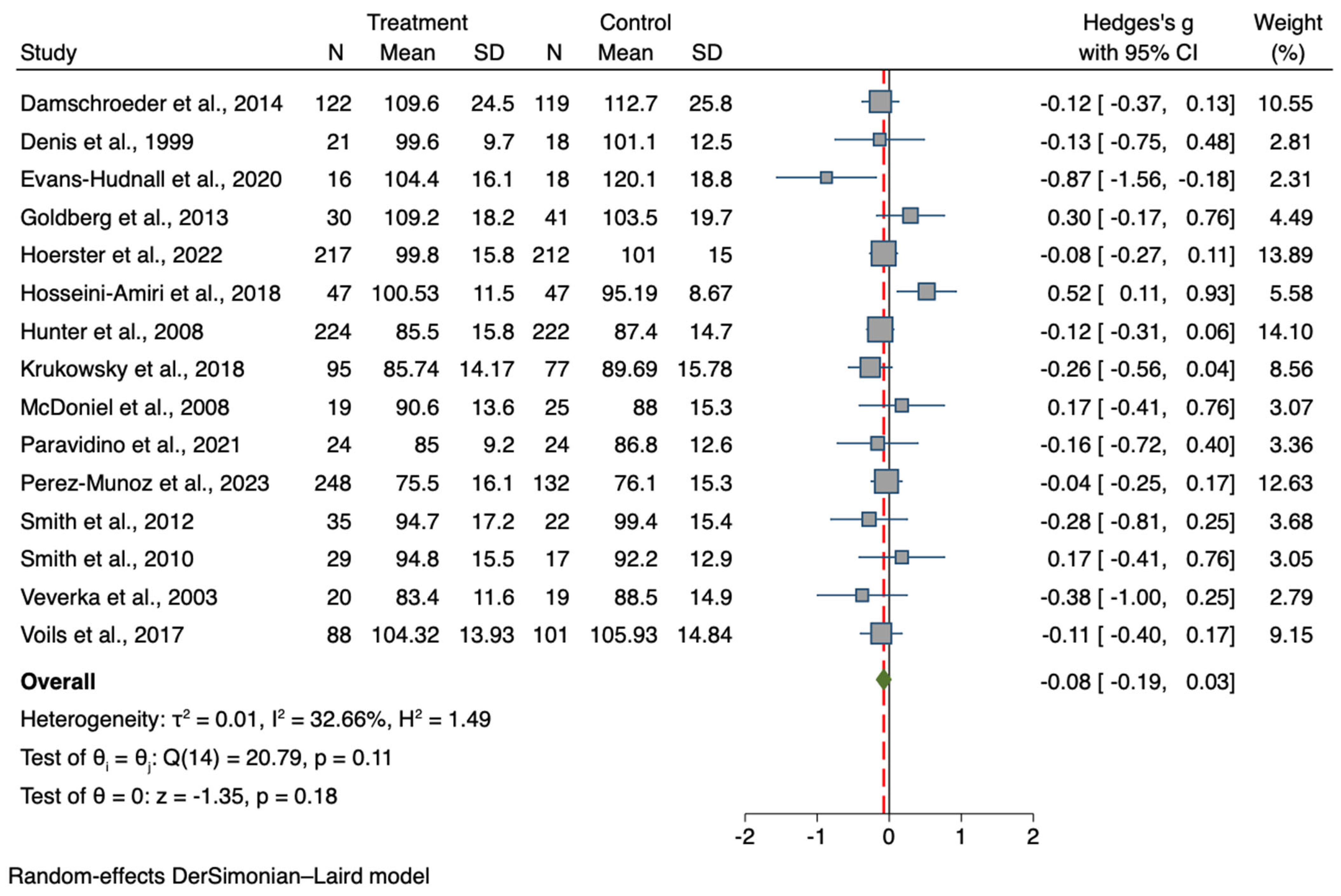
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis and Statistical Analysis
3. Results
3.1. Characteristics of Included Studies and Participants
3.2. Weight Loss Interventions of the Included Studies
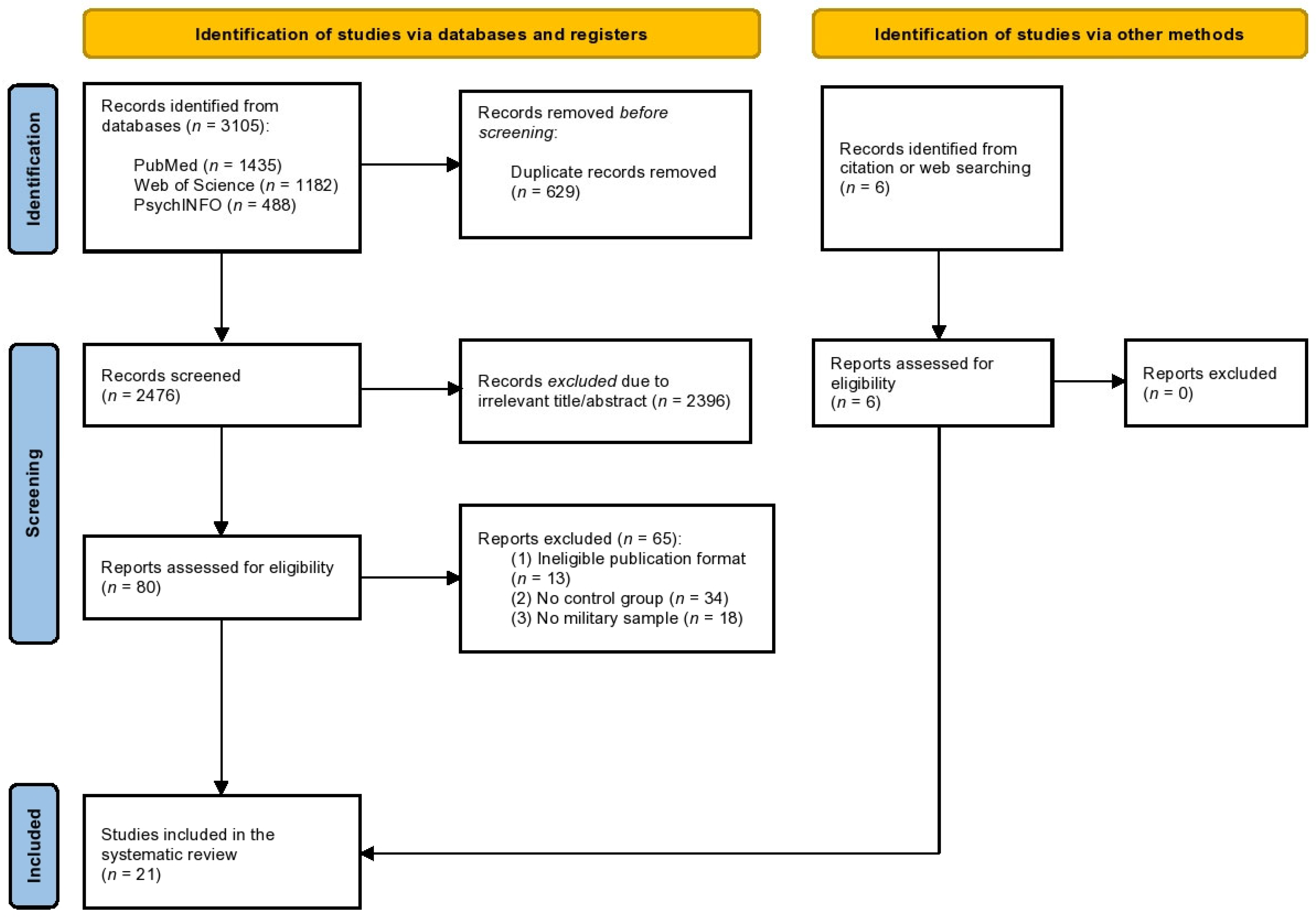
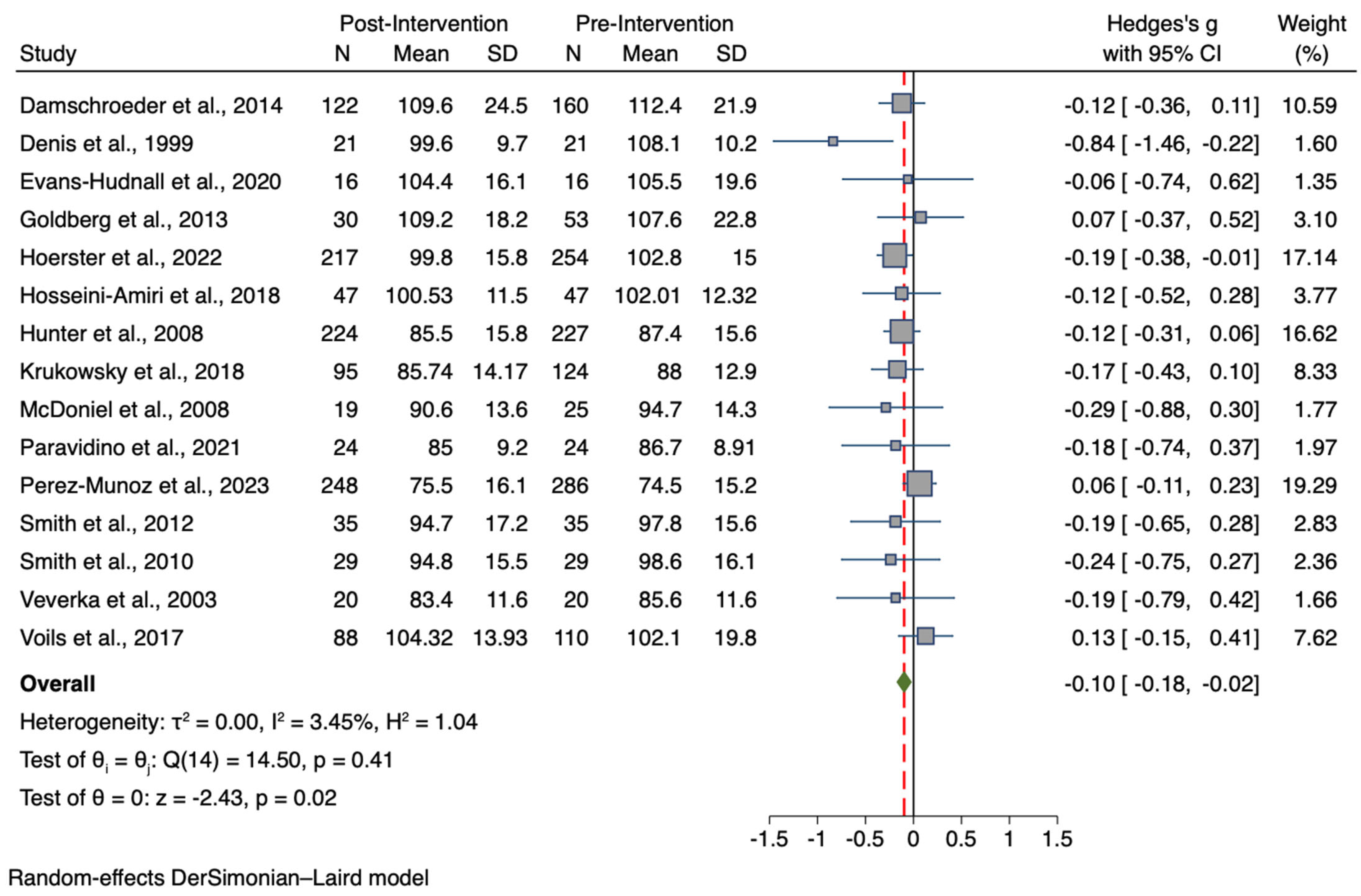
3.3. Results for the Meta-Analysis Comparing Pre-to-Post Intervention for the Treatment Group
Results for the Meta-Analyses Comparing Pre-to-Post Outcomes Per Sample Type
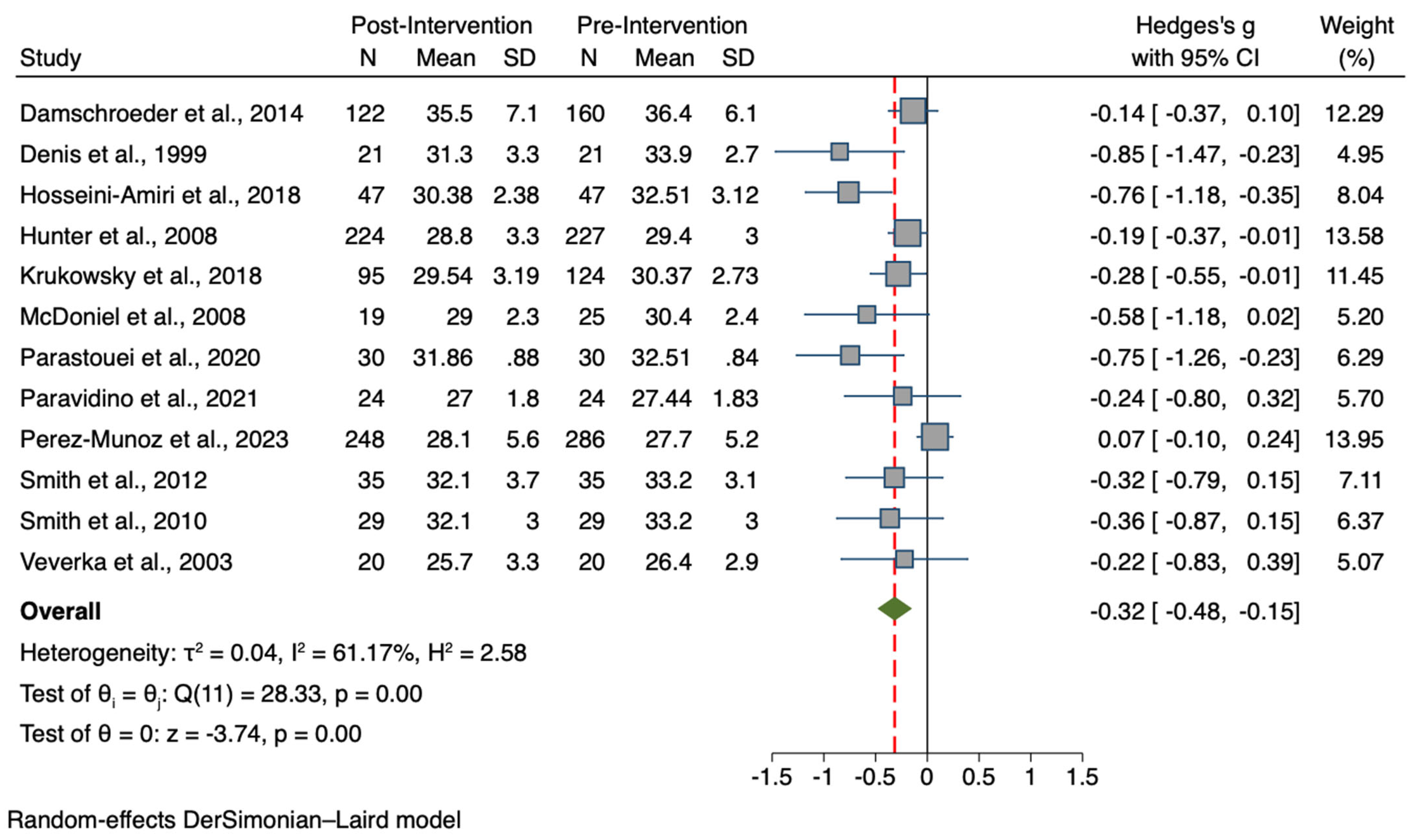
3.4. Results for the Treatment Group vs. Controls Meta-Analyses
Results for Treatment Group vs. Control Meta-Analyses Per Sample Type
3.5. Meta-Regression Analyses
3.6. Sensitivity Analyses
3.7. Results for Separate Meta-Analyses Divided by Intervention Type
3.8. Narrative Synthesis of Additional findings
4. Discussion
5. Clinical Implications and Practical Recommendations
6. Strengths and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; Available online: https://www.who.int/europe/publications/i/item/9789289057738 (accessed on 18 April 2023).
