Abstract
The gut microbiota plays a crucial role in the human microenvironment. Dysbiosis of the gut microbiota is a common pathophysiological phenomenon in critically ill patients. Therefore, utilizing intestinal microbiota to prevent complications and improve the prognosis of critically ill patients is a possible therapeutic direction. The gut microbiome-based therapeutics approach focuses on improving intestinal microbiota homeostasis by modulating its diversity, or treating critical illness by altering the metabolites of intestinal microbiota. There is growing evidence that fecal microbiota transplantation (FMT), selective digestive decontamination (SDD), and microbiota-derived therapies are all effective treatments for critical illness. However, different treatments are appropriate for different conditions, and more evidence is needed to support the selection of optimal gut microbiota-related treatments for different diseases. This narrative review summarizes the curative effects and limitations of microbiome-based therapeutics in different critically ill adult patients, aiming to provide possible directions for gut microbiome-based therapeutics for critically ill patients such as ventilator-associated pneumonia, sepsis, acute respiratory distress syndrome, and COVID-19, etc.
1. Introduction
Intestinal dysbiosis, endotoxemia, and systemic inflammation are major factors contributing to pathophysiological alterations in critically ill patients. Critical illness is characterized by the loss of commensal microbiota and an excessive growth of potentially pathogenic bacteria, resulting in reduced production of short-chain fatty acids (SCFAs) or an inflammatory reaction induced by the gut microbiota [1], leading to prolonged immunosuppression and a high susceptibility to hospital-acquired infections [2,3]. In addition, prophylactic use of broad-spectrum antibiotics to treat infection is a common clinical approach, which aggravates the imbalance between the host immune system and the gut microbiota, suppressing the microbiota [4,5]. Thus, the ability of the original gut microbiota to prevent pathogen colonization is impaired, increasing the risk of infection [6,7]. In a critically ill mouse model, the upregulation of intestinal epithelial apoptosis and the enhancement of barrier hyperpermeability have been reported [8]. Moreover, an association between reduced gut microbiota diversity and increased relative abundance of potentially pathogenic bacteria, including aerobic gram-negative bacteria, has been found in several prospective cohort studies in critically ill patients with sepsis [9,10,11].
The intestinal microbiota inhibits the colonization of potential pathogens in the intestinal epithelium by stimulating the production of IgA, defensins, and antimicrobial peptides in the host, as well as competition for preferred resources among bacteria within specific ecological niches [12,13,14]. However, the increased colonization of pathogens and the disruption of the gut barrier can be affected by the collapse of microbiota diversity [14]. In addition, the intestinal microbiota can communicate with extraintestinal organs (e.g., lung, kidney, and heart, etc.) via bacterial extracellular vesicles [15]. Therefore, the repair of the gut microbiota and its metabolites may be suggested as a direction for the treatment or prevention of critical illness.
In this narrative review, we categorize the therapeutic approaches related to the gut microbiome into three groups: fecal microbiota transplantation (FMT), selective digestive decontamination (SDD), and microbiome-directed therapies including probiotics, prebiotics, synbiotics, and microbiota-derived metabolites and proteins (e.g., SCFAs and flavonoid) (Figure 1). We also summarize the application of different gut microbiome-based therapeutics in different critical illnesses, such as ventilator-associated pneumonia (VAP), sepsis, acute respiratory distress syndrome (ARDS), COVID-19, and other diseases (Table 1).
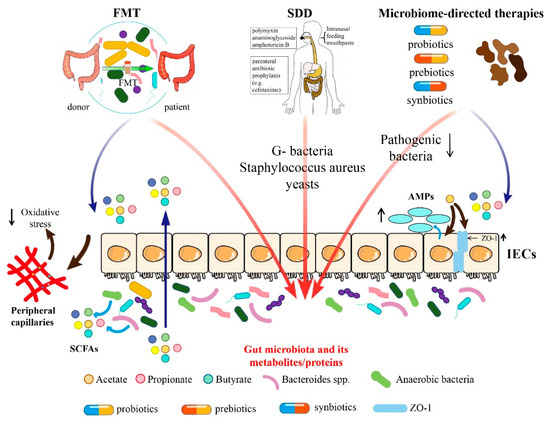
Figure 1.
Summary of mechanism of different gut microbiome-based therapeutic approaches.

Table 1.
The application of gut microbiome-based therapeutics in critically ill adult patients.
Gut microbiome-based therapies include FMT, SDD, supplementation of probiotics, prebiotics, synbiotics, and directly providing microbiota-associated metabolites and proteins. FMT plays a role by regulating gut microbiota and affecting SCFAs. Moreover, SDD mainly affects the colonization of G-bacteria, Staphylococcus aureus, and yeasts in the intestines. Microbiome-directed therapies play roles via decreasing pathogenic bacteria, increasing beneficial bacteria, and regulating the level of SCFAs. SCFAs could increase the expression of intestinal epithelial tight junction proteins and promote anti-microbial peptides (AMPs) secreted by intestinal epithelial cells (IECs), which move into peripheral capillaries and inhibit oxidative stress. FMT, fecal microbiota transplantation; SDD, selectively digestive decontamination; SCFAs, short-chain fatty acids; IECs, intestinal epithelial cells; and AMPs, anti-microbial peptides.
2. Methods/Data Search
The literature search included English-language articles published before September 2023 that belonged to Pubmed-index journals. We also searched the reference lists of the original papers for further relevant articles.
3. FMT
FMT is a therapeutic approach to transfer the micro-manipulated microbiota from healthy donor feces to the patient’s intestine, recovering intestinal diversity, inhibiting the growth of pathogenic bacterial communities in the gut environment, and driving competitive rejection of pathogenic bacteria among the local intestinal microbiota.
FMT reduces the levels of local or foreign bacterial pathogens in the intestinal microbiota of mice and restores the normal function of intestinal microbiota [48,49,50,51]. Early application of FMT reduces mortality in mice with myocardial infarction caused by chronic left anterior descending artery ligation [20]. It has been reported that FMT can alleviate lung inflammation and acute lung injury (ALI) in mice through modulating gut microbiota and metabolic disturbance [52]. In a sepsis mouse model, FMT can regulate the abundance of bacteria such as Firmicutes, Proteobacteria, Escherichia Shigella, and Lactobacillus to a level comparable to that of healthy mice, and downregulate the expression of the NOD-like receptor protein 3 (NLRP3) and Gasdermin-D (GSDMD)-N proteins and the release of inflammatory factors to inhibit cell pyroptosis [53]. Additionally, FMT restores immune homeostasis via providing specific colonies of bacteria which produce SCFAs (e.g., butyrate, etc.) with histone deacetylases inhibitory (HDACi) activity that can be amplified in inflammation and infection-induced systemic immune response, thereby restoring the interferon regulatory factor 3 (IRF3) signaling pathway [50]. In a mouse model of ALI, FMT restored the gut microbiota to reduce oxidative stress and impair the TLR4/NF-κB pathway in the lung, ameliorating lung damage [54]. In addition, in an ALI rat model, FMT could alleviate LPS-induced lung injury by modulating the TGF-β1/Smad/ERK pathway, and was able to regulate gut microbiota, inhibit immune-inflammation, and reduce inflammatory cytokines [55].
FMT application in healthy volunteers revealed a transient suppression of systemic immune cytotoxicity, with decreased T-cytotoxic CD8+ lymphocytes and natural killer cells in circulation, increased T-helper CD4+ cells, and an increased CD4+ to CD8+ ratio [56]. Clinical trials have demonstrated the ability of FMT in melanoma treatment. FMT can effectively shift the intestinal microbiota composition toward taxa favoring anti-programmed death-1 (PD-1) efficacy which is associated with increased intra-tumoral and peripheral antitumor immunity [57,58]. Moreover, FMT may act against antibiotic-resistant CDI [17,59] and contribute to recovery from immunotherapy for colitis and the eradication of colonized antibiotic-resistant bacteria in patients with hematological malignancies [18,60]. In addition, in a study of FMT for critically ill patients with antibiotic-associated diarrhea, good clinical outcomes were observed without infectious complications [61]. With the increasing importance of the microbiota in health, FMT treatment has been further investigated for application in autoimmune diseases, metabolic syndrome, sepsis, and other critical illnesses [62]. Moreover, clinical trials have shown that FMT applied to patients with severe alcoholic hepatitis presenting as acute-on-chronic liver failure (SAH-ACLF) rapidly improves the patients’ clinical severity scores, abnormal liver dysfunction and ascites, reduces pro-inflammatory cytokines levels, and is expected to significantly improve patient survival and the prognosis of hepatic encephalopathy [21]. Taken together, it can clearly be seen that FMT treatment may be a potential therapeutic option for critical illnesses.
