The Synergistic Effect of Quince Fruit and Probiotics (Lactobacillus and Bifidobacterium) on Reducing Oxidative Stress and Inflammation at the Intestinal Level and Improving Athletic Performance during Endurance Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Sample Processing
2.3. Biological Material
2.4. Determination of Dietary Fiber and Extraction of Bound Polyphenols from Quince (Cydonia oblonga Mill.)
2.5. Analysis of Bound Polyphenols via UPLC-PAD/ESI-QqQ MS/MS
2.6. Isolation of Probiotics
2.7. Swimming Exercise Model
2.8. Preparation of Organs for Histology and Immunohistochemistry
2.9. Obtaining the Protein Fraction from Small Intestine and Colon
2.10. Immunodetection of Inflammatory Markers
2.11. Antioxidant Enzymes in the Small Intestine and Colon
2.11.1. Catalase Determination
2.11.2. Glutathione Peroxidase Assay
2.12. Determination of Intestinal Inflammation via Histological Analysis
2.13. Mitochondrial Biogenesis via Immunohistochemical Analysis
2.14. Statistical Analysis
3. Results and Discussion
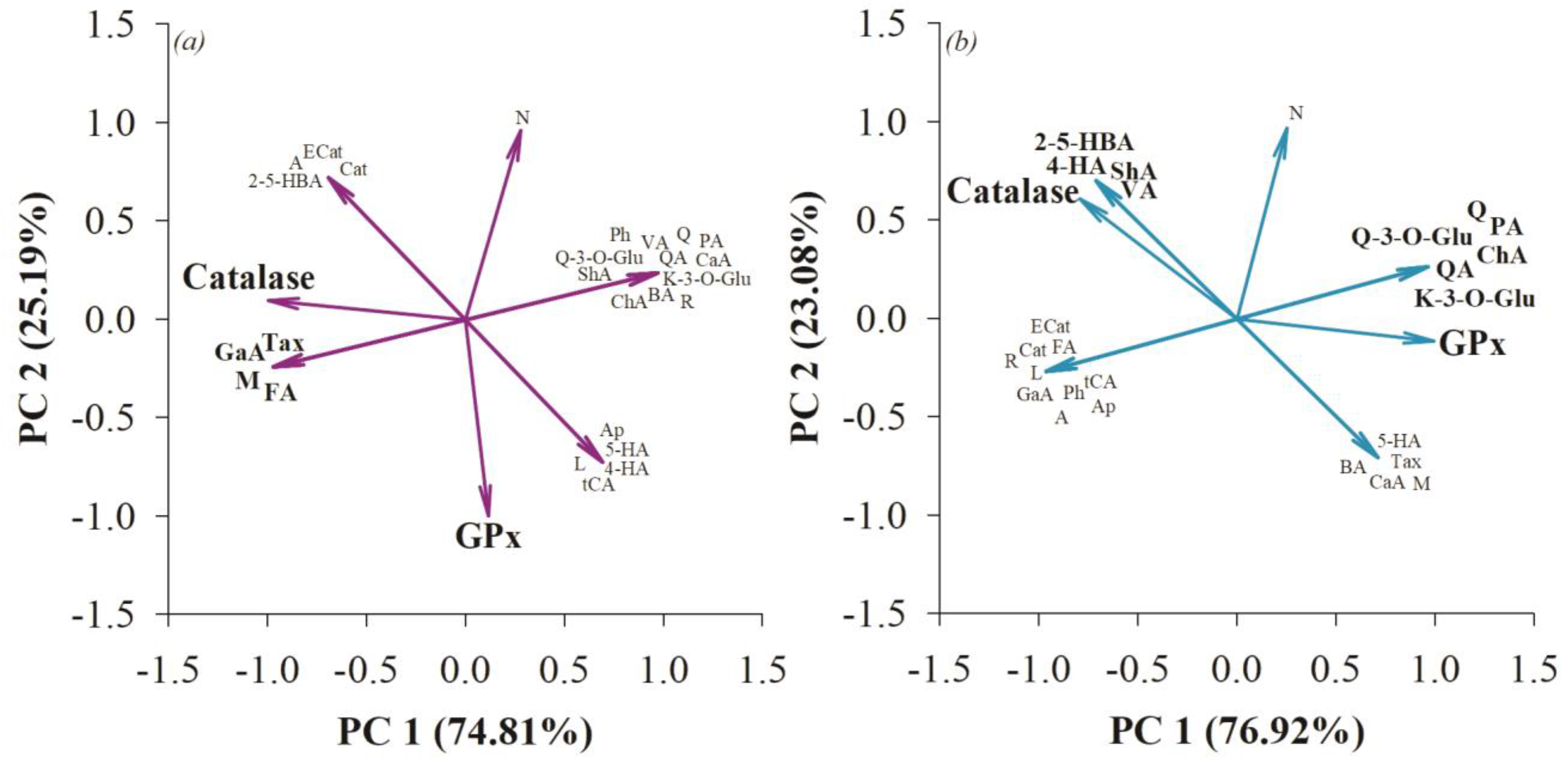
3.1. Dietary Fiber and Bound Polyphenols of Quince (Cydonia oblonga Mill.) as a Prebiotic Source of Synbiotics
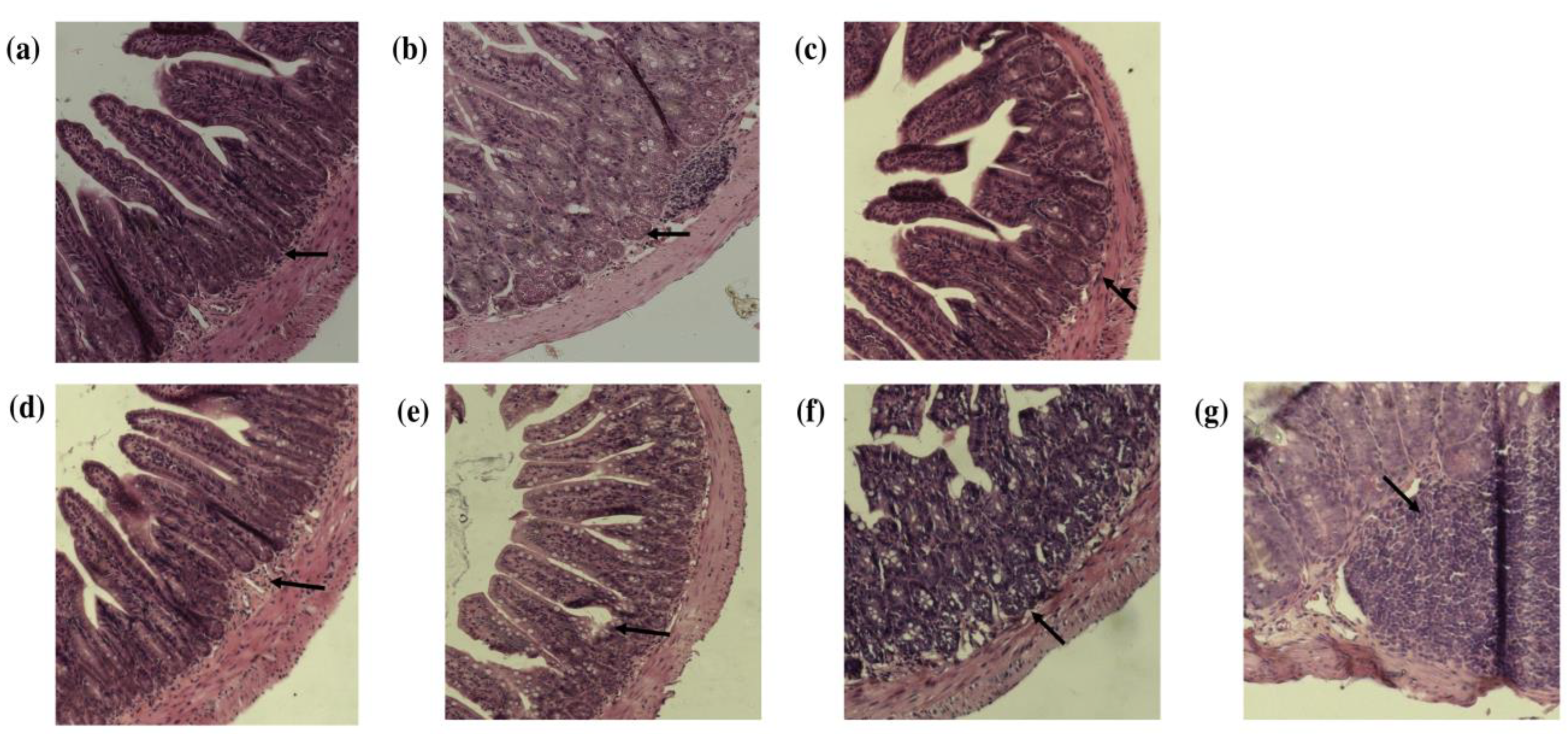
3.2. Histopathologic Evaluation of the Small Intestine and Colon after Consumption of Quince and Probiotic Strains in the Swimming Model
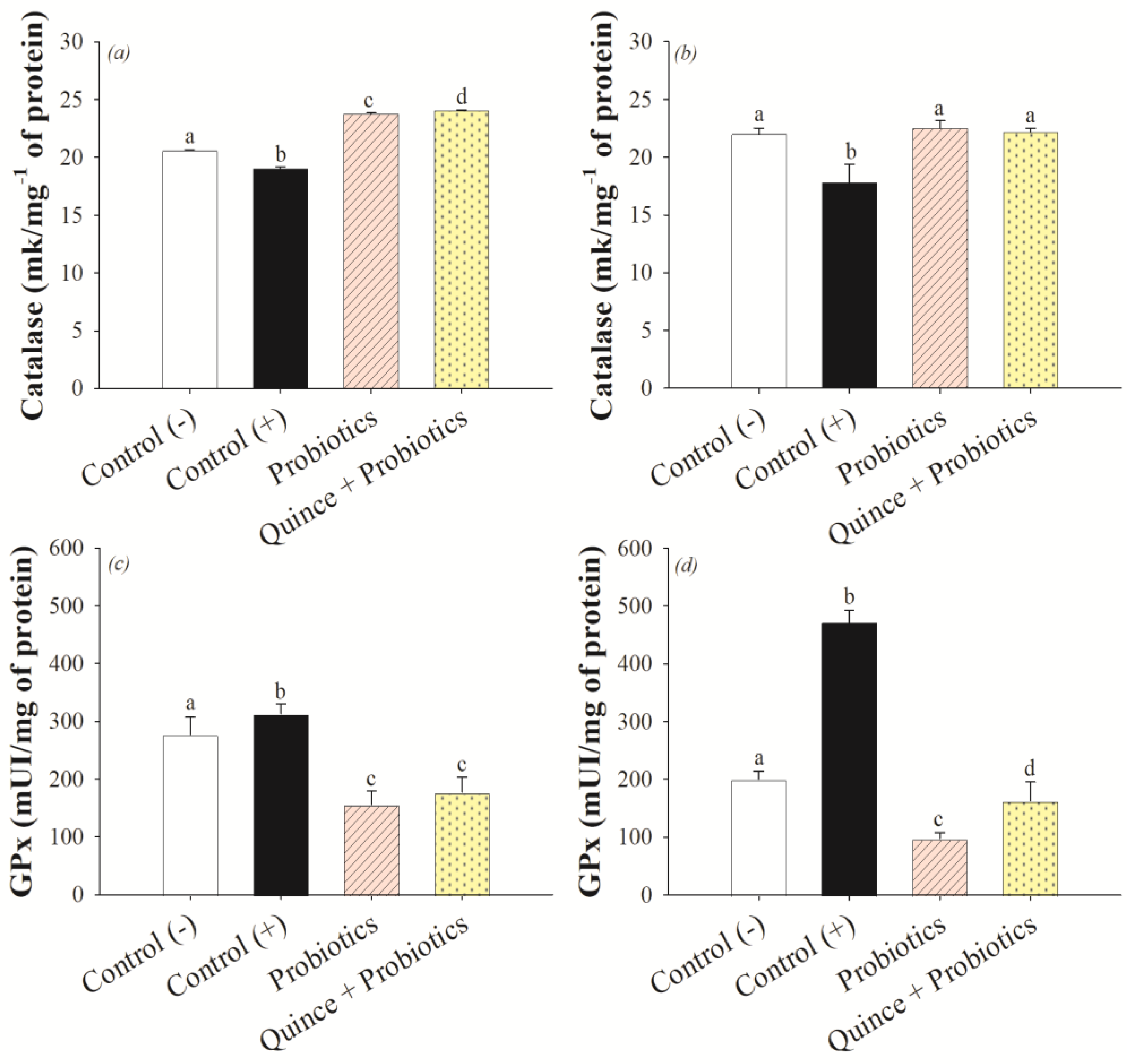
3.3. Antioxidant Effect on the Small Intestine and Colon after Consumption of Quince and Probiotic Strains during Swimming Exercise