- World Obesity Federation, World Obesity Atlas. 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 18 April 2023).
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Johnson, C.L. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002, 288, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Onubi, O.J.; Marais, D.; Aucott, L.; Okonofua, F.; Poobalan, A.S. Maternal obesity in Africa: A systematic review and meta-analysis. J. Public Health 2016, 38, e218–e231. [Google Scholar] [CrossRef]
- Rivera, J.Á.; de Cossío, T.G.; Pedraza, L.S.; Aburto, T.C.; Sánchez, T.G.; Martorell, R. Childhood and adolescent overweight and obesity in Latin America: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Tanofsky-Kraff, M.; Sbrocco, T.; Theim, K.R.; Cohen, L.A.; Mackey, E.R.; Stice, E.; Henderson, J.L.; McCreight, S.J.; Bryant, E.J.; Stephens, M.B. Obesity and the US military family. Obesity 2013, 21, 2205–2220. [Google Scholar] [CrossRef]
- Gaździńska, A.; Jagielski, P.; Turczyńska, M.; Dziuda, Ł.; Gaździński, S. Assessment of Risk Factors for Development of Overweight and Obesity among Soldiers of Polish Armed Forces Participating in the National Health Programme 2016–2020. Int. J. Environ. Res. Public Health 2022, 19, 3069. [Google Scholar] [CrossRef]
- Allman-Farinelli, M.A.; Chey, T.; Merom, D.; E Bauman, A. Occupational risk of overweight and obesity: An analysis of the Australian Health Survey. J. Occup. Med. Toxicol. 2010, 5, 14. [Google Scholar] [CrossRef]
- Schulte, P.A.; Wagner, G.R.; Downes, A.; Miller, D.B. A framework for the concurrent consideration of occupational hazards and obesity. Ann. Occup. Hyg. 2008, 52, 555–566. [Google Scholar]
- Chau, J.Y.; van der Ploeg, H.P.; Merom, D.; Chey, T.; Bauman, A.E. Cross-sectional associations between occupational and leisure-time sitting, physical activity and obesity in working adults. Prev. Med. 2012, 54, 195–200. [Google Scholar] [CrossRef]
- Moore, C.J.; Cunningham, S.A. Social position, psychological stress, and obesity: A systematic review. J. Acad. Nutr. Diet. 2012, 112, 518–526. [Google Scholar] [CrossRef]
- Torres, S.J.; Nowson, C.A. Relationship between stress, eating behavior, and obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.; Lavender, J.M.; Shank, L.M.; Neyland, M.K.H.; Markos, B.; Repke, H.; Haynes, H.; Gallagher-Teske, J.; Schvey, N.A.; Sbrocco, T.; et al. Associations among alexithymia, disordered eating, and depressive symptoms in treatment-seeking adolescent military dependents at risk for adult binge-eating disorder and obesity. Eat Weight Disord. 2022, 27, 3083–3093. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Marriott, B.P.; Dotson, L.; Bathalon, G.P.; Funderburk, L.; White, A.; Hadden, L.; Young, A.J. Overweight and obesity in military personnel: Sociodemographic predictors. Obesity 2012, 20, 1534–1538. [Google Scholar] [CrossRef]
- Shiozawa, B.; Madsen, C.; Banaag, A.; Patel, A.; Koehlmoos, T. Body mass index effect on health service utilization among active duty male United States Army soldiers. Mil. Med. 2019, 184, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.M.; Camlin, C.S.; Fairbank, J.A.; Dunteman, G.H.; Wheeless, S.C. The effects of stress on job functioning of military men and women. Armed Forces Soc. 2001, 27, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Chukwura, C.L.; Santo, T.J.; Waters, C.N.; Andrews, A. ‘Nutrition is out of our control’: Soldiers’ perceptions of their local food environment. Public Health Nutr. 2019, 22, 2766–2776. [Google Scholar] [CrossRef]
- Carlton, J.R.; Manos, G.H.; Van Slyke, J.A. Anxiety and abnormal eating behaviors associated with cyclical readiness testing in a naval hospital active duty population. Mil. Med. 2005, 170, 663–667. [Google Scholar] [CrossRef]
- Sanderson, P.W.; Clemes, S.A.; Biddle, S.J. The correlates and treatment of obesity in military populations: A systematic review. Obes. Facts 2011, 4, 229–237. [Google Scholar] [CrossRef]
- McLaughlin, R.; Wittert, G. The obesity epidemic: Implications for recruitment and retention of defence force personnel. Obes. Rev. 2009, 10, 693–699. [Google Scholar] [CrossRef]
- Miggantz, E.L.; Materna, K.; Herbert, M.S.; Golshan, S.; Hernandez, J.; Peters, J.; Delaney, E.; Webb-Murphy, J.; Wisbach, G.; Afari, N. Characteristics of active duty service members referred to the navy’s weight-management program. Mil. Med. 2023, 188, e174–e181. [Google Scholar] [CrossRef]
- Legg, M.; Stahlman, S.; Chauhan, A.; Patel, D.; Hu, Z.; Wells, N. Obesity prevalence among active component service members prior to and during the COVID-19 pandemic, January 2018–July 2021. MSMR 2022, 29, 8–16. [Google Scholar] [PubMed]
- Armed Forces Health Surveillance Division. Department of Defense Health of the Force 2019. 2020. Available online: https://www.health.mil/Military-Health-Topics/Combat-Support/Armed-Forces-Health-Surveillance-Division/Re-ports-and-Publications (accessed on 20 April 2023).