4. SDD
For more than 30 years, SDD has been proposed as a measure to prevent infection in intensive care unit (ICU) patients, who mostly have respiratory failure, mechanism ventilation, reversible multiple organ failure, or are there after major surgery, a coma, or shock [63].Currently, SDD is only considered as a standard therapy in the Netherlands and is sporadically used in ICUs in other countries [64]. The main aim of SDD is to reduce ICU-acquired infections via eradicating and preventing colonization of the digestive tract by gram-negative bacteria, Staphylococcus aureus, and yeasts, thereby promoting the prognosis of ICU patients [65].
In a randomized controlled trial with 5939 enrolled patients, SDD has been demonstrated to be effective in reducing the mortality rate in ICU patients [66]. In addition, an SDD strategy in ICUs could reduce the clinically relevant infections caused by multidrug-resistant bacteria and decrease colistin- and tobramycin-resistant colonization with a nonsignificant, increasing rate of ICU colonization resistance [67]. A large, well-conducted, cluster, crossover, randomized trial showed that the hospital mortality of critically ill patients receiving mechanism ventilation with SDD was 27.0%, the hospital mortality of other patients without SDD was 29.1%. Although the result had no statistical significance, the authors conclude that the confidence interval includes a clinically relevant benefit [68]. Recently, a meta-analysis comparing the use of SDD with standard care in ICU patients, demonstrated that SDD contributed to a reduced risk of VAP, ICU-acquired bacteremia, and lower hospital mortality [69]. Furthermore, studies associating SDD strategy with COVID-19 have shown that SDD strategies have a beneficial effect on decreasing ICU mortality in mechanically ventilated patients with severe COVID-19 [22,23].
5. Microbiome-Directed Therapies
Loss of commensal microbiota and excessive growth of potentially pathogenic bacteria are the main features of the gut microbiota in critically ill adult patients [2]. Gut microbiota imbalance can increase the risk of secondary infection, immunosuppression, and even organ dysfunction, leading to an increased incidence of opportunistic infections and sepsis, aggravated various target organ damage, and worsened patient condition. Additionally, even after recovery from sepsis, the slow recolonization of patients’ normal microbiota may lead to long-term immunosuppression and poor prognosis. Therefore, different strategies related to the gut microbiota, such as using probiotics and prebiotics alone or in combination (synthetic preparations), have been proposed in order to prevent the further growth of pathogens and improve the outcomes of critically ill patients [25,70,71,72].
5.1. Probiotics
The International Scientific Association for Probiotics and Prebiotics defines probiotics as “live microorganisms that, when given in sufficient amounts, have a beneficial effect on the health of the host” [73]. They protect the intestinal barrier, attenuate pathogen overgrowth, decrease bacterial translocation, reduce serum pro-inflammatory cytokine concentrations while increasing the serum anti-inflammatory cytokine concentrations, and induce host immunomodulation to prevent infection [74,75,76,77]. In addition, probiotics also act through pharmacokinetics. For example, gut Actinobacterium Eggerthella lenta could affect the pharmacokinetic of digoxin and reduce its toxicity in treating congestive heart failure [78]. Moreover, the probiotic E. coli strain Nissle 1917 influences the pharmacokinetics of the antiarrhythmic amiodarone and increases drug absorption [79].
Several probiotics play a role in adult intensive care [80]. Probiotic therapy significantly reduces the incidence of diarrhea, acquired infections, and VAP in critically ill patients [24,26,81]. In sepsis-induced, severe ALI, Akkermansia muciniphila (A. muciniphila) was significantly negatively correlated with TNF-α, IL-1β and IL-6, suggesting that Gut A. muciniphila plays an important role in ALI and that supplementation with A. muciniphila may be a possible therapy for ALI [82]. In addition, the combination of probiotics Bifidobacterium longum, Lactobacillus bulgaricus and Streptococcus thermophilus was more effective as an adjuvant therapy for severe and critically ill patients with COVID-19, shortening the nucleic acid conversion time, and reducing the inflammatory index such as procalcitonin and C-reactive protein [83]. Another probiotic, L. reuteri., can reduce lung inflammation and mortality of ARDS [30]. In uremic dialysis patients, oral administration of Lactobacillus acidophilus led to a decrease in serum dimethylamine, a potential uremic toxin [84]. The administration of probiotics (e.g., Bifidobacterium bifidum, Bifidobacterium catenulatum, Bifidobacterium longum, Lactobacillus plantarum) can also significantly reduce serum proinflammatory endotoxin, decrease cytokine levels, and improve life quality [85]. A double-blind clinical study has shown that the probiotics Lactobacillus rhamnosus GG or a combination application of probiotics (including streptococcus thermophiles, lactobacillus acidophilus, lactobacillus delbrueckii ssp. bulgaricus, lactobacillus paracasei, lactobacillus plantarum, Bifidobacterium longum, Bifidobacterium infantis, and Bifidobacterium breve) has beneficial effects on heart failure caused by adverse cardiac remodeling after myocardial infarction (MI). These probiotics play roles through decreasing the levels of intestinal metabolite trimethylamine N-oxide (TMAO) [34,86].
5.2. Prebiotics
Prebiotics are substrates that can be selectively utilized by host microbes to maintain gut homeostasis and improve health outcomes. Dietary fiber (DF) is a powerful beneficial prebiotic that promotes the production of SCFAs [87].
Several prospective cohort studies have found that the use of DF in critically ill patients is effective in enhancing intestinal barrier function, reducing the systemic inflammatory response, modulating gut microbiota: increasing the abundance of the SCFAs-producing bacteria, and decreasing the level of potentially pathogenic microbiota [35]. The use of DF also improves the clinical outcomes, shortening hospital days, and reducing morbidity and mortality in critically ill patients [35]. Researchers have also found that prebiotics may ameliorate the prognosis of COVID-19 by offering anti-inflammatory nutrition, improving malnutrition, and enhancing immunity through the gut–lung microbial axis [36,37,88]. More importantly, DF-fermented SCFAs increase the production of CD103+DCs, promote the differentiation of activated CD8+T cells to effector cells with a memory phenotype, and improve the outcomes of anti-PD-1 immune checkpoint inhibitor therapy in patients with melanoma [89,90]. Similarly, the SCFAs produced by DF provide energy to the gut microbiota and promote the amino acids to reach the colon for absorption into the body rather than fermenting into uremic solutes [38].
In addition, flavonoid is an important class of natural products widely found in fruits which belongs to a class of plant secondary metabolites [91]. Clinical trials have demonstrated that flavonoids can upregulate the abundance of probiotics, such as Bifidobaterium and Lactobacillus, while downregulating the abundance of some pathogenic bacteria such as Staphylococcus aureus and Clostridium histolyticum [92,93]. Moreover, flavonoids can also promote the production of SCFAs [94].
5.3. Synbiotics
Synbiotics are mixtures of probiotics and prebiotics that exert beneficial effects on the host in two main ways: enhancing the viability of probiotic microorganisms and providing specific health effects [95,96]. Probiotics stimulated by prebiotics could regulate the metabolic activity of the gut, maintain the intestinal biostructure, and promote the growth and multiplication of probiotics and its resistance to reactive oxygen species and bile salts/acids [97]. In addition, synbiotic agents modulate the innate and adaptive immune systems to reduce systemic inflammation and promote extraintestinal organ function [71,97]. Synbiotics lead to lower concentrations of adverse metabolites which results in significantly increased SCFAs levels, which may contribute to a positive effect on the host health [95].
A double-blinded controlled clinical trial demonstrates that a combination of antibiotic intervention led to a significant reduction in pharyngeal aspiration in critically ill patients and an increased level of patient consciousness [98]. In addition, for hospital-acquired infections in critically ill patients, synbiotics may be a safer and more effective way to reduce endotoxin and inflammatory markers in serum and the complications of sepsis [76,81]. Studies have shown that prophylactic synbiotics (e.g., Bifidobacterium breve strain Yakult combined with Lactobacillus casei strain Shiorta, and galactooligosaccharides) increases the number of probiotics (e.g., Bifidobacterium, Lactobacillus) in fecal bacteria and intestinal SCFA levels, especially acetate. These may modulate the gut microbiota and environment, and have preventive effects on the incidence of enterocolitis and VAP in sepsis patients [25]. Moreover, synbiotics are used to maintain a stable intestinal microbiota after SIRS and major surgery including high-risk hepatectomy, colorectal resection surgery, Roux-en-Y gastric bypass (RYGB), and sepsis-associated encephalitis (SAE) [40,99]. Synbiotics could also reduce the incidence of diseases such as VAP and healthcare-associated pneumonia, and shorten ICU length of stay [100,101]. In the late stage of intestinal disorders, supplementation with synbiotics can accelerate the recovery of the microbiota, thus preventing the development of sepsis and the onset and progression of critical diseases such as ARDS to some extent [44]. Synbiotics intake has been demonstrated to reduce plasma levels of uremic toxin and may exert nephroprotective effects [84].