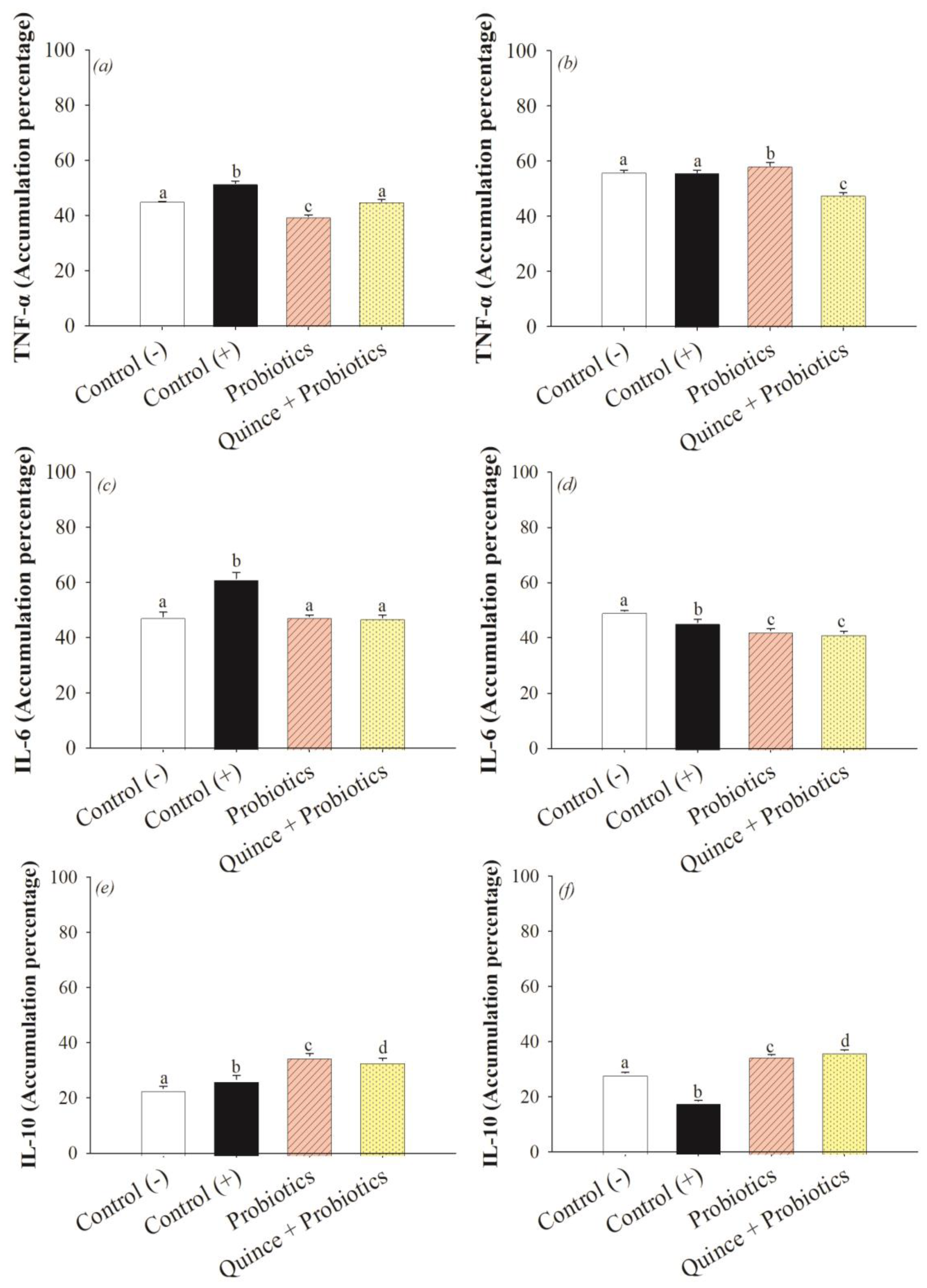
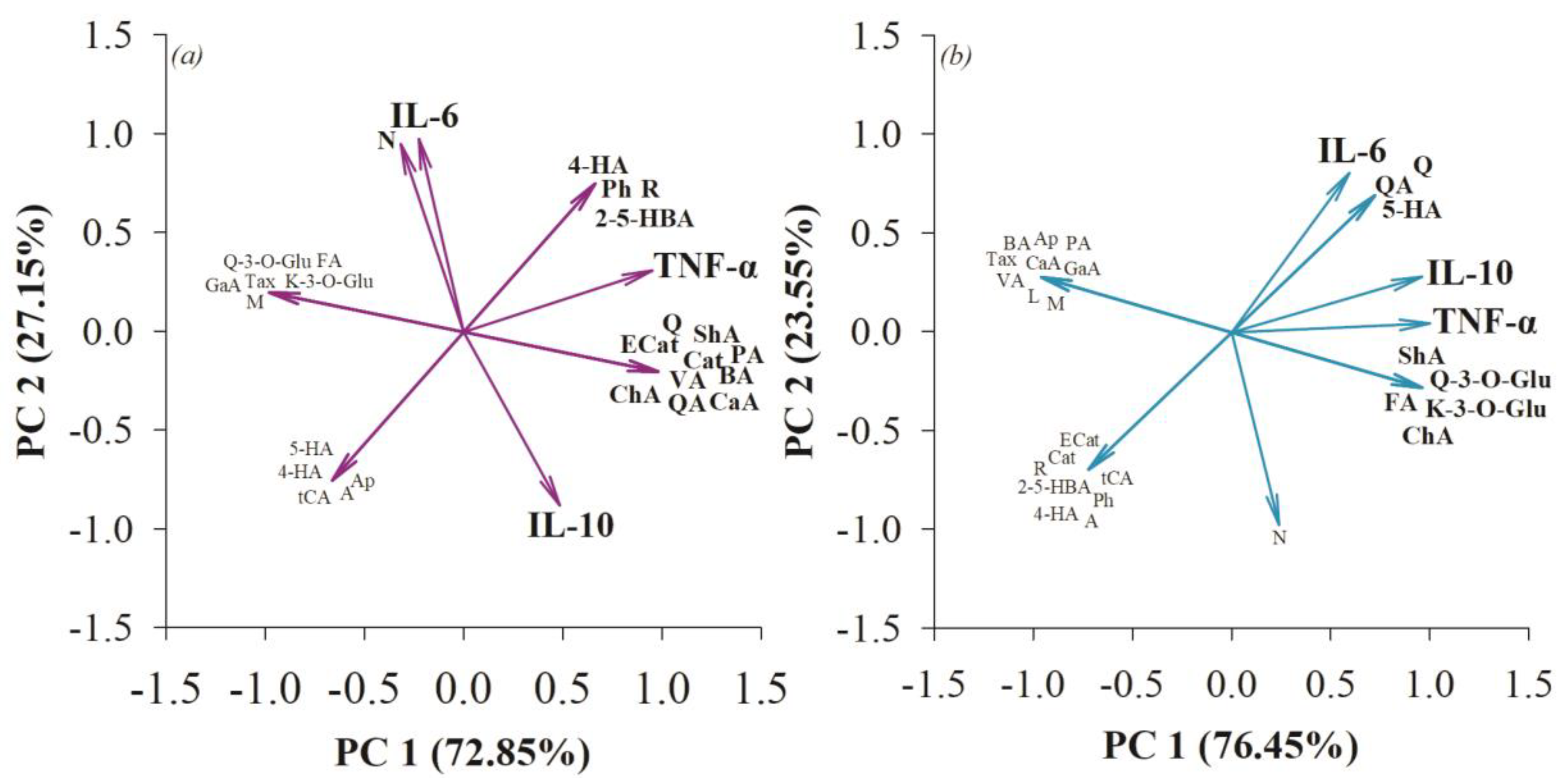
3.4. The Anti-Inflammatory Effect in the Small Intestine and Colon after Consumption of Quince Synbiotics and Probiotic Strains during Swimming Exercise
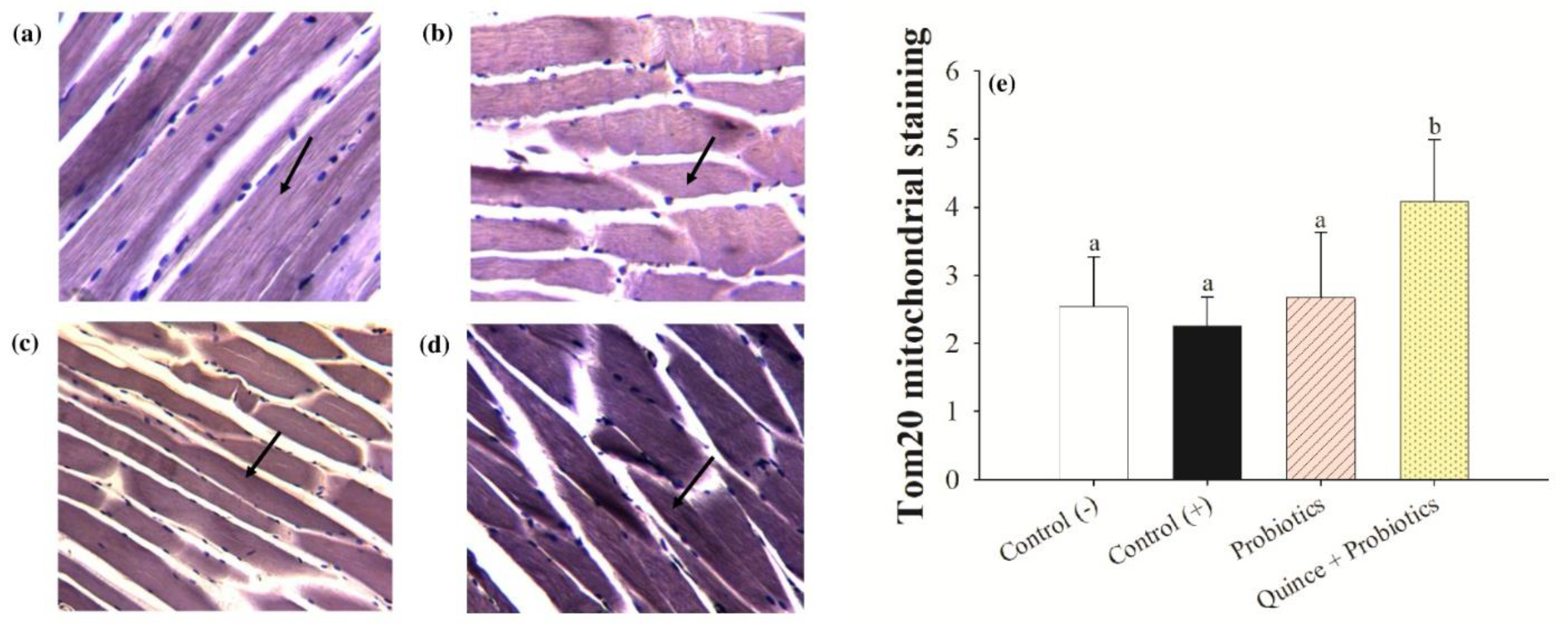
3.5. Increased Exercise Endurance and Mitochondrial Biogenesis in Muscles with the Consumption of Quince Synbiotics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Krzysztofik, M.; Wilk, M.; Wojdała, G.; Gołaś, A. Maximizing muscle hypertrophy: A systematic review of advanced resistance training techniques and methods. Int. J. Environ. Res. Public Health 2019, 16, 4897. [Google Scholar] [CrossRef]
- Đurić, S.; Knezevic, O.M.; Sember, V.; Cuk, I.; Nedeljkovic, A.; Pajek, M.; Mirkov, D.M. Effects of resistance training with constant, inertial, and combined loads on muscle power and strength output. Front. Physiol. 2021, 12, 1875. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of different exercise modalities on oxidative stress: A systematic review. BioMed Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.P.; Burini, R.C. Carbohydrate-dependent, exercise-induced gastrointestinal distress. Nutrients 2014, 6, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Petriz, B.; Marques, G.; Kamilla, L.H.; Franco, O.L. Is there an exercise-intensity threshold capable of avoiding the leaky gut? Front. Nutr. 2021, 8, 627289. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.M.; Chen, S.C.; Kelly, M.R.; Parnell, B.; Torres-McGehee, T.M. Non-steroidal anti-inflammatory drugs on core body temperature during exercise: A systematic review. J. Exerc. Sci. Fit. 2021, 19, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Rauch, C.E.; Mika, A.S.; McCubbin, A.J.; Huschtscha, Z.; Costa, R.J. Effect of prebiotics, probiotics, and synbiotics on gastrointestinal outcomes in healthy adults and active adults at rest and in response to exercise—A systematic literature review. Front. Nutr. 2022, 9, 1003620. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and utility of the antioxidant potential of probiotic Lactobacilli and Bifidobacteria as representatives of the human gut microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Huang, S.Y.; Huang, K.C.; Hsu, C.C.; Yang, K.C.; Li, L.A.; Huang, H.Y. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging 2019, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Fu, Z. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 2019, 63, 1900603. [Google Scholar] [CrossRef]
- Toda, K.; Yamauchi, Y.; Tanaka, A.; Kuhara, T.; Odamaki, T.; Yoshimoto, S.; Xiao, J.Z. Heat-killed bifidobacterium breve B-3 Enhances Muscle Functions: Possible involvement of increases in muscle mass and mitochondrial biogenesis. Nutrients 2020, 12, 219. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T.Y. Bifidobacterium bifidum enhances the intestinal epithelial tight junction barrier and protects against intestinal inflammation by targeting the toll-like receptor-2 pathway in an Nf-κβ-independent manner. Int. J. Mol. Sci. 2021, 22, 8070. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ping, L.; Cao, T.; Sun, L.; Liu, D.; Wang, S.; Li, B. Immunomodulatory effects of the Bifidobacterium longum BL-10 on lipopolysaccharide-induced intestinal mucosal immune injury. Probiotics-modulated intestinal immunity against infectious diseases in animals. Front. Immunol. 2023, 16648714, 274. [Google Scholar] [CrossRef]
- Sadeghpour-Heravi, F.; Hu, H. Bifidobacterium: Host–Microbiome Interaction and Mechanism of Action in Preventing Common Gut-Microbiota-Associated Complications in Preterm Infants: A Narrative Review. Nutrients 2023, 15, 709. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Mateus, N.; de Freitas, V. Polyphenol-Dietary Fiber Conjugates from Fruits and Vegetables: Nature and Biological Fate in a Food and Nutrition Perspective. Foods 2023, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Abliz, A.; Aji, Q.; Abdusalam, E.; Sun, X.; Abdurahman, A.; Zhou, W.; Umar, A. Effect of Cydonia oblonga Mill. leaf extract on serum lipids and liver function in a rat model of hyperlipidaemia. J. Ethnopharmacol. 2014, 151, 970–974. [Google Scholar] [CrossRef]
- Minaiyan, M.; Ghannadi, A.; Etemad, M.; Mahzouni, P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced ulcerative colitis in rats. Res. Pharm. Sci. 2012, 7, 103–110. [Google Scholar]
- Herrera-Rocha, K.M.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Larrosa-Pérez, M.; Moreno-Jiménez, M.R. Phenolic Acids and Flavonoids in Acetonic Extract from Quince (Cydonia oblonga Mill.): Nutraceuticals with Antioxidant and Anti-Inflammatory Potential. Molecules 2022, 27, 2462. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Santos, A.R.D.O.D.; Carvalho, A.C.A.D.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.D.A.; Barbalho, S.M. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, M.U.D.; Behl, T.; Nijhawan, P.; Sachdeva, M.; Khan, N. Investigation of anti-depressant effect of aqueous and ethanolic extract of Cydonia oblonga in rats. Obes. Med. 2020, 18, 100202. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Valls-Fonayet, J.; Krisa, S.; Garcia, F.; Saucier, C.; Richard, T.; Hornedo-Ortega, R. Polyphenolic characterization of Merlot, Tannat and Syrah skin extracts at different degrees of maturity and anti-inflammatory potential in RAW 264.7 cells. Foods 2021, 10, 541. [Google Scholar] [CrossRef]
- Goñi, I.; Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Towards an updated methodology for measurement of dietary fiber, including associated polyphenols, in food and beverages. Food Res. Int. 2009, 42, 840–846. [Google Scholar] [CrossRef]
- Díaz-Rivas, J.O.; González-Laredo, R.F.; Chávez-Simental, J.A.; Montoya-Ayón, J.B.; Moreno-Jiménez, M.R.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E. Comprehensive characterization of extractable phenolic compounds by UPLC-PDA-ESI-QqQ of Buddleja scordioides plants elicited with salicylic acid. J. Chem. 2018, 2018, 4536970. [Google Scholar] [CrossRef]
- Mexican Official Standard NOM-062-ZOO-1999; Technical Specifications for the Production, Care and Use of Laboratory Animals. SAGARPA: Mexico City, Mexico, 1999.