- Hruby, A.; Hill, O.T.; Bulathsinhala, L.; McKinnon, C.J.; Montain, S.J.; Young, A.J.; Smith, T.J. Trends in overweight and obesity in soldiers entering the US Army, 1989–2012. Obesity 2015, 23, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, P.W.; Clemes, S.A.; Biddle, S.J. Prevalence and socio-demographic correlates of obesity in the British Army. Ann. Hum. Biol. 2014, 41, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Sundin, J.; Fear, N.T.; Wessely, S.; Rona, R.J. Obesity in the UK Armed Forces: Risk factors. Mil. Med. 2011, 176, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Quertier, D.; Goudard, Y.; Goin, G.; Régis-Marigny, L.; Sockeel, P.; Dutour, A.; Pauleau, G.; De La Villéon, B. Overweight and obesity in the French army. Mil. Med. 2022, 187, e99–e105. [Google Scholar] [CrossRef]
- Salimi, Y.; Taghdir, M.; Sepandi, M.; Zarchi, A.-A.K. The prevalence of overweight and obesity among Iranian military personnel: A systematic review and meta-analysis. BMC Public Health 2019, 19, 162. [Google Scholar] [CrossRef]
- Gaździńska; Jagielski, P.; Baran, P. Evaluation of nutritional status and the level of physical fitness of military flying personnel staying at the training camp. Pol. J. Aviat. Med. Psychol. 2018, 24, 12–18. [Google Scholar] [CrossRef]
- Al-Qahtani, D.A.; Imtiaz, M.L.; Shareef, M.M. Obesity and cardiovascular risk factors in Saudi adult soldiers. Saudi Med. J. 2005, 26, 1260–1268. [Google Scholar]
- Fogelholm, M.; Malmberg, J.; Suni, J.; Santtila, M.; Kyröläinen, H.; Mäntysaari, M. Waist circumference and BMI are independently associated with the variation of cardio-respiratory and neuromuscular fitness in young adult men. Int. J. Obes. 2006, 30, 962–969. [Google Scholar] [CrossRef]
- Yokota, M.; Bathalon, G.P.; Berglund, L.G. Assessment of male anthropometric trends and the effects on simulated heat stress responses. Eur. J. Appl. Physiol. 2008, 104, 297–302. [Google Scholar] [CrossRef]
- Loube, D.I.; Loube, A.A.; Mitler, M.M. Weight loss for obstructive sleep apnea: The optimal therapy for obese patients. J. Am. Diet. Assoc. 1994, 94, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Charteris, J. Load stress; carrier strain: Implications for military and recreational back packing. Ergonomics 2000, 1, 25–47. [Google Scholar]
- Lyons, J.; Allsopp, A.; Bilzon, J. Influences of body composition upon the relative metabolic and cardiovascular demands of load-carriage. Occup. Med. 2005, 55, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Kress, A.M.; Peterson, M.R.; Hartzell, M.C. Association between obesity and depressive symptoms among US Military active duty service personnel 2002. J. Psychosom. Res. 2006, 60, 263–271. [Google Scholar] [CrossRef]
- Vieweg, W.V.R.; Julius, D.A.; Benesek, J.; Satterwhite, L.; Fernandez, A.; Feuer, S.J.; Pandurangi, A.K. Posttraumatic stress disorder and body mass index in military veterans: Preliminary findings. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1150–1154. [Google Scholar] [CrossRef]
- Cypel, Y.S.; DePhilippis, D.; Davey, V. Substance Use in US Vietnam War Era Veterans and Nonveterans: Results from the Vietnam Era Health Retrospective Observational Study. Subst. Use Misuse 2023, 58, 858–870. [Google Scholar] [CrossRef]
- Lesnewich, L.M.; Lu, S.-E.; Weinreb, K.S.; Sparks, S.O.; Litke, D.R.; Helmer, D.A.; Pigeon, W.R.; McAndrew, L.M. Associations between risky alcohol use, disability, and problem-solving impairment among Veterans with Gulf War Illness: Secondary data analysis of a randomized clinical trial. J. Psychosom. Res. 2023, 170, 111336. [Google Scholar] [CrossRef]
- Development USDoVAOoRa. VA Research on Obesity, The U.S. Department of Veterans Affairs 2014. Available online: https://www.research.va.gov/topics/obesity (accessed on 23 April 2023).
- Litwack, S.D.; Mitchell, K.S.; Sloan, D.M.; Reardon, A.F.; Miller, M.W. Eating disorder symptoms and comorbid psychopathology among male and female veterans. Gen. Hosp. Psychiatry 2014, 36, 406–410. [Google Scholar] [CrossRef]
- Littman, A.J.; Damschroder, L.J.; Verchinina, L.; Lai, Z.; Kim, H.M.; Hoerster, K.D.; Klingaman, E.A.; Goldberg, R.W.; Owen, R.R.; Goodrich, D.E. National evaluation of obesity screening and treatment among veterans with and without mental health disorders. Gen. Hosp. Psychiatry 2015, 37, 7–13. [Google Scholar] [CrossRef]
- Breland, J.Y.; Donalson, R.; Dinh, J.V.; Maguen, S. Trauma exposure and disordered eating: A qualitative study. Women Health 2018, 58, 160–174. [Google Scholar] [CrossRef]
- Tanielian, T.; Jaycox, L.; Schell, T.; Marshall, G.; Burnam, M.; Eibner, C.; Karney, B.; Meredith, L.; Ringel, J.; Vaiana, M. Invisible Wounds of War: Summary and Recommendations for Addressing Psychological and Cognitive Injuries; RAND: Santa Monica, CA, USA, 2008. [Google Scholar]
- Herrmann, T.; Preib, E.; French, M.; Beckstrom, J.; Nazarenko, E.; Lackner, R.; Marchand, W.R.; Yabko, B. Veterans’ experiences with mindfulness-based eating: A mixed methods study on MB-SAVOR. Complementary Ther. Clin. Pract. 2022, 47, 101548. [Google Scholar] [CrossRef] [PubMed]
- Spieker, E.A.; Sbrocco, T.; Theim, K.R.; Maurer, D.; Johnson, D.; Bryant, E.; Bakalar, J.L.; Schvey, N.A.; Ress, R.; Seehusen, D.; et al. Preventing Obesity in the Military Community (POMC): The development of a clinical trials research network. Int. J. Environ. Res. Public Health 2015, 12, 1174–1195. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, R.A.; Hare, M.E.; Talcott, G.W.; Gladney, L.A.; Johnson, K.C.; Richey, P.A.; Kocak, M.; Keller, P.L.; Hryshko-Mullen, A.; Klesges, R.C. Dissemination of the Look AHEAD intensive lifestyle intervention in the United States Air Force: Study rationale, design and methods. Contemp. Clin. Trials 2015, 40, 232–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Group, L.A.R. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity 2006, 14, 737–752. [Google Scholar]
- Earles, J.E.; Kerr, B.; James, L.C.; Folen, R.A. Clinical effectiveness of the LE 3 AN program: A military healthy lifestyle program. J. Clin. Psychol. Med. Settings 2007, 14, 51–57. [Google Scholar] [CrossRef]
- Kahwati, L.C.; Lance, T.X.; Jones, K.R.; Kinsinger, L.S. RE-AIM evaluation of the Veterans Health Administration’s MOVE! weight management program. Transl. Behav. Med. 2011, 1, 551–560. [Google Scholar] [CrossRef]
- Piche, B.M.; Stankorb, S.M.; Salgueiro, M. Attitudes, beliefs, and behaviors of active duty soldiers attending the Army MOVE! weight management program. Mil. Med. 2014, 179, 906–912. [Google Scholar] [CrossRef]
- Shahnazari, M.; Ceresa, C.; Foley, S.; Fong, A.; Zidaru, E.; Moody, S. Nutrition-focused wellness coaching promotes a reduction in body weight in overweight US veterans. J. Acad. Nutr. Diet. 2013, 113, 928–935. [Google Scholar] [CrossRef]
- Arsenault, J.E.; Singleton, M.C.; Funderburk, L.K. Use of the Go-for-Green nutrition labeling system in military dining facilities is associated with lower fat intake. J. Acad. Nutr. Diet. 2014, 114, 1067–1071. [Google Scholar] [CrossRef]
- Stewart, T.; Han, H.; Allen, H.R.; Bathalon, G.; Ryan, D.H.; Newton, R.L.; Williamson, D.A. HEALTH: Efficacy of an internet/population-based behavioral weight management program for the US Army. J. Diabetes Sci. Technol. 2011, 5, 178–187. [Google Scholar] [CrossRef]
- Farage, G.; Simmons, C.; Kocak, M.; Klesges, R.C.; Talcott, G.W.; Richey, P.; Hare, M.; Johnson, K.C.; Sen, S.; Krukowski, R. Assessing the contribution of self-monitoring through a commercial weight loss app: MEdiation and predictive modeling study. JMIR mHealth uHealth 2021, 9, e18741. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.M.; Peterson, A.L.; Alvarez, L.M.; Poston, W.C.; Brundige, A.R.; Haddock, C.K.; Van Brunt, D.L.; Foreyt, J.P. Weight management using the internet: A randomized controlled trial. Am. J. Prev. Med. 2008, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bowles, S.V.; Picano, J.; Epperly, T.; Myer, S. The LIFE program: A wellness approach to weight loss. Mil. Med. 2006, 171, 1089–1094. [Google Scholar] [CrossRef]
- Davis, M.K. A comprehensive weight-loss program for soldiers. Mil. Med. 1996, 161, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Afari, N.; Cuneo, J.G.; Herbert, M.; Miller, I.; Webb-Murphy, J.; Delaney, E.; Peters, J.; Materna, K.; Miggantz, E.; Godino, J.; et al. Design for a cohort-randomized trial of an acceptance and commitment therapy-enhanced weight management and fitness program for Navy personnel. Contemp. Clin. Trials Commun. 2019, 15, 100408. [Google Scholar] [CrossRef]
- Nadolsky, K.Z. Rationale for Utilization of Obesity Pharmacotherapy in the Active Duty Population. Mil. Med. 2018, 183, 45–50. [Google Scholar] [CrossRef]
- Mailloux, O.; Tassé, N.; Tchernof, A.; Nadeau, M.; Dawe, P.; Beckett, A.; Biertho, L. Bariatric Surgery Should Be Offered to Active-Duty Military Personnel: A Retrospective Study of the Canadian Armed Forces’ Experience. Obes. Surg. 2023, 33, 1092–1098. [Google Scholar] [CrossRef]
- Walter, F.A.; Hoyt, T.; Martinez, H.; Dziura, J. Preoperative psychological assessment and weight loss outcomes in bariatric surgery patients at a military treatment facility: A retrospective profile analysis. Mil. Med. 2022, 187, e1169–e1175. [Google Scholar] [CrossRef]
- Malkawi, A.M.; Meertens, R.M.; Kremers, S.P.J.; Sleddens, E.F.C. Dietary, physical activity, and weight management interventions among active-duty military personnel: A systematic review. Mil. Med. Res. 2018, 5, 43. [Google Scholar] [CrossRef]
- Csizmar, G.T.; Irwin, M. Efficacy of weight loss interventions in United States active duty military populations: A systematic review. Mil. Med. 2021, 186, 1093–1099. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.4; (updated August 2023). Cochrane, 2023. Available online: www.training.cochrane.org/handbook. (accessed on 3 November 2023).