Based on the above literature, we briefly summarize the different scenarios in which FMT, SDD, probiotics, prebiotics, and synbiotics act directly by regulating the gut microbiota (Table 2).

Table 2.
Appropriate situations for different therapeutic approaches.
5.4. Microbiota-Derived Metabolites and Proteins
Transfer of sterile filtrates from donor feces, rather than fecal microbiota, has been shown to be sufficient to restore normal bowel habits and eliminate symptoms [106]. Therefore, researchers speculate that gut metabolites may contribute to critical illness and dysbiosis. There is observational data that correlates critical illness, dysbiosis, and altered gut metabolites, including SCFAs, flavonoids, indole derivations, amines, bile acids, etc. [107]. In this section, several microbiota-derived metabolites and proteins therapies will be summarized.
5.4.1. Short-Chain Fatty Acid
Typically, undigested DF, as well as proteins and peptides, can be fermented by gut bacteria in the cecum and colon. The main products of these fermentative reactions are SCFAs, consisting of groups of fatty acids with less than six carbons. SCFAs include formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4), and valeric acid (C5). The major SCFAs in the gut are C2, C3, and C4, accounting for more than 95% of all SCFAs [108]. SCFAs are key mediators in the regulation of myocardial tissue repair by gut microbiota [20]. Decreased microbiota abundance has been shown to alter immune cell responses to infectious damage, usually resulting in a pro-inflammatory phenotype [109], which further aggravates disease progression. Normal levels of SCFAs support the activity of innate lymphocytes, T cells, and B cells in the gut, thereby improving immune tolerance in the gut, strengthening the gut immune barrier, and enhancing its ability to clear pathogens [14,110]. Furthermore, a large proportion of gut-derived SCFAs are transported out of the gut to affect other organs through the gut–lung axis, gut–brain axis, gut–liver axis, gut–kidney axis, gut–bone axis, gut–skin axis, gut–fat axis, gut–heart axis, and so on [111,112,113,114,115,116]. Available studies have shown that gut microbiota dysbiosis and a lack of SCAFs are significantly associated with the severity of COVID-19 [45]. Therefore, maintaining healthy gut microbiota and normal levels of SCFAs contribute to critical illness prevention and prognosis. However, little is known about their therapeutic mechanism in critically ill patients, except for mouse models, so verifying the therapeutic mechanism of SCFAs in clinical trials may be a possible direction in subsequent research. In the following sections, we introduce the beneficial role that major SCFAs play in critical illness.
Butyrate
Previous work on mouse models has shown that butyrate, a specific type of SCFAs readily produced from fiber-rich diets through microbial fermentation, is critical for the maintenance of intestinal homeostasis [117]. Thus, we speculate that butyrate acid plays a crucial role in critically ill patients.
In a mouse model of sepsis-associated encephalitis, butyrate is a major metabolite of intestinal microbiota and may have a neuroprotective effect in the process of sepsis through the GPR109A/Nrf2/HO-1 pathway [118].
Butyrate acid promotes the proliferation and differentiation of intestinal epithelial cells (IECs) and the synthesis of intestinal epithelial tight junction protein, such as the increased expression of Zo-1 and Occludin, reduces cell apoptosis, and inhibits intestinal permeability, resulting in enhanced intestinal mucosa mechanical barrier function [119,120]. In addition, butyrate acid enhances the intestinal mucosal immune barrier. It can maintain immune homeostasis by restoring the IRF3 signaling pathway [50]. Moreover, butyrate acid promotes the production of anti-microbial peptides (AMPs) [121]. AMPs are small molecular peptides produced by IECs with broad-spectrum antimicrobial activities, such as butyrate acid which promotes RegIIIγ and β-defensins in a GPR43-dependent manner, which play important roles in limiting bacteria and manipulating species composition [122,123]. Furthermore, butyrate acid strengthens the intestinal biological barrier function. Elevated levels of butyrate acid lowers colonic PH and inhibits the growth and colonization of pathogenic bacteria [124]. It also prevents bacterial translocation across the intestinal barrier by promoting the antimicrobial activity of intestinal macrophages [125].
As a histone deacetylase inhibitor, it affects the gene expression profile of intestinal epithelial cells and immune cells through histone deacetylases [126,127,128]. It can alter the gene expression profile of immune cells such as Treg cells, intestinal macrophages, and bone marrow-derived macrophages through G protein-coupled receptors (GPCR), affecting their response to microbial stimulation [125,129]. Butyrate acid can relieve inflammation and clinical symptoms in critically ill patients by activating Treg cells [130]. In addition to directly affecting the function of the intestine itself, butyrate acid can also affect the organs via the gut–lung axis and gut–brain axis. Studies have shown that the GPCR overlap with each other in extraintestinal or intestinal organs [131]. For example, GPR109A viewed as GPCR common to both lung and intestine can be activated by butyrate [108]. Butyrate acid affects the intestinal epithelial barrier function and immune function regulation through GPR109A, with similar effects on lung tissue (Table 3). Intraperitoneal injection of sodium butyrate acid can decrease the expression of hypermobility protein 1 (HP-1), pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and inhibit the activation of the NF-κB signaling pathway in an ALI/ARDS mouse model [60,132].

Table 3.
Association of G protein-coupled receptor with SCFAs.
Propionate
Propionate is mainly produced by Bacteroides spp. and is used as a gluconeogenic substrate in the liver and intestinal to provide energy to the body [124]. Propionic acid is an anti-inflammatory cytokine, its level in serum may predict the severity and prognosis in critically ill patients and may be a cytokine regulatory marker for critical illnesses such as sepsis [144].
Acetate
Acetate is mainly derived from gut microbes and is a metabolite released into the intestinal lumen by anaerobic bacteria from the gut microbiota, which is then absorbed by IEC and distributed to peripheral capillaries [145]. In recent years, studies in mice have found that acetate inhibits the permeability of the alveolar–capillary barrier, reduces pulmonary edema, inhibits oxidative stress, suppresses inflammatory cell recruitment and inflammatory mediator production, and regulates MAPK pathway activation; thus leading to amelioration of ALI and ARDS [146]. Elevated levels of acetate may prevent IEC translocation by inhibiting endotoxin and increasing claudin, thereby reducing the incidence of sepsis [25,71,147].
5.4.2. Flavonoid Metabolites
Researchers have found that flavonoids ingested with food enter the circulatory system through the intestines to exert their beneficial effects [148]. Generally, flavonoids are absorbed as metabolites, and gut microbiota participate in this metabolism [149,150]. Flavonoid metabolites can shape the gut microbiota by inhibiting the growth of various pathogens and increasing beneficial genera; these could reduce the endotoxin, maintain gut immune homeostasis, and promote nutrients absorption [151,152]. Flavonoids metabolites have anti-inflammatory effects and play a part in local and systemic immunity. In mouse models, flavonoid metabolites can improve intestinal barrier function by reducing intestinal mucosal inflammation and maintaining the intestinal tight junction barrier and structure [46,153,154]. In addition, flavonoid metabolites regulate inflammatory mediators, such as through inhibiting endothelial activation, NLRP3 inflammasome, toll-like receptors (TLRs), or bromodomain-containing protein 4 (BRD4), as well as activating nuclear factor erythroid-derived 2-related factor 2 (Nrf2), thereby restoring cytokine storm in critical illness including SARS-CoV-2 infection [47]. DAT, a kind of flavonoid metabolite, has been shown to treat upper respiratory tract infections in mice and protect mice from bacterial endotoxin-induced septic shock [155,156]. Therefore, the researchers suggest that flavonoid metabolites produced by intestinal microbial metabolism play important roles when critically ill patients and hosts are infected with viruses and lethal bacterial infections.