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Bertevello, P.L.; Logullo, Â.F.; Nonogaki, S.; Campos, F.M.; Chiferi, V.; Alves, C.C.; Waitzberg, D.L. Immunohistochemical assessment of mucosal cytokine profile in acetic acid experimental colitis. Clinics 2005, 60, 277–286. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557. [Google Scholar] [PubMed]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.; Motilva, M.; Tomas, M.; et al. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Compr. Rev. Food Sci. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.; Gan, R.; Li, H.B. Health benefits and side effects of short-chain fatty acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, G.; Yang, X.; Chen, Z.; Zhou, L. Behavioral abnormalities in C57BL/6 mice with chronic ulcerative colitis induced by DSS. BMC Gastroenterol. 2023, 23, 84. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay between exercise and gut microbiome in the context of human health and performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef] [PubMed]
- Satka, S.; Frybortova, V.; Zapletalova, I.; Anzenbacher, P.; Anzenbacherova, E.; Kozakova, H.; Jourova, L. Effect of DSS-Induced Ulcerative Colitis and Butyrate on the Cytochrome P450 2A5: Contribution of the Microbiome. Int. J. Mol. Sci. 2022, 23, 11627. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Martin, S.A.; Williams, C.; Whitlock, K.; Wallig, M.A.; Pence, B.D.; Woods, J.A. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013, 33, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Curgali, K.; Toth, S.; Jonecova, Z.; Maretta, M.; Kalpakidis, T.; Petriskova, I.; Kruzliak, P. Quercetin protects jejunal mucosa from experimental intestinal ischemia reperfusion injury by activation of CD68 positive cells. Acta Histochem. 2018, 120, 28–32. [Google Scholar] [CrossRef]
- Wu, B.; Bhatnagar, R.; Indukuri, V.V.; Chopra, S.; March, K.; Cordero, N.; Reddivari, L. Intestinal mucosal barrier function restoration in mice by maize diet containing enriched Flavan-4-Ols. Nutrients 2020, 12, 896. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E. Mutual influence of polyphenols and Lactobacillus spp. bacteria in food: A review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Lu, P.D.; Zhao, Y.H. Targeting NF-κB pathway for treating ulcerative colitis: Comprehensive regulatory characteristics of Chinese medicines. Chin. Med. 2020, 15, 15. [Google Scholar] [CrossRef]
- Ayuda-Durán, B.; Sánchez-Hernández, E.; González-Manzano, S.; Santos-Buelga, C.; González-Paramás, A.M. The effects of polyphenols against oxidative stress in Caenorhabditis elegans are determined by coexisting bacteria. Front. Nutr. 2022, 9, 989427. [Google Scholar] [CrossRef]
- Karaca, B.; Yilmaz, M.; Gursoy, U.K. Targeting Nrf2 with Probiotics and Postbiotics in the Treatment of Periodontitis. Biomolecules 2022, 12, 729. [Google Scholar] [CrossRef]
- Van Wijck, K.; Lenaerts, K.; Van Loon, L.J.; Peters, W.H.; Buurman, W.A.; Dejong, C.H. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders. Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef] [PubMed]
- Sukhovskaya, I.V.; Kantserova, N.P.; Lysenko, L.A.; Morozov, A.A. Taxifolin modulates transcriptomic response to heat stress in rainbow trout, Oncorhynchus mykiss. Animals 2022, 12, 1321. [Google Scholar] [CrossRef]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Ribeiro, V.M.; Bedê, T.P.; Rocha, G.S.; Barroso, S.; Valença, S.; De Azeredo, V.B. High fat diet and high polyphenols beverages effects in enzymatic and non-enzymatic antioxidant activity. Nutr. Hosp. 2018, 35, 169–175. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yin, Y.; Li, D.N.; Zhao, D.Y.; Huang, J.Q. Biological Activities of p-Hydroxycinnamic Acids in Maintaining Gut Barrier Integrity and Function. Foods 2023, 12, 2636. [Google Scholar] [CrossRef]
- Xu, X.; Luo, A.; Lu, X.; Liu, M.; Wang, H.; Song, H.; Duan, X. p-Hydroxybenzoic acid alleviates inflammatory responses and intestinal mucosal damage in DSS-induced colitis by activating ERβ signaling. J. Funct. Foods. 2021, 87, 104835. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Golubnitschaja, O. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression—3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y.; Jiang, C.; Liu, L.; Li, J.; Li, D.; Tang, Y. Intensive running enhances NF-κB activity in the mice liver and the intervention effects of quercetin. Nutrients 2020, 12, 2770. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Zhang, J. Naringenin: A flavanone with anti-inflammatory and anti-infective properties. Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef]
- Geng, Y.; Li, L.; Wang, X.; He, F.; Zhou, Y.; Yang, M.; Xu, Y. Interleukin-10 polymorphisms affect the key periodontal pathogens in Chinese periodontitis patients. Sci. Rep. 2018, 8, 9068. [Google Scholar] [CrossRef] [PubMed]
