Nanoparticles of Lactiplantibacillus plantarum K8 Reduce Staphylococcus aureus Respiratory Infection and Tumor Necrosis Factor Alpha- and Interferon Gamma-Induced Lung Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of K8 Nanoparticles, K8NPs
2.2. In Vivo Study
2.2.1. Bacterial Culture and Infection of Mice
2.2.2. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis Using Mouse Serum
2.2.3. Hematoxylin and Eosin Staining of Lung Tissue
Immunohistochemistry
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. ELISA Analysis Using Cell Culture Supernatants
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
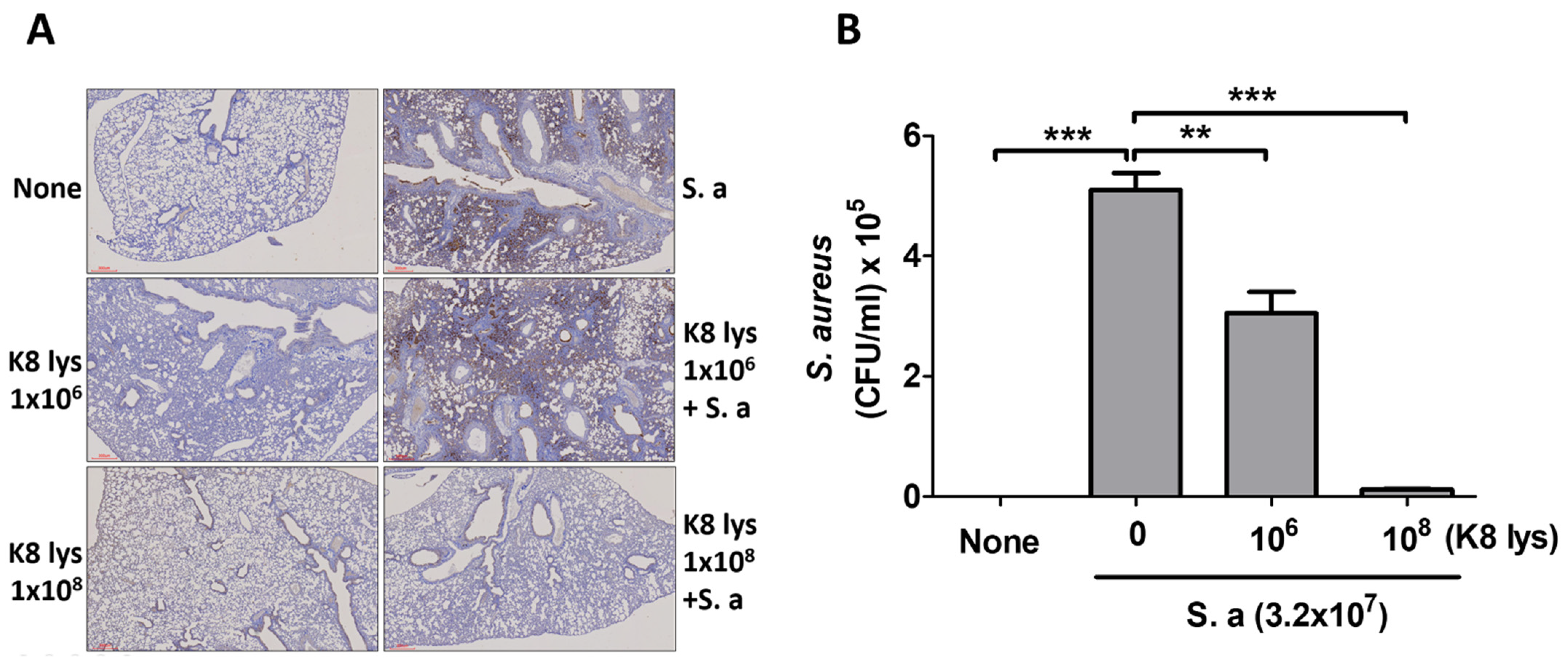
3.1. K8NPs Reduced S. aureus Lung Infection in Mice
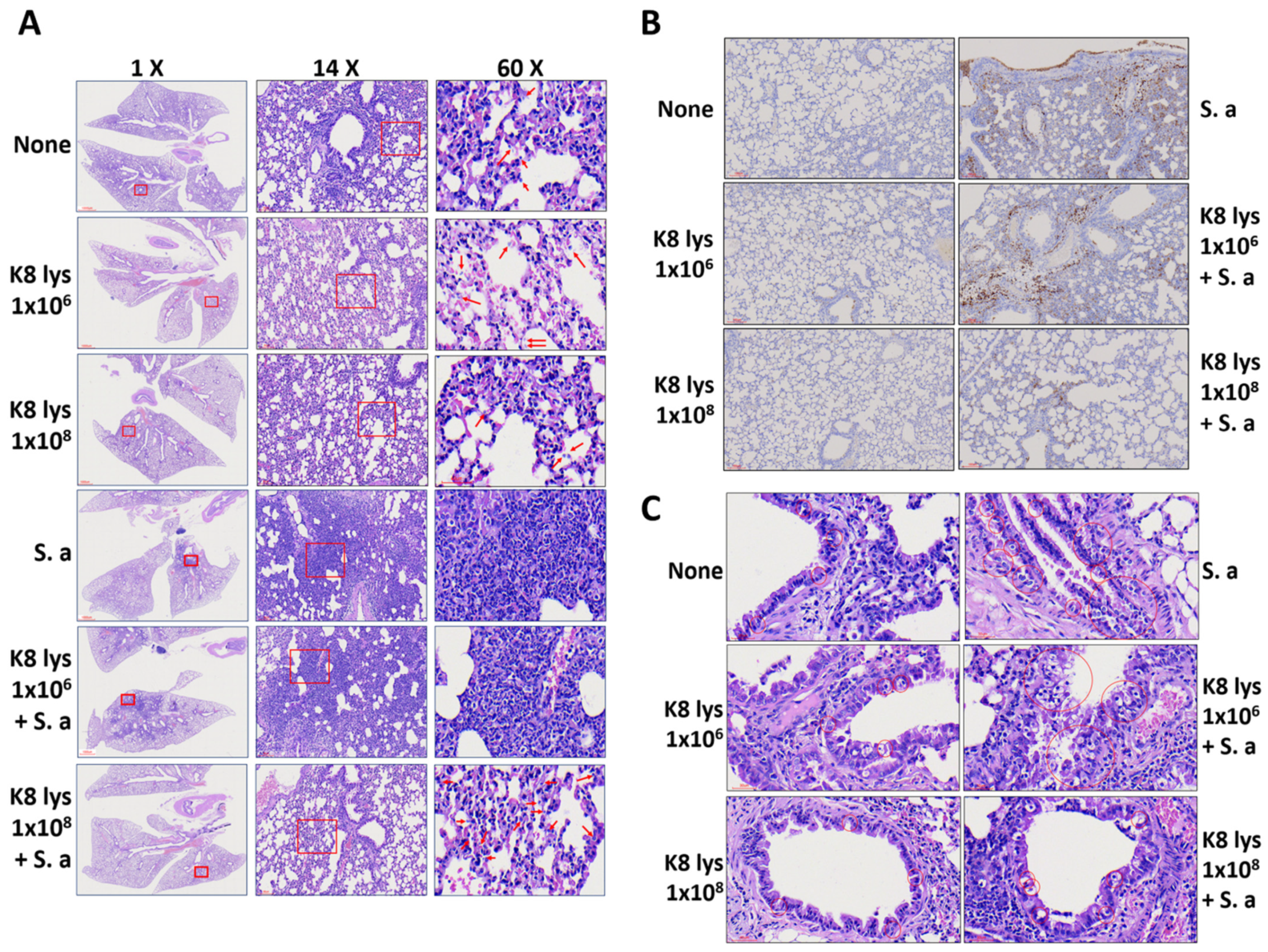