5.4.3. Others
Other than SCFAs and flavonoid metabolites, other microbiota-derived metabolites and proteins also play a critical role in maintaining the balance of intestinal mucosa and contributing to the treatment of critical illness. Indole-3-propionic acid (IPA) could modulate gut microbiota in normal mice, increase the levels of some probiotics (e.g., Akkermansiaceae, Bifidobacteriaceae), strengthen the mucus barrier, and attenuate LPS-induced inflammatory factors in sepsis by increasing mucins and goblet cell secretion products [157]. Studies on mouse models of sepsis suggest that the anti-inflammatory activity of IPA may associated with the increased abundance of Bifidobacteriaceae and inhibited expansion of Enterobacteriaceae, contributing to an improvement in mortality in sepsis [158]. Aromatic microbial metabolites (AMMs), such as phenyl lactic and 4-hydroxyphenyllactic acids, have been observed at a much higher level than normal in the serum of septic patients [159]. A prospective observational pilot study found that high level of AMMs were associated with severity and mortality in critically ill patients, and may become a possible direction to improve the prognosis of critical illness [160]. TMAO has been found to be higher in individuals with heart failure than in controls, suggesting that TMAO is a new and novel risk factor in heart failure development and can lead to cardiac hypertrophy and cardiac fibrosis [161]. Beta-lactamase, produced primarily by extended-spectrum β-lactam Enterobacteriaceae, has been shown to reduce the jejunal concentration of antibiotics and to prevent antibiotics from reaching the colon, thus alleviating the effect of antibiotics on gut microbiota disturbance [162,163].
6. Limitations
The gut microbiome has been recognized as a potential tool for the prevention and treatment of critical illness. However, although different microbiome-based therapeutics have proven to be effective in the treatment of critical illness, there remain limitations and challenges for future development (Figure 2).
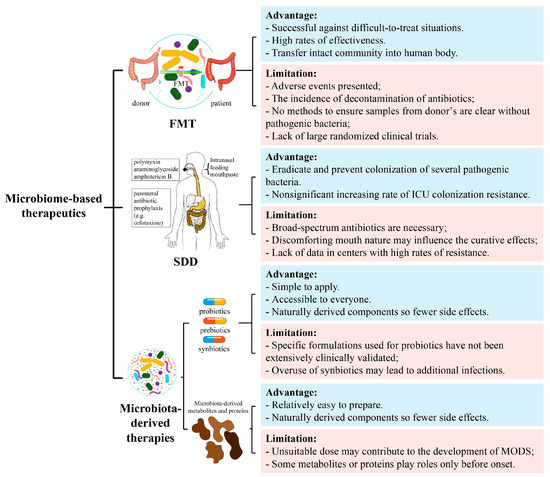
Figure 2.
Advantages and limitations of gut microbiome-based therapeutics. Including FMT, SDD, and microbiota-derived therapies. FMT, fecal microbiota transplantation; SDD, selectively digestive decontamination; MODS, multiple organ dysfunction syndrome.
6.1. Limitations of FMT
Adverse events in FMT include excessive flatulence, reflux, and the requirement of discontinuation of antibiotics in patients, which greatly increase complications such as E. coli bacteremia, lactobacillus bacteremia, and bacterial peritonitis [62,164,165]. More importantly, it is not entirely clear which bacteria are inhibited after FMT, and there is no suitable method to screen for potentially pathogenic bacteria in donor samples [14]. In addition, the lack of large randomized clinical trials remains a non-negligible limitation [103].
6.2. Limitations of SDD
Although SDD has been reported as an effective strategy to reduce ICU inpatient mortality, there are still some barriers to its widespread clinical use. One of the barriers is the concern that the widespread use of broad-spectrum antibiotics might promote antimicrobial-resistant organisms [166]. Additionally, it has been noted that, in patients on prolonged ventilation in the ICU, dosing procedures can be optimized due to the discomforting nature of oral creams and the reduced access of gastric suspensions to the upper gastrointestinal tract, which may bias in clinical trials [68]. Indeed, little data is available in centers with high rates of resistance and without long-term follow-ups, and the effect of SDD on the incidence of antimicrobial-resistant organisms is still unsolved [69]. Further studies will also need to assess the impact on secondary resistance and disruption patterns in the gut microbiome.
6.3. Limitations of Probiotics, Prebiotics and Synbiotics Therapies
Not all studies have shown optimistic results for microbiome-directed therapies [165,167,168], and there are also several risk factors in critical illness. On the one hand, the current clinical trial results of microbiota-directed therapy do not fully support their preventive role in critically ill patients [168,169,170]. The suitable probiotics for each dysbiosis situation are difficult to find and using them alone reduces their efficacy. Thus, combinations with other components are needed, but the specific formulation used for probiotics have not been extensively clinically validated [171]. On the other hand, overuse of synbiotics in microbiome-directed therapy not only fails to treat nosocomial infections in critically ill patients, but also leads to additional infectious complications [75].
6.4. Limitations of Intestinal Microbial Metabolite Therapy
High levels of SCFAs may have direct cytotoxic effects on pathogens and contribute to the development of MODS [144,172]. Moreover, after rectal administration or oral administration of butyrate acid, the proportion of Bacteroidetes phylum increases but the proportion of the thick-walled phylum decreases, which is detrimental to the restoration of gut microbiota homeostasis in critically ill patients [173,174]. In a variety of diseases, such as influenza, DAT treatment activates the immune system; however, it was only effective when DAT was given before the onset of infection. If it was given after the infection, it may exacerbate the progression of the disease [155].
7. Conclusions and Further Directions
FMT and SDD, as well as probiotics, prebiotics, and synbiotics, can restore the original intestinal microecology in critically ill patients, reduce the inflammatory response, and decrease the incidence of infectious complications. Gut microbial metabolites may improve the clinical condition of critically ill patients by enhancing immune tolerance and alleviating inflammatory response. Therefore, gut microbiome-based therapeutics may be applicable to critically ill adult patients.
Although current evidence suggests that gut microbiome-based therapeutics are beneficial for critically ill adult patients, there are still some issues that need to be validated in further studies in human and mouse models to continue exploring mechanisms. In our view, firstly, the toxicity and appropriate therapeutic doses of probiotics, prebiotics, synbiotics, and gut microbiota metabolites should be evaluated. Second, the appropriate composition of FMT grafts should be determined to ensure patient safety. Third, more clinical trials should be conducted for more types of critical illness, and the number of patients or cases needs to be expanded. Fourth, further molecular mechanisms of action need to be explored, which may contribute to the new therapy targets. Finally, the application of gut microbiome-based therapeutics for prevention of critical illness may be more desirable than the treatment of critical illness.
In addition to deeper discussions about the mechanisms and studies that need to be validated by mouse models, further studies of new and useful materials, technologies, and methodologies, such as lactomodulin, symbiotic microbial consortia, and engineered symbiotic bacteria, may also be a direction in gut microbiome-based therapeutics of critical illness [175].