- Stromsnes, K.; Lagzdina, R.; Olaso-Gonzalez, G.; Gimeno-Mallench, L.; Gambini, J. Pharmacological properties of polyphenols: Bioavailability, mechanisms of action, and biological effects in in vitro studies, animal models, and humans. Biomedicines 2021, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Hees, J.T.; Harbauer, A.B. Metabolic Regulation of Mitochondrial Protein Biogenesis from a Neuronal Perspective. Biomolecules 2022, 12, 1595. [Google Scholar] [CrossRef]
- Vitali, D.G.; Drwesh, L.; Cichocki, B.A.; Kolb, A.; Rapaport, D. The biogenesis of mitochondrial outer membrane proteins show variable dependence on import factors. IScience 2020, 23, 100779. [Google Scholar] [CrossRef]
- Chang, W.T.; Huang, S.C.; Cheng, H.L.; Chen, S.C.; Hsu, C.L. Rutin and gallic acid regulates mitochondrial functions via the SIRT1 pathway in C2C12 myotubes. Antioxidants 2021, 10, 286. [Google Scholar] [CrossRef]
- Ommati, M.M.; Farshad, O.; Mousavi, K.; Khalili, M.; Jamshidzadeh, A.; Heidari, R. Chlorogenic acid supplementation improves skeletal muscle mitochondrial function in a rat model of resistance training. Biologia 2020, 75, 1221–1230. [Google Scholar] [CrossRef]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Perkins, G.; Murphy, A.N.; Naviaux, R.; Villarreal, F. Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: Effects of epicatechin rich cocoa. Clin. Transl. Sci. 2012, 5, 43–47. [Google Scholar] [CrossRef]
- Frampton, J.; Cobbold, B.; Nozdrin, M.; Oo, H.T.; Wilson, H.; Murphy, K.G.; Chambers, E.S. The effect of a single bout of continuous aerobic exercise on glucose, insulin and glucagon concentrations compared to resting conditions in healthy adults: A systematic review, meta-analysis and meta-regression. Sports Med. 2021, 51, 1949–1966. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Van Krimpen, S.J.; Jansen, F.A.; Ottenheim, V.L.; Belzer, C.; van der Ende, M.; van Norren, K. The effects of pro-, pre-, and synbiotics on muscle wasting, a systematic review—Gut permeability as potential treatment target. Nutrients 2021, 13, 1115. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Mihara, T.; Oki, M.; Kumagai, T. Effects of heat-killed Lactobacillus casei subsp. casei 327 intake on defecation in healthy volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. Bioscience of Microbiota. Nutr. Health 2018, 37, 17–025. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsu, Y.J.; Ho, H.H.; Chang, Y.C.; Kuo, Y.W.; Yeh, Y.T.; Lee, M.C. Bifidobacterium longum subsp. longum OLP-01 supplementation during endurance running training improves exercise performance in middle-and long-distance runners: A double-blind controlled trial. Nutrients 2020, 12, 1972. [Google Scholar] [CrossRef]
- Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Huang, C.C. Live and Heat-Killed Probiotic Lactobacillus paracasei PS23 Accelerated the Improvement and Recovery of Strength and Damage Biomarkers after Exercise-Induced Muscle Damage. Nutrients 2022, 14, 4563. [Google Scholar] [CrossRef]
- McDermott, C.E.; Vincent, H.K.; Mathews, A.E.; Cautela, B.G.; Sandoval, M.; Tremblay, A.; Langkamp-Henken, B. Impact of probiotic supplementation on exercise endurance among non-elite athletes: Study protocol for a randomized, placebo-controlled, double-blind, clinical trial. Trials 2022, 23, 603. [Google Scholar] [CrossRef]

| Prebiotics | Total Abundance |
|---|---|
| Total of dietary fiber | 0.820 ± 0.70 g/100 g |
| Soluble fiber | 45.02 ± 0.80% |
| Insoluble fiber | 54.97 ± 0.05% |
| Total of bound polyphenols | 30,218 ± 104 µg/g |
| Probiotics | Total Dose of Bacteria |
| L. casei | 2 × 109 cel/mL |
| L. paracasei | 2 × 109 cel/mL |
| B. longum | 2 × 109 cel/mL |
| B. breve | 2 × 109 cel/mL |
| B. bifidum | 2 × 109 cel/mL |
| Total dose of probiotics | 1 × 1010 cel/mL |
| Level | Type of Injury and/or Damage |
|---|---|
| 0 | Normal, no damage |
| 1 | Inflammation in the superficial epithelium or mild cellular alterations such as atrophy |
| 2 | Inflammation in the lamina propria and epithelium |
| 3 | Inflammation in the lamina propria, epithelium, and muscle phase, leukocyte infiltration |
| 4 | Inflammation in the lamina propria, epithelium, muscle phase, leukocyte infiltration, and presence of ulcers |
| 5 | Inflammation in the lamina propria, epithelium, muscle phase, ulcers, and presence of microabsessions |
| No. | Compound | Acronym | Retention Time (min) | Molecular Weight | Main Transition (m/z) | λ Max (nm) | Abundance |
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||||
| 1 | Gallic acid | GaA | 1.07 | 169 | 79 > 125 | 270 | 4.17 ± 0.72 |
| 2 | Vanillic acid | VA | 3.71 | 167 | 123 >152 | 270 | 46.37 ± 4.63 |
| 3 | Shikimic acid | ShA | 0.57 | 173 | 93 > 111 | 270 | 233.77 ± 10.35 |
| 4 | Proteocatechuic acid | PA | 2.11 | 153 | 109 > 91 | 270 | 169.54 ± 5.95 |
| 5 | 2,5-dihydroxybenzoic acid | 2-5-DHA | 2.83 | 153 | 109 > 81 | 270 | 15.66 ± 1.46 |
| 6 | 4-hydroxybenzoic acid | 4-HA | 3.13 | 137 | 93 > 65 | 270 | 651.49 ± 17.32 |
| 7 | 5-hydroxybenzoic acid | 5-HA | 6.15 | 153 | 136 > 93 | 270 | 30.90 ± 0.59 |
| 8 | Benzoic acid | BA | 6.29 | 121 | 77 > 92 | 270 | 2954.54 ± 19.30 |