3.2. K8NPs Restored Alveolar Structure Damaged by S. aureus Infection
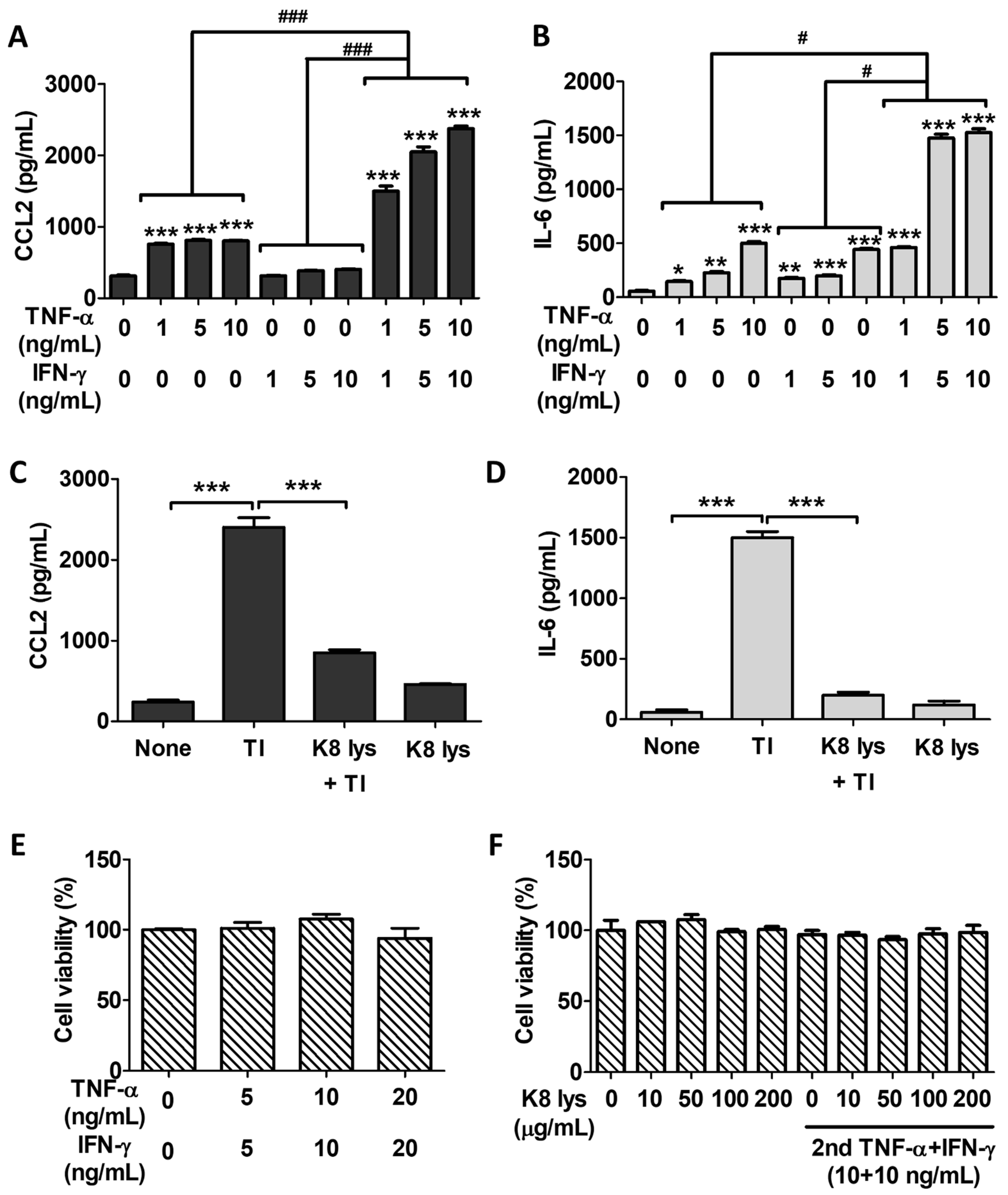
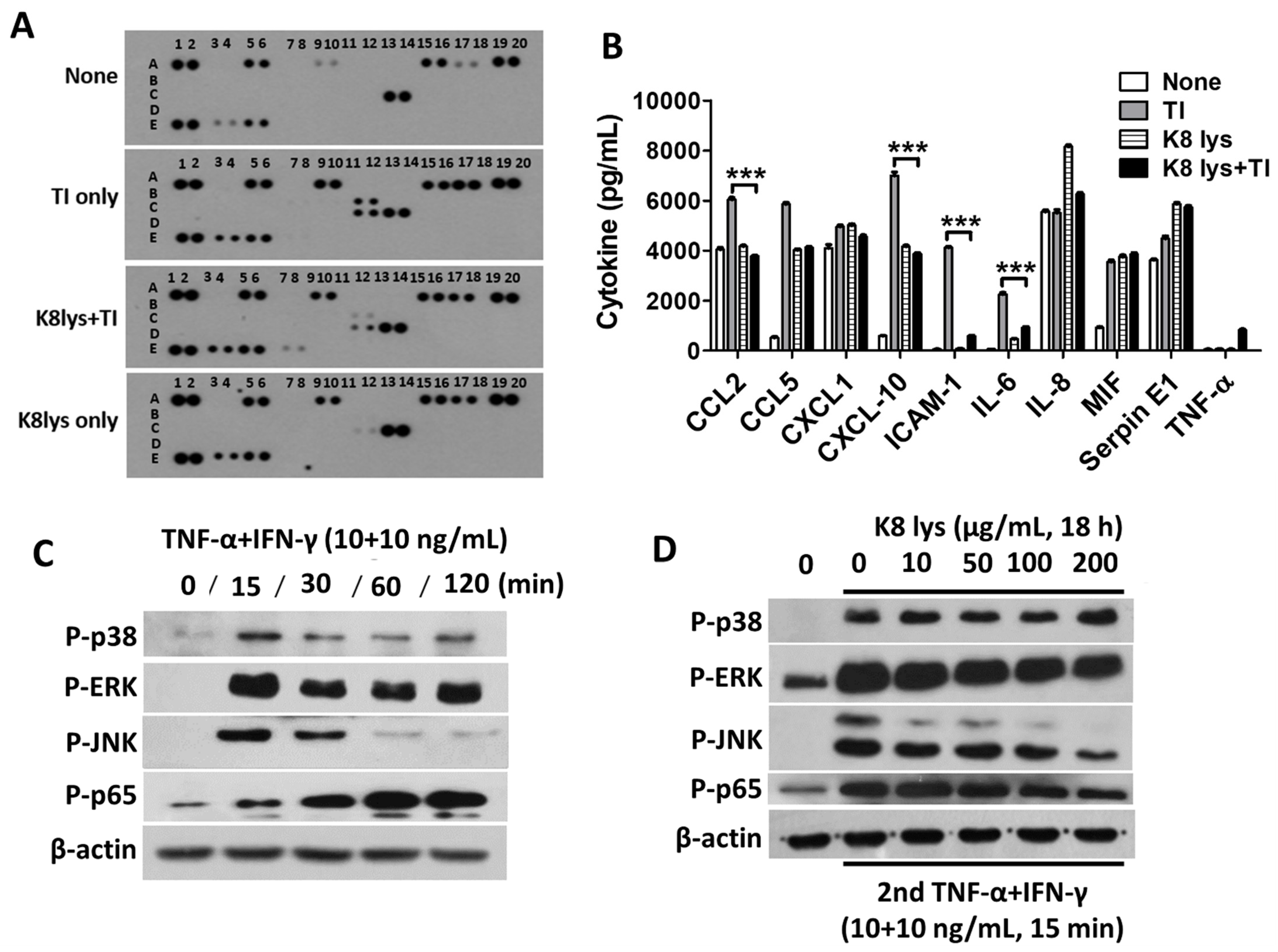
3.3. Oral Administration of K8NPs Reduced CCL2 Production, Which Was Increased by S. aureus Infection or TI Injection
3.4. K8NPs Inhibited TI-Induced CCL2 and IL-6 Production in A549 Cells
3.5. Inhibition of Pro-Inflammatory Cytokines in TI-Treated A549 Cells Mediated by the Inactivation of Mitogen-Activated Protein Kinases and Nuclear Factor Kappa B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bates, D.V.; Hansen-Flaschen, J. Respiratory Disease. Encyclopedia Britannica. Available online: https://www.britannica.com/science/respiratory-disease (accessed on 24 August 2023).