Author Contributions
S.H. wrote the initial manuscript. X.H. and F.L. participated in reviewing and critically correcting the manuscript. P.P. contributed to the manuscript structure and supervised the work. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from The Scientific Research Program of FuRong Laboratory (No. 2023SK2101); The national key clinical specialist construction programs of China (Grant Number z047-02); the National Natural Science Foundation of China (No. 82200039); Natural Science Foundation of ChangSha (No. kq2208368); Natural Science Foundation of Hunan Province of China (No. 2023JJ30930, No. 2023JJ40982).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yamada, T.; Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Yamakawa, K.; Hamasaki, T.; Nakahori, Y.; Ohnishi, M.; Kuwagata, Y.; et al. Rapid and Sustained Long-Term Decrease of Fecal Short-Chain Fatty Acids in Critically Ill Patients With Systemic Inflammatory Response Syndrome. J. Parenter. Enter. Nutr. 2015, 39, 569–577. [Google Scholar] [CrossRef]
- Wolff, N.S.; Hugenholtz, F.; Wiersinga, W.J. The emerging role of the microbiota in the ICU. Crit. Care 2018, 22, 78. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ojima, M.; Ogura, H. Gut Microbiota and Probiotics/Synbiotics for Modulation of Immunity in Critically Ill Patients. Nutrients 2021, 13, 2439. [Google Scholar] [CrossRef]
- Mittal, R.; Coopersmith, C.M. Redefining the gut as the motor of critical illness. Trends Mol. Med. 2014, 20, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Baggs, J.; Jernigan, J.A.; Halpin, A.L.; Epstein, L.; Hatfield, K.M.; McDonald, L.C. Risk of Subsequent Sepsis Within 90 Days After a Hospital Stay by Type of Antibiotic Exposure. Clin. Infect. Dis. 2018, 66, 1004–1012. [Google Scholar] [CrossRef]
- Guidry, C.A.; Shah, P.M.; Dietch, Z.C.; Elwood, N.R.; Krebs, E.D.; Mehaffey, J.H.; Sawyer, R.G. Recent Anti-Microbial Exposure Is Associated with More Complications after Elective Surgery. Surg. Infect. 2018, 19, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Coopersmith, C.M. Gut integrity in critical illness. J. Intensive Care 2019, 7, 17. [Google Scholar] [CrossRef]
- Adelman, M.W.; Woodworth, M.H.; Langelier, C.; Busch, L.M.; Kempker, J.A.; Kraft, C.S.; Martin, G.S. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit. Care 2020, 24, 278. [Google Scholar] [CrossRef]
- Chernevskaya, E.; Klimenko, N.; Pautova, A.; Buyakova, I.; Tyakht, A.; Beloborodova, N. Host-Microbiome Interactions Mediated by Phenolic Metabolites in Chronically Critically Ill Patients. Metabolites 2021, 11, 122. [Google Scholar] [CrossRef]
- Ravi, A.; Halstead, F.D.; Bamford, A.; Casey, A.; Thomson, N.M.; van Schaik, W.; Snelson, C.; Goulden, R.; Foster-Nyarko, E.; Savva, G.M.; et al. Loss of microbial diversity and pathogen domination of the gut microbiota in critically ill patients. Microb. Genom. 2019, 5, e000293. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol. 2019, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Keskey, R.; Cone, J.T.; DeFazio, J.R.; Alverdy, J.C. The use of fecal microbiota transplant in sepsis. Transl. Res. 2020, 226, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, J.; Wang, J.; Yang, Y.; Huang, J.; Gong, H.; Cui, H.; Chen, D. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit. Care 2016, 20, 332. [Google Scholar] [CrossRef]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, J.; Grzesiowski, P.; Sorensen, N.; Madry, K.; Muszynski, J.; Robak, K.; Wroblewska, M.; Dzieciatkowski, T.; Dulny, G.; Dwilewicz-Trojaczek, J.; et al. Fecal Microbiota Transplantation in Patients With Blood Disorders Inhibits Gut Colonization With Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin. Infect. Dis. 2017, 65, 364–370. [Google Scholar] [CrossRef]
- Ianiro, G.; Murri, R.; Sciumè, G.D.; Impagnatiello, M.; Masucci, L.; Ford, A.C.; Law, G.R.; Tilg, H.; Sanguinetti, M.; Cauda, R.; et al. Incidence of Bloodstream Infections, Length of Hospital Stay, and Survival in Patients With Recurrent Clostridioides difficile Infection Treated With Fecal Microbiota Transplantation or Antibiotics: A Prospective Cohort Study. Ann. Intern. Med. 2019, 171, 695–702. [Google Scholar] [CrossRef]
- Ho, K.M.; Kalgudi, S.; Corbett, J.M.; Litton, E. Gut microbiota in surgical and critically ill patients. Anaesth. Intensive Care 2020, 48, 179–195. [Google Scholar] [CrossRef]
- Sharma, A.; Roy, A.; Premkumar, M.; Verma, N.; Duseja, A.; Taneja, S.; Grover, S.; Chopra, M.; Dhiman, R.K. Fecal microbiota transplantation in alcohol-associated acute-on-chronic liver failure: An open-label clinical trial. Hepatol. Int. 2022, 16, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, O.; Del Campo-Albendea, L.; de Aledo, A.L.G.; Añón, J.M.; Rodríguez-Solís, C.; Mancebo, J.; Vera, P.; Ballesteros, D.; Jiménez, J.; Maseda, E.; et al. Long-term survival of mechanically ventilated patients with severe COVID-19: An observational cohort study. Ann. Intensive Care 2021, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, S.B.; Figaroa, G.; van der Voort, P.H.J.; Nijsten, M.W.; Pillay, J. Ventilator-associated pneumonia in critically-ill patients with COVID-19 in a setting of selective decontamination of the digestive tract. Crit. Care 2021, 25, 445. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Hirose, T.; Ogura, H. Efficacy of probiotics in the prevention of diarrhea in ventilated critically ill ICU patients: Meta-analysis of randomized control trials. J. Intensive Care 2021, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Yamada, T.; Ogura, H.; Mohri, T.; Kiguchi, T.; Fujimi, S.; Asahara, T.; Yamada, T.; Ojima, M.; Ikeda, M.; et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: A randomized controlled trial. Crit. Care 2018, 22, 239. [Google Scholar] [CrossRef]
- Batra, P.; Soni, K.D.; Mathur, P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: An updated systematic review and meta-analysis of randomized control trials. J. Intensive Care 2020, 8, 81. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Hamishehkar, H.; Asghari, R.; Abri, R.; Shadvar, K.; Sanaie, S. Effect of a Probiotic Preparation on Ventilator-Associated Pneumonia in Critically Ill Patients Admitted to the Intensive Care Unit: A Prospective Double-Blind Randomized Controlled Trial. Nutr. Clin. Pract. 2019, 34, 156–162. [Google Scholar] [CrossRef]
- Mullish, B.H.; Marchesi, J.R.; McDonald, J.A.K.; Pass, D.A.; Masetti, G.; Michael, D.R.; Plummer, S.; Jack, A.A.; Davies, T.S.; Hughes, T.R.; et al. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: Should we be considering probiotics during viral pandemics? Gut Microbes 2021, 13, 1900997. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Borrazzo, C.; Pinacchio, C.; Santinelli, L.; Innocenti, G.P.; Cavallari, E.N.; Celani, L.; Marazzato, M.; Alessandri, F.; Ruberto, F.; et al. Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study. Front. Nutr. 2020, 7, 613928. [Google Scholar] [CrossRef]
- Alghetaa, H.; Mohammed, A.; Zhou, J.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Resveratrol-mediated attenuation of superantigen-driven acute respiratory distress syndrome is mediated by microbiota in the lungs and gut. Pharmacol. Res. 2021, 167, 105548. [Google Scholar] [CrossRef]
- Li, L.; Fang, Z.; Liu, X.; Hu, W.; Lu, W.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes. PLoS ONE 2020, 15, e0231865. [Google Scholar] [CrossRef]
- Neamah, W.H.; Busbee, P.B.; Alghetaa, H.; Abdulla, O.A.; Nagarkatti, M.; Nagarkatti, P. AhR Activation Leads to Alterations in the Gut Microbiome with Consequent Effect on Induction of Myeloid Derived Suppressor Cells in a CXCR2-Dependent Manner. Int. J. Mol. Sci. 2020, 21, 9613. [Google Scholar] [CrossRef]