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Boutelle, K.N.; Afari, N.; Obayashi, S.; Eichen, D.M.; Strong, D.R.; Peterson, C.B. Design of the CHARGE study: A randomized control trial evaluating a novel treatment for Veterans with binge eating disorder and overweight and obesity. Contemp. Clin. Trials 2023, 130, 107234. [Google Scholar] [CrossRef] [PubMed]
- Damschroder, L.J.; Lutes, L.D.; Kirsh, S.; Kim, H.M.; Gillon, L.; Holleman, R.G.; Goodrich, D.E.; Lowery, J.C.; Richardson, C.R. Small-Changes Obesity Treatment among Veterans 12-Month Outcomes. Am. J. Prev. Med. 2014, 47, 541–553. [Google Scholar] [CrossRef]
- Dennis, K.E.; Pane, K.W.; Adams, B.K.; Qi, B.B. The impact of a shipboard weight control program. Obes. Res. 1999, 7, 60–67. [Google Scholar] [CrossRef]
- Erickson, Z.D.; Kwan, C.L.; Gelberg, H.A.; Arnold, I.Y.; Chamberlin, V.; Rosen, J.A.; Shah, C.; Nguyen, C.T.; Hellemann, G.; Aragaki, D.R.; et al. A randomized, controlled multisite study of behavioral interventions for veterans with mental illness and antipsychotic medication-associated obesity. J. Gen. Intern. Med. 2017, 32, 32–39. [Google Scholar] [CrossRef]
- Evans-Hudnall, G.; O Odafe, M.; Johnson, A.; Armenti, N.; O’neil, J.; Lawson, E.; Trahan, L.H.; Rassu, F.S. Using an Adjunctive Treatment to Address Psychological Distress in a National Weight Management Program: Results of an Integrated Pilot Study. Mil. Med. 2020, 185, E1662–E1670. [Google Scholar] [CrossRef]
- Goldberg, R.W.; Reeves, G.; Tapscott, S.; Medoff, D.; Dickerson, F.; Goldberg, A.P.; Ryan, A.S.; Fang, L.J.; Dixon, L.B.; Muralidharan, A.; et al. “MOVE!”: Outcomes of a weight loss program modified for veterans with serious mental illness. Psychiatr. Serv. 2013, 64, 737–744. [Google Scholar] [CrossRef]
- Hoerster, K.D.; Hunter-Merrill, R.; Nguyen, T.; Rise, P.; Barón, A.E.; McDowell, J.; Donovan, L.M.; Gleason, E.; Lane, A.; Plumley, R.; et al. Effect of a Remotely Delivered Self-directed Behavioral Intervention on Body Weight and Physical Health Status among Adults with Obesity: The D-ELITE Randomized Clinical Trial. JAMA 2022, 328, 2230–2241. [Google Scholar] [CrossRef]
- Hosseini-Amiri, M.; Aliyari, S.; Zareiyan, A.; Dabbagh-Moghadam, A. The Effects of Extended Parallel Process Model on Obese Soldiers’ Knowledge, Attitudes, and Practices about Obesity Management: A Randomized Controlled Clinical Trial. Iran. J. Nurs. Midwifery Res. 2018, 23, 458–464. [Google Scholar] [PubMed]
- Krukowski, R.A.; Hare, M.E.; Talcott, G.W.; Johnson, K.C.; Richey, P.A.; Kocak, M.; Balderas, J.; Colvin, L.; Keller, P.L.; Waters, T.M.; et al. Dissemination of the Look AHEAD Intensive Lifestyle Intervention in the United States Military: A Randomized Controlled Trial. Obesity 2018, 26, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Lutes, L.D.; Damschroder, L.J.; Masheb, R.; Kim, H.M.; Gillon, L.; Holleman, R.G.; Goodrich, D.E.; Lowery, J.C.; Janney, C.; Kirsh, S.; et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA small changes randomized trial. J. Gen. Intern. Med. 2017, 32, 40–47. [Google Scholar] [CrossRef]
- McDoniel, S.O.; Nelson, H.A.; Thomson, C.A. Employing RMR technology in a 90-day weight control program. Obes. Facts. 2008, 1, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Parastouei, K.; Saeidipoor, S.; Sepandi, M.; Abbaszadeh, S.; Taghdir, M. Effects of synbiotic supplementation on the components of metabolic syndrome in military personnel: A double-blind randomised controlled trial. BMJ Mil. Health 2022, 168, 362–367. [Google Scholar] [CrossRef]
- Paravidino, V.B.; Mediano, M.F.F.; Silva, I.C.M.; Wendt, A.; Del Vecchio, F.B.; Neves, F.A.; Terra, B.d.S.; Gomes, E.A.C.; Moura, A.S.; Sichieri, R. Effect of physical exercise on spontaneous physical activity energy expenditure and energy intake in overweight adults (the EFECT study): A study protocol for a randomized controlled trial. Trials 2018, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Muñoz, A.; Hare, M.E.; Andres, A.; Klesges, R.C.; Wayne Talcott, G.; Little, M.A.; Waters, T.M.; Harvey, J.R.; Bursac, Z.; Krukowski, R.A. A Postpartum Weight Loss-focused Stepped-care Intervention in a Military Population: A Randomized Controlled Trial. Ann. Behav. Med. 2023, 2023, kaad014. [Google Scholar] [CrossRef]
- Smith, T.J.; Sigrist, L.D.; Bathalon, G.P.; McGraw, S.; Karl, J.P.; Young, A.J. Efficacy of a meal-replacement program for promoting blood lipid changes and weight and body fat loss in US Army soldiers. J. Am. Diet. Assoc. 2010, 110, 268–273. [Google Scholar] [CrossRef]
- Smith, T.J.; Crombie, A.; Sanders, L.F.; Sigrist, L.D.; Bathalon, G.P.; McGraw, S.; Young, A.J. Efficacy of orlistat 60 mg on weight loss and body fat mass in US Army soldiers. J. Acad. Nutr. Diet. 2012, 112, 533–540. [Google Scholar] [CrossRef]
- Staudter, M.; Dramiga, S.; Webb, L.; Hernandez, D.; Cole, R. Effectiveness of pedometer use in motivating active duty and other military healthcare beneficiaries to walk more. US Army Med. Dep. J. 2011, 108–119. [Google Scholar]
- Veverka, D.V.; Anderson, J.; Auld, G.W.; Coulter, G.R.; Kennedy, C.; Chapman, P.L. Use of the stages of change model in improving nutrition and exercise habits in enlisted Air Force men. Mil. Med. 2003, 168, 373–379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Voils, C.I.; Olsen, M.K.; Gierisch, J.M.; McVay, M.A.; Grubber, J.M.; Gaillard, L.; Bolton, J.; Maciejewski, M.L.; Strawbridge, E.; Yancy, W.S., Jr. Maintenance of weight loss after initiation of nutrition training: A randomized trial. Ann. Intern. Med. 2017, 166, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Aboul-Enein, B.H.; Bernstein, J.; Kruk, J. Selected weight management interventions for military populations in the United States: A narrative report. Nutr. Health 2017, 23, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Department of Veterans Affairs: Va/DoD Clinical Practice Guideline for Screening and Management of Overweight and Obesity Submitted 04/18/14. Available online: https://www.healthquality.va.gov/guidelines (accessed on 3 November 2023).