| Hydroxycinnamic acids | |||||||
| 9 | Quinic acid | QA | 0.56 | 191 | 93 > 85 | 320 | 759.23 ± 6.38 |
| 10 | Caffeic acid | CaA | 3.85 | 179 | 135 > 89 | 320 | 8370.80 ± 166.66 |
| 11 | Chlorogenic acid | ChA | 3.42 | 353 | 191 > 85 | 320 | 14.60 ± 0.42 |
| 12 | Ferulic acid | FA | 5.57 | 193 | 178 > 134 | 320 | 79.01 ± 4.11 |
| 13 | t-cinnamic acid | tCA | 8.44 | 148 | 148 > 149 | 320 | 481.59 ± 8.48 |
| Flavonols | |||||||
| 14 | Quercetin | Q | 8.28 | 480 | 479 > 303 | 360 | 175.94 ± 10.08 |
| 15 | Quercetin-3-O-glucuronide | Q-3-O-Glu | 5.94 | 480 | 479 > 303 | 360 | 9894.17 ± 98.47 |
| 16 | Kaempferol-3-O-glucuronide | K-3-O-Glu | 6.62 | 447 | 255 > 284 | 360 | 5859.11 ± 137.59 |
| 17 | Rutin | R | 5.84 | 610 | 609 > 300 | 360 | 553.92 ± 20.64 |
| Flavanols | |||||||
| 18 | Catechin | Cat | 3.16 | 289 | 245 > 123 | 280 | 47.70 ± 7.20 |
| 19 | Epicatechin | ECat | 4.38 | 298 | 245 > 123 | 280 | 276.84 ± 10.23 |
| Flavanones | |||||||
| 20 | Naringenin | N | 9.06 | 271 | 151 > 119 | 280 | 3.89 ± 0.90 |
| Flavones | |||||||
| 21 | Luteolin | L | 8.22 | 286 | 285 > 133 | 280 | 65.85 ± 5.52 |
| 22 | Acacetin | A | 11.37 | 269 | 148 > 117 | 320 | 25.88 ± 4.06 |
| 23 | Apigenin | Ap | 9.07 | 270 | 268 > 116 | 320 | 13.41 ± 3.90 |
| Dihydrochalcones | |||||||
| 24 | Phloretin | P | 9.21 | 273 | 123 > 167 | 280 | 0.00 ± 0.00 |
| 25 | Phloridzin | Ph | 7.35 | 436 | 435 > 167 | 280 | 33.08 ± 6.21 |
| Flavanonols | |||||||
| 26 | Taxifolin | Tax | 5.95 | 125 | 303 > 285 | 290 | 4.03 ± 0.84 |
| Xanthonoid | |||||||
| 27 | Mangiferin | M | 4.17 | 422 | 422 > 303 | 370 | 0.22 ± 0.00 |
| Treatments | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|
| Control (−) | 0.94 ± 0.13 b | 0.78 ± 0.18 c | 1.19 ± 0.58 a | 1.42 ± 0.48 a |
| Control (+) | 0.53 ± 0.12 a | 0.59 ± 0.10 c | 0.21 ± 0.05 c | 0.80 ± 0.13 d |
| Probiotics | 0.37 ± 0.08 a | 0.41 ± 0.08 c | 0.60 ± 0.08 d | 0.54 ± 0.05 c |
| Quince synbiotic | 0.56 ± 0.11 a | 0.52 ± 0.15 c | 0.51 ± 0.11 d | 0.59 ± 0.12 c |
| Small Intestine | ||||
|---|---|---|---|---|
| Level Damage | 0 | 1 | 2 | 3 |
| Control (−) | 8 (10) | 2 (10) | 0 (10) | 0 (10) |
| Control (+) | 7 (10) | 3 (10) | 0 (10) | 0 (10) |
| Probiotics | 3 (10) | 6 (10) | 1 (10) | 0 (10) |
| Quince synbiotic | 7 (10) | 3 (10) | 0 (10) | 0 (10) |
| Colon | ||||
| Level damage | 0 | 1 | 2 | 3 |
| Control (−) | 5 (10) | 3 (10) | 2 (10) | 0 (10) |
| Control (+) | 1 (10) | 4 (10) | 5 (10) | 0 (10) |
| Probiotics | 3 (10) | 5 (10) | 1 (10) | 1 (10) |
| Quince synbiotic | 3 (10) | 3 (10) | 3 (10) | 1 (10) |
| Groups | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| Control (−) | 9.47 ± 1.18 | 7.14 ± 0.67 | 5.43 ± 0.46 | 5.01 ± 0.29 | 2.20 ± 0.35 |
| Control (+) | 5.76 ± 0.69 | 4.05 ± 0.49 | 3.93 ± 0.34 | 3.44 ± 0.39 | 3.78 ± 0.60 |
| Probiotics | 9.48 ± 0.51 | 8.76 ± 0.68 | 8.32 ± 0.72 | 9.18 ± 1.14 | 5.66 ± 0.66 |
| Quince synbiotic | 9.07 ± 1.22 | 4.51 ± 0.28 | 7.88 ± 0.92 | 8.44 ± 1.12 | 2.52 ± 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Rocha, K.M.; Manjarrez-Juanes, M.M.; Larrosa, M.; Barrios-Payán, J.A.; Rocha-Guzmán, N.E.; Macías-Salas, A.; Gallegos-Infante, J.A.; Álvarez, S.A.; González-Laredo, R.F.; Moreno-Jiménez, M.R. The Synergistic Effect of Quince Fruit and Probiotics (Lactobacillus and Bifidobacterium) on Reducing Oxidative Stress and Inflammation at the Intestinal Level and Improving Athletic Performance during Endurance Exercise. Nutrients 2023, 15, 4764. https://doi.org/10.3390/nu15224764
Herrera-Rocha KM, Manjarrez-Juanes MM, Larrosa M, Barrios-Payán JA, Rocha-Guzmán NE, Macías-Salas A, Gallegos-Infante JA, Álvarez SA, González-Laredo RF, Moreno-Jiménez MR. The Synergistic Effect of Quince Fruit and Probiotics (Lactobacillus and Bifidobacterium) on Reducing Oxidative Stress and Inflammation at the Intestinal Level and Improving Athletic Performance during Endurance Exercise. Nutrients. 2023; 15(22):4764. https://doi.org/10.3390/nu15224764
Chicago/Turabian StyleHerrera-Rocha, Karen Marlenne, María Magdalena Manjarrez-Juanes, Mar Larrosa, Jorge Alberto Barrios-Payán, Nuria Elizabeth Rocha-Guzmán, Alejo Macías-Salas, José Alberto Gallegos-Infante, Saul Alberto Álvarez, Rubén Francisco González-Laredo, and Martha Rocío Moreno-Jiménez. 2023. "The Synergistic Effect of Quince Fruit and Probiotics (Lactobacillus and Bifidobacterium) on Reducing Oxidative Stress and Inflammation at the Intestinal Level and Improving Athletic Performance during Endurance Exercise" Nutrients 15, no. 22: 4764. https://doi.org/10.3390/nu15224764
APA StyleHerrera-Rocha, K. M., Manjarrez-Juanes, M. M., Larrosa, M., Barrios-Payán, J. A., Rocha-Guzmán, N. E., Macías-Salas, A., Gallegos-Infante, J. A., Álvarez, S. A., González-Laredo, R. F., & Moreno-Jiménez, M. R. (2023). The Synergistic Effect of Quince Fruit and Probiotics (Lactobacillus and Bifidobacterium) on Reducing Oxidative Stress and Inflammation at the Intestinal Level and Improving Athletic Performance during Endurance Exercise. Nutrients, 15(22), 4764. https://doi.org/10.3390/nu15224764