- Tungsanga, S.; Katavetin, P.; Panpetch, W.; Udompornpitak, K.; Saisorn, W.; Praditpornsilpa, K.; Eiam-Ong, S.; Tungsanga, K.; Tumwasorn, S.; Leelahavanichkul, A. Lactobacillus rhamnosus L34 attenuates chronic kidney disease progression in a 5/6 nephrectomy mouse model through the excretion of anti-inflammatory molecules. Nephrol. Dial. Transplant. 2022, 37, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Saiedi, S.; Ebrahimi, B.; Alizadeh, M.; Khajebishak, Y.; Ghadimi, S.S. Probiotics Supplementation on Cardiac Remodeling Following Myocardial Infarction: A Single-Center Double-Blind Clinical Study. J. Cardiovasc. Transl. Res. 2021, 14, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Moscoso, D.I.; Porter, J.; Krishnareddy, S.; Abrams, J.A.; Seres, D.; Chong, D.H.; Freedberg, D.E. Relationship Between Dietary Fiber Intake and Short-Chain Fatty Acid-Producing Bacteria During Critical Illness: A Prospective Cohort Study. J. Parenter. Enter. Nutr. 2020, 44, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Toraldo, D.M. Targeting the gut-lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Ther. Adv. Respir. Dis. 2020, 14, 1753466620937170. [Google Scholar] [CrossRef] [PubMed]
- Hawryłkowicz, V.; Lietz-Kijak, D.; Kaźmierczak-Siedlecka, K.; Sołek-Pastuszka, J.; Stachowska, L.; Folwarski, M.; Parczewski, M.; Stachowska, E. Patient Nutrition and Probiotic Therapy in COVID-19: What Do We Know in 2021? Nutrients 2021, 13, 3385. [Google Scholar] [CrossRef] [PubMed]
- Cigarran Guldris, S.; González Parra, E.; Cases Amenós, A. Gut microbiota in chronic kidney disease. Nefrologia 2017, 37, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zeng, Q.; Li, K.X.; Wang, Y.; Wang, L.; Sun, M.W.; Zeng, J.; Jiang, H. Efficacy of probiotics or synbiotics for critically ill adult patients: A systematic review and meta-analysis of randomized controlled trials. Burn. Trauma 2022, 10, tkac004. [Google Scholar] [CrossRef] [PubMed]
- Rohith, G.; Sureshkumar, S.; Anandhi, A.; Kate, V.; Rajesh, B.S.; Abdulbasith, K.M.; Nanda, N.; Palanivel, C.; Vijayakumar, C. Effect of Synbiotics in Reducing the Systemic Inflammatory Response and Septic Complications in Moderately Severe and Severe Acute Pancreatitis: A Prospective Parallel-Arm Double-Blind Randomized Trial. Dig. Dis. Sci. 2023, 68, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.; Pregartner, G.; Schemmer, P. Effects of both Pro- and Synbiotics in Liver Surgery and Transplantation with Special Focus on the Gut-Liver Axis-A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2461. [Google Scholar] [CrossRef]
- Lopes, R.; Theodoro, J.M.V.; da Silva, B.P.; Queiroz, V.A.V.; de Castro Moreira, M.E.; Mantovani, H.C.; Hermsdorff, H.H.; Martino, H.S.D. Synbiotic meal decreases uremic toxins in hemodialysis individuals: A placebo-controlled trial. Food Res. Int. 2019, 116, 241–248. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Kabata, D.; Shintani, A.; Tasaki, O.; Ojima, M.; Ikeda, M.; Shimazu, T. Association of prophylactic synbiotics with reduction in diarrhea and pneumonia in mechanically ventilated critically ill patients: A propensity score analysis. J. Infect. Chemother. 2018, 24, 795–801. [Google Scholar] [CrossRef]
- Haak, B.W.; Prescott, H.C.; Wiersinga, W.J. Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis. Front. Immunol. 2018, 9, 2042. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Zuo, T.; Yeoh, Y.K.; Liu, Q.; Zhang, L.; Zhan, H.; Lu, W.; Xu, W.; Lui, G.C.Y.; et al. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients With COVID-19. Gastroenterology 2022, 162, 548–561.e544. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, J.; Kou, Y.; Liu, M.; Meng, L.; Zheng, X.; Xu, S.; Liang, M.; Sun, H.; Liu, Z.; et al. The intestinal microbial metabolite desaminotyrosine is an anti-inflammatory molecule that modulates local and systemic immune homeostasis. FASEB J. 2020, 34, 16117–16128. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Al-Ishaq, R.K.; Abotaleb, M.; Nosal, V.; Kajo, K.; Ashrafizadeh, M.; et al. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021, 138, 111430. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef]
- Kim, S.M.; DeFazio, J.R.; Hyoju, S.K.; Sangani, K.; Keskey, R.; Krezalek, M.A.; Khodarev, N.N.; Sangwan, N.; Christley, S.; Harris, K.G.; et al. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat. Commun. 2020, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Li, Y.; Zhao, H.; He, C.; Wang, Z.; Zhao, H. Fecal Microbiota Transplantation Protects the Intestinal Mucosal Barrier by Reconstructing the Gut Microbiota in a Murine Model of Sepsis. Front. Cell. Infect. Microbiol. 2021, 11, 736204. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Shi, L.; Kong, X.L.; Li, K.Y.; Li, H.; Jiang, D.X.; Zhang, F.; Zhou, Z.G. Gut Microbiota Protected Against pseudomonas aeruginosa Pneumonia via Restoring Treg/Th17 Balance and Metabolism. Front. Cell. Infect. Microbiol. 2022, 12, 856633. [Google Scholar] [CrossRef]
- Lou, X.; Xue, J.; Shao, R.; Yang, Y.; Ning, D.; Mo, C.; Wang, F.; Chen, G. Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances. Front. Immunol. 2022, 13, 1063543. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yin, G.F.; Wang, Y.L.; Tan, Y.M.; Huang, C.L.; Fan, X.M. Impact of fecal microbiota transplantation on TGF-β1/Smads/ERK signaling pathway of endotoxic acute lung injury in rats. 3 Biotech. 2020, 10, 52. [Google Scholar] [CrossRef]
- Goloshchapov, O.V.; Olekhnovich, E.I.; Sidorenko, S.V.; Moiseev, I.S.; Kucher, M.A.; Fedorov, D.E.; Pavlenko, A.V.; Manolov, A.I.; Gostev, V.V.; Veselovsky, V.A.; et al. Long-term impact of fecal transplantation in healthy volunteers. BMC Microbiol. 2019, 19, 312. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Moore, T.; Rodriguez, A.; Bakken, J.S. Fecal microbiota transplantation: A practical update for the infectious disease specialist. Clin. Infect. Dis. 2014, 58, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, X.X.; Hong, M.; Huang, X.Z.; Chen, H.; Xu, J.H.; Wang, C.; Zhang, Y.X.; Zhong, J.X.; Nie, H.; et al. Sodium butyrate alleviates LPS-induced acute lung injury in mice via inhibiting HMGB1 release. Int. Immunopharmacol. 2018, 56, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, Y.; Chen, W.; Buch, H.; Shan, Y.; Chang, L.; Bai, Y.; Shen, C.; Zhang, X.; Huo, Y.; et al. Rescue fecal microbiota transplantation for antibiotic-associated diarrhea in critically ill patients. Crit. Care 2019, 23, 324. [Google Scholar] [CrossRef]
- Giles, E.M.; D’Adamo, G.L.; Forster, S.C. The future of faecal transplants. Nat. Rev. Microbiol. 2019, 17, 719. [Google Scholar] [CrossRef] [PubMed]
- Stoutenbeek, C.P.; van Saene, H.K.; Miranda, D.R.; Zandstra, D.F. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med. 1984, 10, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.J.; Duncan, E.M.; Prior, M.E.; Maclennan, G.; Marshall, A.P.; Wells, E.C.; Todd, L.; Rose, L.; Campbell, M.K.; Webster, F.; et al. Comparison of four methods for assessing the importance of attitudinal beliefs: An international Delphi study in intensive care settings. Br. J. Health Psychol. 2014, 19, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M. Selective Decontamination of the Digestive Tract: An Answer at Last? Jama 2022, 328, 2310–2311. [Google Scholar] [CrossRef]
- de Smet, A.M.; Kluytmans, J.A.; Cooper, B.S.; Mascini, E.M.; Benus, R.F.; van der Werf, T.S.; van der Hoeven, J.G.; Pickkers, P.; Bogaers-Hofman, D.; van der Meer, N.J.; et al. Decontamination of the digestive tract and oropharynx in ICU patients. N. Engl. J. Med. 2009, 360, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramírez, C.; Hípola-Escalada, S.; Cabrera-Santana, M.; Hernández-Viera, M.A.; Caipe-Balcázar, L.; Saavedra, P.; Artiles-Campelo, F.; Sangil-Monroy, N.; Lübbe-Vázquez, C.F.; Ruiz-Santana, S. Long-term use of selective digestive decontamination in an ICU highly endemic for bacterial resistance. Crit. Care 2018, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, J.A.; Seppelt, I.M.; Goodman, F.; Billot, L.; Correa, M.; Davis, J.S.; Gordon, A.C.; Hammond, N.E.; Iredell, J.; Li, Q.; et al. Effect of Selective Decontamination of the Digestive Tract on Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation: A Randomized Clinical Trial. Jama 2022, 328, 1911–1921. [Google Scholar] [CrossRef]
- Hammond, N.E.; Myburgh, J.; Seppelt, I.; Garside, T.; Vlok, R.; Mahendran, S.; Adigbli, D.; Finfer, S.; Gao, Y.; Goodman, F.; et al. Association Between Selective Decontamination of the Digestive Tract and In-Hospital Mortality in Intensive Care Unit Patients Receiving Mechanical Ventilation: A Systematic Review and Meta-analysis. Jama 2022, 328, 1922–1934. [Google Scholar] [CrossRef]