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for medical care of patients with obesity. Endocr. Pract. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef]
- Del Re, A.; Frayne, S.M.; Harris, A.H. Antiobesity medication use across the veterans health administration: Patient-level predictors of receipt. Obesity 2014, 22, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Rasu, R.S.; Hunter, C.M.; Peterson, A.L.; Maruska, H.M.; Foreyt, J.P. Economic evaluation of an Internet-based weight management program. Am. J. Manag. Care 2010, 16, e98–e104. [Google Scholar] [CrossRef]
- Robbins, A.S.; Chao, S.Y.; Baumgartner, N.; Runyan, C.N.; Oordt, M.S.; Fonseca, V.P. A low-intensity intervention to prevent annual weight gain in active duty Air Force members. Mil. Med. 2006, 171, 556–561. [Google Scholar] [CrossRef]
- Stewart, T.; May, S.; Allen, H.R.; Bathalon, G.P.; Lavergne, G.; Sigrist, L.; Ryan, D.; Williamson, D.A. Development of an internet/population-based weight management program for the US Army. J. Diabetes Sci. Technol. 2008, 2, 116–126. [Google Scholar] [CrossRef]
- McGraw, S.M.; Bathalon, G.P.; Ellison, B.K.; Graff, J.D.; Burrell, L.M.; Carr, R.E.; Ross, C.M.; Williamson, D.A.; Young, A.J. Dieting practices of soldiers in the U.S. Army weight control program. Med. Sci. Sports Exerc. 2005, 37, S142. [Google Scholar]
- Winett, R.A.; Tate, D.F.; Anderson, E.S.; Wojcik, J.R.; Winett, S.G. Long-term weight gain prevention: A theoretically based Internet approach. Prev. Med. 2005, 41, 629–641. [Google Scholar] [CrossRef]
- McNulty, A.F. Prevalence and contributing factors of eating disorder behaviors in active duty service women in the Army, Navy, Air Force, and Marines. Mil. Med. 2001, 166, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ruan, B.; Jiang, H.; Le, S.; Liu, Y.; Ao, X.; Huang, Y.; Shi, X.; Xue, R.; Fu, X.; et al. The Weight-loss Effect of GLP-1RAs Glucagon-Like Peptide-1 Receptor Agonists in Non-diabetic Individuals with Overweight or Obesity: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2023, 118, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Mesarič, K.K.; Pajek, J.; Zakrajšek, B.L.; Bogataj, Š.; Kodrič, J. Cognitive behavioral therapy for lifestyle changes in patients with obesity and type 2 diabetes: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 12793. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Bathke, M.; Baxter, S.D.; Halliday, T.M.; Lynch, A.; Malik, N.; Raynor, H.A.; Garay, J.L.; Rozga, M. Weight Management Interventions Provided by a Dietitian for Adults with Overweight or Obesity: An Evidence Analysis Center Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2023, 123, 520–545.e10. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Theodoulou, A.; Oke, J.L.; Butler, A.R.; Bastounis, A.; Dunnigan, A.; Byadya, R.; Cobiac, L.J.; Scarborough, P.; Hobbs, F.R.; et al. Long-term effect of weight regain following behavioral weight management programs on cardiometabolic disease incidence and risk: Systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2023, 16, e009348. [Google Scholar] [CrossRef]
- Theodoulou, A.; Hartmann-Boyce, J.; Gorenberg, J.; Oke, J.L.; Butler, A.R.; Bastounis, A.; Jebb, S.A.; Aveyard, P. Weight regain and mental health outcomes following behavioural weight management programmes: A systematic review with meta-analyses. Clin. Obes. 2023, 13, e12575. [Google Scholar] [CrossRef]
- Crane, N.; Hagerman, C.; Horgan, O.; Butryn, M. Patterns and Predictors of Engagement with Digital Self-Monitoring during the Maintenance Phase of a Behavioral Weight Loss Program: Quantitative Study. JMIR mHealth uHealth 2023, 18, e45057. [Google Scholar] [CrossRef]
- Applewhite, B.; Olivola, M.; Tweed, C.; Wesemann, U.; Himmerich, H. Body dysmorphic disorder, muscle dysmorphia, weight and shape dissatisfaction and the use of appearance-enhancing drugs in the military: A systematic review. BMJ Mil. Health 2022, 8, e002135. [Google Scholar] [CrossRef]
- Clerc, P.G.; Mayer, S.B.; Graybill, S. Overweight BMI (25–29) in active duty military: Excess fat or more lean mass? A look at the evidence. Mil. Med. 2022, 187, 201–203. [Google Scholar] [CrossRef]
- Mayer, S.B.; Graybill, S.; Raffa, S.D.; Tracy, C.; Gaar, E.; Wisbach, G.; Goldstein, M.G.; Sall, J. Synopsis of the 2020 US VA/DoD clinical practice guideline for the management of adult overweight and obesity. Mil. Med. 2021, 186, 884–896. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Obesity: Identification, Assessment and Management; NICE Guideline (CG189); National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of pharmacological treatments for obesity with weight loss and adverse events: A systematic review and meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Kahan, S. Maintenance of lost weight and long-term management of obesity. Med. Clin. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
| Study, Country | Year | Population | Sample (N) | Age (Years, M ± SD) | Study Design | Intervention | BW (M ± SD) T0 T1 | BMI (M ± SD) T0 T1 | Duration (Weeks) |
|---|---|---|---|---|---|---|---|---|---|
Afari et al., [60] USA (Study ongoing) | 2019 | US Navy | 178 | 29.7 ± 6.9 | RCT | ACT + SS | 94.8 ± 18.8 NR | 33.1 ± 3.9 NR | 8 |
Boutelle et al., [70] USA (Study ongoing) | 2023 | US Veterans | 129 | 47.1 ± 11.3 | RCT | CHARGE | NR NR | 34.8 ± 4.7 NR | 20 |
Damschroder et al., [71] USA | 2014 | US Veterans | 481 | 55.0 ± 10.0 | RCT | ASPIRE | 113.2 ± 23.2 111.1 ± 25.1 | 36.6 ± 6.2 35.9 ± 7.3 | 52 |
Dennis et al., [72] USA | 1999 | US Navy | 39 | 31.2 ± 6.5 | RCT | Shipboard Weight Control Program | 107.5 ± 11.0 100.3 ± 11.0 | 33.5 ± 2.8 31.2 ± 3.2 | 26 |
Erickson et al., [73] USA | 2017 | US Veterans | 121 | 51.3 ± 9.2 | RCT | LB Intervention | 103.1 ± NR 101.8 ± NR | NR NR | 52 |
Evans-Hudnall et al., [74] USA | 2020 | US Veterans | 34 | 58.7 ± 9.1 | RCT | HERO | 112.8 ± 23.0 112.7 ± 17.5 | 36.7 ± 7.0 NR | 16 |
Goldberg et al., [75] USA | 2013 | US Veterans | 109 | 52.0 ± 9.1 | RCT | MOVE! | 106.0 ± 21.7 105.9 ± 19.1 | NR NR | 26 |
Hoerster et al., [76] USA | 2022 | US Veterans | 511 | 57.4 ± 13.9 | RCT | D-ELITE | 102.3 ± 14.5 100.4 ± 15.4 | NR NR | 52 |
Hosseini-Amiri et al., [77] Iran | 2018 | Active Soldiers | 94 | 23.3 ± 1.6 | RCT | EPPM | 100.1 ±10.7 97.9 ±10.1 | 31.9 ± 2.7 30.9 ± 2.6 | 4 |
Hunter et al., [57] USA | 2008 | US Air Force active-duty personnel | 446 | 34.0 ± 7.3 | RCT | BIT | 87.0 ± 15.2 86.4 ± 15.3 | 29.3 ± 3.0 29.1 ± 3.1 | 26 |
Krukowski et al., [78] USA | 2018 | Active duty-military personnel | 248 | 34.6 ± 7.5 | RCT | Look AHEAD ILI | 89.0 ± 14.3 87.5 ± 14.9 | 30.4 ± 2.9 29.9 ± 3.2 | 52 |
Lutes et al., [79] USA | 2017 | US Veterans | 332 | 55.9 ± 9.5 | RCT | ASPIRE-SC | 113.0 ± 22.4 111.4 ± NR | 36.2 ± 6.0 NR | 104 |
McDoniel et al., [80] USA | 2008 | US Air Force active-duty personnel | 54 | 28.0 ± 7.3 | RCT | “Sensible Weight” Program | 90.5 ± 14.4 89.1 ± 14.6 | 29.8 ± 2.4 29.1 ± 2.5 | 13 |
Parastouei et al., [81] Iran | 2020 | Military Personnel | 60 | 41.5 ± 7.2 | RCT | Synbiotic Supplementation | NR NR | 32.1 ± 0.8 31.8 ± 0.9 | 8 |
Paravidino et al., [82] Brazil | 2021 | Military trainer of Naval Academy | 72 | 21.0 ± 2.0 | RCT | EFECT study | 87.3 ± 9.6 86.4 ± 10.2 | 27.9 ± 2.1 27.6 ±2.1 | 2 |
| Perez-Munoz et al., [83] USA | 2023 | Active-duty Military Women and TRICARE beneficiaries | 430 | 30.6 ± 4.9 | RCT | PPWL Intervention | 74.2 ± 15.0 74.7 ± 15.0 | 27.6 ± 5.2 28.2 ± 5.6 | 26 |
Smith et al., [84] USA | 2010 | US Army Soldiers | 113 | 28.4 ± 7.4 | RCT | Meal-Replacement Program | 97.2 ± 15.1 93.8 ± 15.5 | 32.8 ±3.0 32.0 ± 3.0 | 26 |
Smith et al., [85] USA | 2012 | Active-duty Soldiers | 435 | NR | RCT | Orlistat | 99.6 ± 15.8 96.5 ± 16.5 | 33.3 ± 3.4 32.3 ± 3.7 | 26 |
Staudter et al. [86] USA | 2011 | US Active-duty military | 106 | 50.0 ± 9.3 | RCT | Pedometer Intervention | 87.6 ± 16.3 NR | 32.5 ± 5.4 NR | 12 |
Veverka et al., [87] USA | 2003 | US Air Force active-duty personnel | 39 | NR | RCT | Stages of Change Model | 85.5 ± 12.9 85.9 ± 13.2 | 26.9 ± 3.3 26.7 ± 3.6 | 26 |
Voils et al., [88] USA | 2017 | US Veterans | 222 | 61.8 ± 8.3 | RCT | Maintenance Intervention | 103.6 ± 20.4 105.2 ± 14.4 | 34.0 ± 6.1 NR | 56 |
| Group | N | SMD | 95% CI | Z | p | Heterogeneity |
|---|---|---|---|---|---|---|
| Pre-to-post intervention | (Pre, Post) | |||||
| Overall | ||||||
| BW (n = 15) | 1431, 1235 | −0.10 | −0.18, −0.02 | −2.43 | 0.015 * | I2 = 3.45%; p = 0.413 |
| BMI (n = 12) | 1028, 914 | −0.32 | −0.48, −0.15 | −3.74 | <0.001 * | I2 = 61.2%; p < 0.001 ** |
| Active-duty personnel | ||||||
| BW (n = 10) | 838, 726 | −0.12 | −0.23, −0.00 | −2.04 | 0.041 * | I2 = 12.6%; p = 0.327 |
| BMI (n = 11) | 868, 792 | −0.35 | −0.54, −0.16 | −3.62 | <0.001 * | I2 = 64.3%; p <0.002 ** |
| Veterans | ||||||
| BW (n = 5) | 593, 476 | −0.09 | −0.21, −0.04 | −1.36 | 0.174 | I2 = 4.71%; p = 0.380 |
| Treatment vs. controls | (Treatment, Control) | |||||
| Overall | ||||||
| BW (n = 15) | 1235, 1094 | −0.08 | −0.19, 0.03 | −1.35 | 0.178 | I2 = 32.7%; p = 0.107 |