- Haak, B.W.; Levi, M.; Wiersinga, W.J. Microbiota-targeted therapies on the intensive care unit. Curr. Opin. Crit. Care 2017, 23, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.M.; Wischmeyer, P.E. Probiotic and synbiotic therapy in the critically ill: State of the art. Nutrition 2019, 59, 29–36. [Google Scholar] [CrossRef]
- McClave, S.A.; Patel, J.; Bhutiani, N. Should fecal microbial transplantation be used in the ICU? Curr. Opin. Crit. Care 2018, 24, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Lemieux, M.; Langlois, P.L.; Wischmeyer, P.E. Probiotic and synbiotic therapy in critical illness: A systematic review and meta-analysis. Crit. Care 2016, 19, 262. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Seifi, N.; Sedaghat, A.; Nematy, M.; Khadem-Rezaiyan, M.; Shirazinezhad, R.; Ranjbar, G.; Safarian, M. Effects of synbiotic supplementation on the serum endotoxin level, inflammatory status, and clinical outcomes of adult patients with critical illness: A randomized controlled trial. Nutr. Clin. Pract. 2022, 37, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Mousavi, S.M.; Sadeghi, A.; Salari-Moghaddam, A.; Parohan, M.; Larijani, B.; Esmaillzadeh, A. The effect of probiotics on inflammatory biomarkers: A meta-analysis of randomized clinical trials. Eur. J. Nutr. 2020, 59, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Haiser, H.J.; Gootenberg, D.B.; Chatman, K.; Sirasani, G.; Balskus, E.P.; Turnbaugh, P.J. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 2013, 341, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Matuskova, Z.; Anzenbacherova, E.; Vecera, R.; Tlaskalova-Hogenova, H.; Kolar, M.; Anzenbacher, P. Administration of a probiotic can change drug pharmacokinetics: Effect of E. coli Nissle 1917 on amidarone absorption in rats. PLoS ONE 2014, 9, e87150. [Google Scholar] [CrossRef]
- Sudeep, K.C.; Angurana, S.K. Probiotic therapy in critical illness: Does it hold water? Intensive Care Med. 2021, 47, 922–923. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; Gao, Z.; Zhang, J.; Chen, H.; Ma, S.; Liu, A.; Mo, M.; Wu, C.; Chen, D.; et al. Synbiotic Therapy Prevents Nosocomial Infection in Critically Ill Adult Patients: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials Based on a Bayesian Framework. Front. Med. 2021, 8, 693188. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xuan, W.; Yang, X.; Liu, W.; Zhang, H.; Jiang, G.; Cao, B.; Jiang, Y. Ficolin A knockout alleviates sepsis-induced severe lung injury in mice by restoring gut Akkermansia to inhibit S100A4/STAT3 pathway. Int. Immunopharmacol. 2023, 121, 110548. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Lu, C.; Qiu, L.; Song, X.; Jia, H.; Cui, D.; Zhang, G. The efficacy of probiotics in patients with severe COVID-19. Ann. Palliat. Med. 2021, 10, 12374–12380. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.K.; Wu, Y.Y.; Yang, Y.F.; Ting, I.W.; Lin, C.C.; Yen, T.H.; Chen, J.H.; Wang, C.H.; Huang, C.C.; Lin, H.C. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2015, 6, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; Neilson, A.P.; Osterberg, K.L.; Smithson, A.T.; Englund, T.R.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity 2015, 23, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Benahmed, A.G.; Bjørklund, G. The microbiota-mediated dietary and nutritional interventions for COVID-19. Clin. Immunol. 2021, 226, 108725. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Zarour, H.M. Facts and Hopes for Gut Microbiota Interventions in Cancer Immunotherapy. Clin. Cancer Res. 2022, 28, 4370–4384. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Molan, A.L.; Liu, Z.; Plimmer, G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef]
- Yu, Q.; Yu, F.; Li, Q.; Zhang, J.; Peng, Y.; Wang, X.; Li, T.; Yin, N.; Sun, G.; Ouyang, H.; et al. Anthocyanin-Rich Butterfly Pea Flower Extract Ameliorating Low-Grade Inflammation in a High-Fat-Diet and Lipopolysaccharide-Induced Mouse Model. J. Agric. Food Chem. 2023, 71, 11941–11956. [Google Scholar] [CrossRef]
- Olas, B. Probiotics, Prebiotics and Synbiotics-A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Saghafi, F.; Bordbari, Z.; Zare-Kamali, J.; Jafari-Nedooshan, J.; Sahebnasagh, A. Investigating the effect of oral synbiotic on enteral feeding tolerance in critically ill patients: A double-blinded controlled clinical trial of gut microbiota. Nutr. Clin. Pract. 2023, 38, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cui, H.; Wang, F.; Zhang, Y.; Xu, Q.; Liu, D.; Wang, K.; Hou, S. Role of gut microbiota in postoperative complications and prognosis of gastrointestinal surgery: A narrative review. Medicine 2022, 101, e29826. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Greer, A.; Skorupski, C.; Hao, Q.; Johnstone, J.; Dionne, J.C.; Lau, V.; Manzanares, W.; Eltorki, M.; Duan, E.; et al. Probiotics in Critical Illness: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2022, 50, 1175–1186. [Google Scholar] [CrossRef]
- Lan, S.H.; Hung, S.H.; Chang, S.P.; Lu, L.C.; Lai, C.C.; Lin, W.T. Pro-, pre- and synbiotics for the prevention of incidental ventilator-associated pneumonia among critically ill patients: A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti-Infect. Ther. 2022, 20, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e1416. [Google Scholar] [CrossRef] [PubMed]
- Matzaras, R.; Nikopoulou, A.; Protonotariou, E.; Christaki, E. Gut Microbiota Modulation and Prevention of Dysbiosis as an Alternative Approach to Antimicrobial Resistance: A Narrative Review. Yale J. Biol. Med. 2022, 95, 479–494. [Google Scholar] [PubMed]
- Saha, S.; Tariq, R.; Tosh, P.K.; Pardi, D.S.; Khanna, S. Faecal microbiota transplantation for eradicating carriage of multidrug-resistant organisms: A systematic review. Clin. Microbiol. Infect. 2019, 25, 958–963. [Google Scholar] [CrossRef]
- Campos-Madueno, E.I.; Moradi, M.; Eddoubaji, Y.; Shahi, F.; Moradi, S.; Bernasconi, O.J.; Moser, A.I.; Endimiani, A. Intestinal colonization with multidrug-resistant Enterobacterales: Screening, epidemiology, clinical impact, and strategies to decolonize carriers. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 229–254. [Google Scholar] [CrossRef]
- Ott, S.J.; Waetzig, G.H.; Rehman, A.; Moltzau-Anderson, J.; Bharti, R.; Grasis, J.A.; Cassidy, L.; Tholey, A.; Fickenscher, H.; Seegert, D.; et al. Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients With Clostridium difficile Infection. Gastroenterology 2017, 152, 799–811.e797. [Google Scholar] [CrossRef] [PubMed]
- Van Treuren, W.; Dodd, D. Microbial Contribution to the Human Metabolome: Implications for Health and Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 345–369. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Yin, Y.; Sichler, A.; Ecker, J.; Laschinger, M.; Liebisch, G.; Höring, M.; Basic, M.; Bleich, A.; Zhang, X.J.; Kübelsbeck, L.; et al. Gut microbiota promote liver regeneration through hepatic membrane phospholipid biosynthesis. J. Hepatol. 2023, 78, 820–835. [Google Scholar] [CrossRef]
- Hua, Q.; Han, Y.; Zhao, H.; Zhang, H.; Yan, B.; Pei, S.; He, X.; Li, Y.; Meng, X.; Chen, L.; et al. Punicalagin alleviates renal injury via the gut-kidney axis in high-fat diet-induced diabetic mice. Food Funct. 2022, 13, 867–879. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Han, Y.; Chen, X.; Gong, P.; Zhai, P.; Yao, W.; Ba, Q.; Wang, H. Eucommia bark/leaf extract improves HFD-induced lipid metabolism disorders via targeting gut microbiota to activate the Fiaf-LPL gut-liver axis and SCFAs-GPR43 gut-fat axis. Phytomedicine 2023, 110, 154652. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Matsiras, D.; Bezati, S.; Ventoulis, I.; Verras, C.; Parissis, J.; Polyzogopoulou, E. Gut Failure: A Review of the Pathophysiology and Therapeutic Potentials in the Gut-Heart Axis. J. Clin. Med. 2023, 12, 2567. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Tian, M.; Zhang, X.; Jin, X.; Jiang, Y.; Sun, X.; Wang, Y.; Peng, P.; Liu, J.; Xia, C.; et al. Butyrate enhances CPT1A activity to promote fatty acid oxidation and iTreg differentiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2014681118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, J.; Wu, Q.; Fang, H.; Shao, X.; Ouyang, X.; He, Z.; Deng, Y.; Chen, C. Gut Microbiota Mediates the Susceptibility of Mice to Sepsis-Associated Encephalopathy by Butyric Acid. J. Inflamm. Res. 2022, 15, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2017, 66, 548–563. [Google Scholar] [CrossRef]