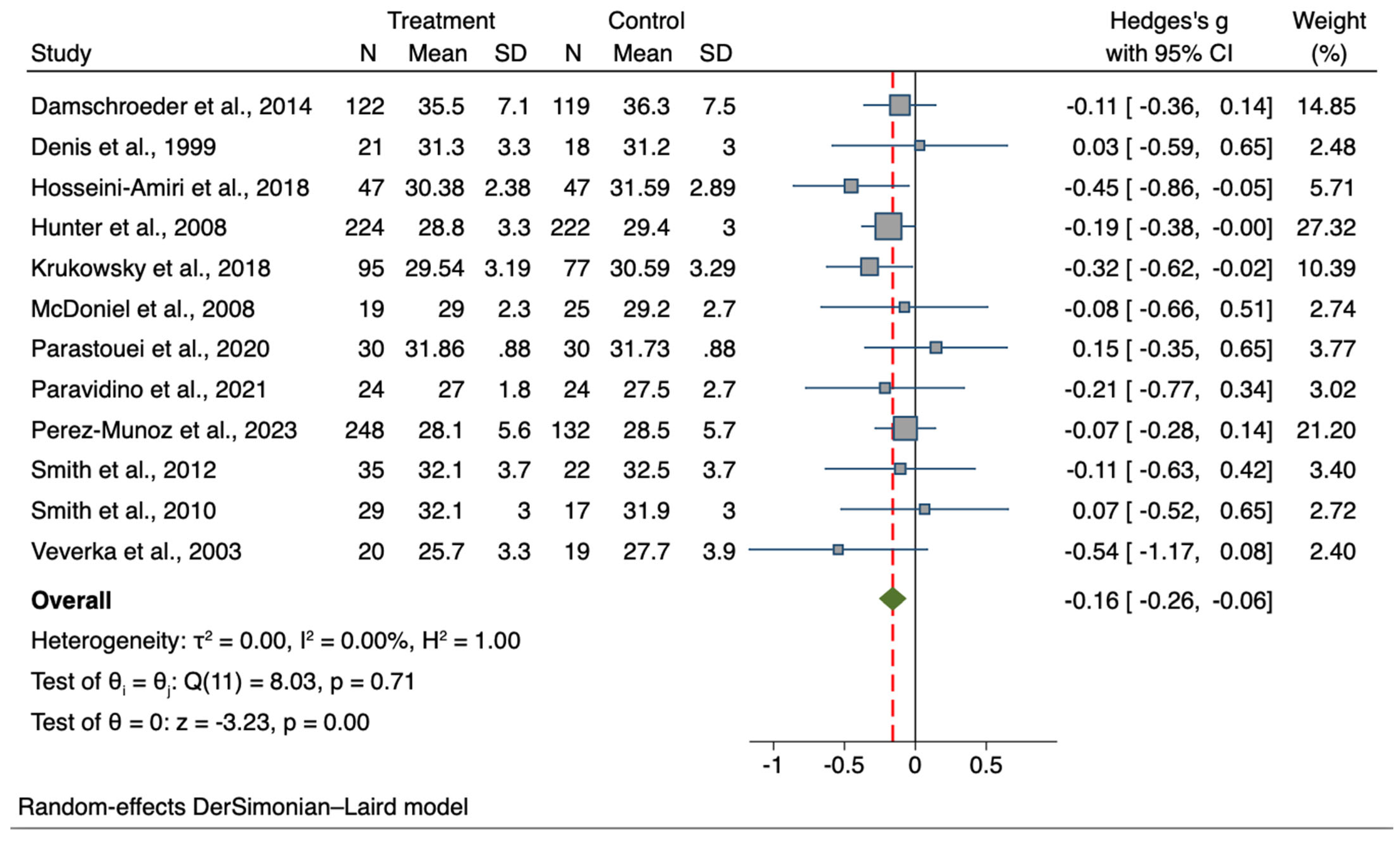
| BMI (n = 12) | 914, 752 | −0.16 | −0.26, −0.06 | −3.23 | 0.001 * | I2 = 0.00%; p = 0.711 |
| Active-duty personnel | ||||||
| BW (n = 10) | 762, 603 | −0.06 | −0.21, 0.09 | −0.77 | 0.439 | I2 = 31.1%; p = 0.159 |
| BMI (n = 11) | 792; 633 | −0.17 | −0.27, −0.06 | −3.14 | 0.001 * | I2 = 0.00%; p = 0.643 |
| Veterans | ||||||
| BW (n = 5) | 473, 491 | −0.10 | −0.30, −0.09 | −1.05 | 0.294 | I2 = 47.6%; p = 0.106 |
| Group | Variable | N Studies Included | β (SD) | 95% CIs | p |
|---|---|---|---|---|---|
| Intervention Group | |||||
| BW | |||||
| Age | 13 | −0.002 (0.004) | −0.009, 0.005 | 0.609 | |
| BW at baseline | 15 | 0.005 (0.003) | −0.001, 0.011 | 0.135 | |
| Duration of the intervention | 15 | −0.000 (0.003) | −0.005, 0.005 | 0.944 | |
| BMI | |||||
| Age | 10 | 0.002 (0.021) | −0.028, 0.015 | 0.545 | |
| BMI at baseline | 12 | 0.034 (0.028) | −0.021, 0.089 | 0.224 | |
| Duration of the intervention | 12 | −0.008 (0.005) | −0.018, 0.003 | 0.142 |
| Type of Intervention | BMI Longitudinal Meta-Analyses | BMI Cross-Sectional Meta-Analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Hedge’s g | 95% CI | p | N | Hedge’s g | 95% CI | p | |
| Behavioral and Lifestyle | 10 | −0.28 | −0.45, −0.11 | 0.001 ** | 10 | −0.18 | −0.28, −0.08 | < 0.001 ** |
| Diet and Nutritional | 9 | −0.30 | −0.50, −0.11 | 0.002 ** | 9 | −0.15 | −0.26, −0.03 | 0.010 * |
| Self-Monitoring | 7 | −0.26 | −0.48, −0.04 | 0.021 * | 7 | −0.17 | −0.28, −0.06 | 0.003 ** |
| Counseling Provided | 6 | −0.30 | −0.47, −0.14 | 0.001 ** | 6 | −0.13 | −0.29, 0.03 | 0.113 |
| Internet-Based | 3 | −0.22 | −0.37, −0.07 | 0.004 ** | 3 | −0.25 | −0.40, −0.09 | 0.002 ** |
| Topic | Clinical Recommendations | Practical Implications | Level of Evidence | RCTs (n) |
|---|---|---|---|---|
| Short-term weight loss intervention for obesity (up to 6–12 months). | Individual or group-based comprehensive lifestyle intervention | Physical activity (aerobics, resistance, or high intensity); no sufficient evidence from RCTs regarding the superior effectiveness of one type, frequency, or intensity of physical activity. | High | 18 |
| Dietary and nutritional interventions such as meal replacements promoting low caloric balance intake and healthy meal plans provided by a registered dietitian (when available) and individualized to each patient. | High | 12 | ||
| Cognitive behavioral therapy, psychoeducational strategies, and motivational techniques for cognitive, emotional, and social factors that influence weight management. | High | 12 | ||
| Structured outcome monitoring over time (clinical or self-monitoring): body weight, BMI, fat percentage, waist-to-hip ratio, abdominal circumference. | High | 12 | ||
| Internet-based intervention when in-person programs are not available. | Good | 5 | ||
| Behavioral therapy plus the use of technology (e.g., pedometer). | Weak | 2 | ||
| Pharmacological intervention (e.g., Orlistat). | Weak | 1 | ||
| Long-term weight loss intervention for obesity | Military personnel who have lost weight should be enrolled in a comprehensive weight loss maintenance program. | Lack of evidence for weight maintenance programs in military populations. | Weak | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravina, D.; Keeler, J.L.; Akkese, M.N.; Bektas, S.; Fina, P.; Tweed, C.; Willmund, G.-D.; Treasure, J.; Himmerich, H. Randomized Controlled Trials to Treat Obesity in Military Populations: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4778. https://doi.org/10.3390/nu15224778
Gravina D, Keeler JL, Akkese MN, Bektas S, Fina P, Tweed C, Willmund G-D, Treasure J, Himmerich H. Randomized Controlled Trials to Treat Obesity in Military Populations: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(22):4778. https://doi.org/10.3390/nu15224778
Chicago/Turabian StyleGravina, Davide, Johanna Louise Keeler, Melahat Nur Akkese, Sevgi Bektas, Paula Fina, Charles Tweed, Gerd-Dieter Willmund, Janet Treasure, and Hubertus Himmerich. 2023. "Randomized Controlled Trials to Treat Obesity in Military Populations: A Systematic Review and Meta-Analysis" Nutrients 15, no. 22: 4778. https://doi.org/10.3390/nu15224778
APA StyleGravina, D., Keeler, J. L., Akkese, M. N., Bektas, S., Fina, P., Tweed, C., Willmund, G.-D., Treasure, J., & Himmerich, H. (2023). Randomized Controlled Trials to Treat Obesity in Military Populations: A Systematic Review and Meta-Analysis. Nutrients, 15(22), 4778. https://doi.org/10.3390/nu15224778