- Li, X.Y.; He, C.; Zhu, Y.; Lu, N.H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 2020, 26, 2187–2193. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e437. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Melhem, H.; Kaya, B.; Ayata, C.K.; Hruz, P.; Niess, J.H. Metabolite-Sensing G Protein-Coupled Receptors Connect the Diet-Microbiota-Metabolites Axis to Inflammatory Bowel Disease. Cells 2019, 8, 450. [Google Scholar] [CrossRef]
- Liu, J.; Chang, G.; Huang, J.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X. Sodium Butyrate Inhibits the Inflammation of Lipopolysaccharide-Induced Acute Lung Injury in Mice by Regulating the Toll-Like Receptor 4/Nuclear Factor κB Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1674–1682. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Hebeda, C.B.; Farsky, S.H.; Curi, R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009, 117, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Hatanaka, E.; Lambertucci, R.H.; Newsholme, P.; Curi, R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem. Funct. 2009, 27, 48–55. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Wattanaphichet, P.; Chatsudthipong, V.; Muanprasat, C. GPR40 receptor activation promotes tight junction assembly in airway epithelial cells via AMPK-dependent mechanisms. Tissue Barriers 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, F.; Zhang, C.; Zhang, Q.; Su, X.; Zhu, X.; Liu, A.; Shi, W.; Lin, W.; Jin, Z.; et al. Melatonin alleviates titanium nanoparticles induced osteolysis via activation of butyrate/GPR109A signaling pathway. J. Nanobiotechnology 2021, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Wu, H.; Xu, Z.; Xi, H.; Chen, C.; Chen, D.; Gong, Y.; Hua, Y.; Wang, Z. The role of propionic acid at diagnosis predicts mortality in patients with septic shock. J. Crit. Care 2018, 43, 95–101. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Xu, M.; Wang, C.; Li, N.; Wang, J.; Zhang, Y.; Deng, X. Intraperitoneal Injection of Acetate Protects Mice Against Lipopolysaccharide (LPS)-Induced Acute Lung Injury Through Its Anti-Inflammatory and Anti-Oxidative Ability. Med. Sci. Monit. 2019, 25, 2278–2288. [Google Scholar] [CrossRef]
- Ji, J.J.; Sun, Q.M.; Nie, D.Y.; Wang, Q.; Zhang, H.; Qin, F.F.; Wang, Q.S.; Lu, S.F.; Pang, G.M.; Lu, Z.G. Probiotics protect against RSV infection by modulating the microbiota-alveolar-macrophage axis. Acta Pharmacol. Sin. 2021, 42, 1630–1641. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, H.; Wen, X.; Ho, C.T.; Li, S. Citrus flavonoids and the intestinal barrier: Interactions and effects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Joardder, M.U.H.; Kumar, C.; Karim, M.A. Prediction of porosity of food materials during drying: Current challenges and directions. Crit. Rev. Food Sci. Nutr. 2018, 58, 2896–2907. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2691–2709. [Google Scholar] [CrossRef]
- Lambert, M.A.; Moss, C.W. Production of p-hydroxyhydrocinnamic acid from tyrosine by Peptostreptococcus anaerobius. J. Clin. Microbiol. 1980, 12, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Hu, L.; Wu, C.; Zhang, Z.; Liu, M.; Maruthi Prasad, E.; Chen, Y.; Wang, K. Pinocembrin Protects Against Dextran Sulfate Sodium-Induced Rats Colitis by Ameliorating Inflammation, Improving Barrier Function and Modulating Gut Microbiota. Front. Physiol. 2019, 10, 908. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of Flavonoids on Inflammasome Activation during Inflammatory Responses. Mol. Nutr. Food Res. 2018, 62, e1800147. [Google Scholar] [CrossRef] [PubMed]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef]
- Somerville, V.S.; Braakhuis, A.J.; Hopkins, W.G. Effect of Flavonoids on Upper Respiratory Tract Infections and Immune Function: A Systematic Review and Meta-Analysis. Adv. Nutr. 2016, 7, 488–497. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef]
- Fang, H.; Fang, M.; Wang, Y.; Zhang, H.; Li, J.; Chen, J.; Wu, Q.; He, L.; Xu, J.; Deng, J.; et al. Indole-3-Propionic Acid as a Potential Therapeutic Agent for Sepsis-Induced Gut Microbiota Disturbance. Microbiol. Spectr. 2022, 10, e0012522. [Google Scholar] [CrossRef]
- Beloborodova, N.V.; Olenin, A.Y.; Pautova, A.K. Metabolomic findings in sepsis as a damage of host-microbial metabolism integration. J. Crit. Care 2018, 43, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Chernevskaya, E.; Beloborodova, N.; Klimenko, N.; Pautova, A.; Shilkin, D.; Gusarov, V.; Tyakht, A. Serum and fecal profiles of aromatic microbial metabolites reflect gut microbiota disruption in critically ill patients: A prospective observational pilot study. Crit. Care 2020, 24, 312. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail. 2015, 21, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Szychowiak, P.; Villageois-Tran, K.; Patrier, J.; Timsit, J.F.; Ruppé, É. The role of the microbiota in the management of intensive care patients. Ann. Intensive Care 2022, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Corriero, A.; Gadaleta, R.M.; Puntillo, F.; Inchingolo, F.; Moschetta, A.; Brienza, N. The central role of the gut in intensive care. Crit. Care 2022, 26, 379. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Flett, K.B.; Merakou, C.; Mehrotra, P.; Stam, J.; Snesrud, E.; Hinkle, M.; Lesho, E.; McGann, P.; McAdam, A.J.; et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 2019, 25, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Sarwar, S.; Fowler, R.A.; Cuthbertson, B.H. Effect of selective decontamination on antimicrobial resistance in intensive care units: A systematic review and meta-analysis. Lancet. Infect. Dis. 2013, 13, 328–341. [Google Scholar] [CrossRef]
- Majid, H.A.; Cole, J.; Emery, P.W.; Whelan, K. Additional oligofructose/inulin does not increase faecal bifidobacteria in critically ill patients receiving enteral nutrition: A randomised controlled trial. Clin. Nutr. 2014, 33, 966–972. [Google Scholar] [CrossRef]
- Johnstone, J.; Meade, M.; Lauzier, F.; Marshall, J.; Duan, E.; Dionne, J.; Arabi, Y.M.; Heels-Ansdell, D.; Thabane, L.; Lamarche, D.; et al. Effect of Probiotics on Incident Ventilator-Associated Pneumonia in Critically Ill Patients: A Randomized Clinical Trial. Jama 2021, 326, 1024–1033. [Google Scholar] [CrossRef]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar] [CrossRef]
- Millar, M.; Seale, J.; Greenland, M.; Hardy, P.; Juszczak, E.; Wilks, M.; Panton, N.; Costeloe, K.; Wade, W.G. The Microbiome of Infants Recruited to a Randomised Placebo-controlled Probiotic Trial (PiPS Trial). EBioMedicine 2017, 20, 255–262. [Google Scholar] [CrossRef]
- Wan, Y.D.; Zhu, R.X.; Wu, Z.Q.; Lyu, S.Y.; Zhao, L.X.; Du, Z.J.; Pan, X.T. Gut Microbiota Disruption in Septic Shock Patients: A Pilot Study. Med. Sci. Monit. 2018, 24, 8639–8646. [Google Scholar] [CrossRef] [PubMed]
- Bohnhoff, M.; Miller, C.P.; Martin, W.R. Resistance of the mouse’s intestinal tract to experimental salmonella infection. i. factors which interfere with the initiation of infection by oral inoculation. J. Exp. Med. 1964, 120, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Jiminez, J.A.; Uwiera, T.C.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Butyrate Supplementation at High Concentrations Alters Enteric Bacterial Communities and Reduces Intestinal Inflammation in Mice Infected with Citrobacter rodentium. mSphere 2017, 2, e00243-17. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Ghemrawi, R.; Abu-Izneid, T.; Ramadan, A.; Al-Marzooq, F. Discovery of Lactomodulin, a Unique Microbiome-Derived Peptide That Exhibits Dual Anti-Inflammatory and Antimicrobial Activity against Multidrug-Resistant Pathogens. Int. J. Mol. Sci. 2023, 24, 6901. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).