- Pomés, A.; Chapman, M.D.; Wünschmann, S. Indoor Allergens and Allergic Respiratory Disease. Curr. Allergy Asthma Rep. 2016, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Dondi, A.; Carbone, C.; Manieri, E.; Zama, D.; Del Bono, C.; Betti, L.; Biagi, C.; Lanari, M. Outdoor Air Pollution and Childhood Respiratory Disease: The Role of Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 4345. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Prince, A. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin. Immunopathol. 2012, 34, 281–297. [Google Scholar] [CrossRef]
- Flora, M.; Perrotta, F.; Nicolai, A.; Maffucci, R.; Pratillo, A.; Mollica, M.; Bianco, A.; Calabrese, C. Staphylococcus aureus in chronic airway diseases: An overview. Respir. Med. 2019, 155, 66–71. [Google Scholar] [CrossRef]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2001, 34, 50s–59s. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef]
- Lai, C.; Wang, K.; Zhao, Z.; Zhang, L.; Gu, H.; Yang, P.; Wang, X. C-C Motif Chemokine Ligand 2 (CCL2) Mediates Acute Lung Injury Induced by Lethal Influenza H7N9 Virus. Front. Microbiol. 2017, 8, 587. [Google Scholar] [CrossRef]
- Patel, R.; Naqvi, S.A.; Griffiths, C.; Bloom, C.I. Systemic adverse effects from inhaled corticosteroid use in asthma: A systematic review. BMJ Open Respir. Res. 2020, 7, e000756. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Cakler, P.C.; Reed, S.C. The impact of the long-chain n-3 polyunsaturated fatty acids on human health. Nutr. Res. Rev. 2005, 18, 113–129. [Google Scholar] [CrossRef]
- Hultqvist, M.; Olofsson, P.; Gelderman, K. A new arthritis therapy with oxydative burst inducers. PLoS Med. 2006, 3, e348. [Google Scholar] [CrossRef]
- Sanchez-Mendoza, M.; Castillo-Henkel, C.; Navarrete, A. Relaxant action mechanism of berberine identified as the active principle of Argemone ochroleuca Seet in Guinea-pig tracheal smooth muscle. J. Pharm. Pharmacol. 2008, 60, 229–236. [Google Scholar] [CrossRef]
- Du, T.; Lei, A.; Zhang, N.; Zhu, C. The Beneficial Role of Probiotic Lactobacillus in Respiratory Diseases. Front. Immunol. 2022, 13, 908010. [Google Scholar] [CrossRef]
- Szydłowska, A.; Sionek, B. Probiotics and Postbiotics as the Functional Food Components Affecting the Immune Response. Microorganisms 2022, 11, 104. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.R.; Jeong, B.J.; Lee, S.S.; Kim, T.R.; Jeong, J.H.; Lee, M.; Lee, S.; Lee, J.S.; Chung, D.K. Effects of oral intake of kimchi-derived Lactobacillus plantarum K8 lysates on skin moisturizing. J. Microbiol. Biotechnol. 2015, 25, 74–80. [Google Scholar] [CrossRef]
- Kim, G.; Choi, K.H.; Kim, H.; Chung, D.K. Alleviation of LPS-Induced Inflammation and Septic Shock by Lactiplantibacillus plantarum K8 Lysates. Int. J. Mol. Sci. 2021, 22, 5921. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, Y.H.; Gho, J.Y.; Kang, G.A.; Kang, S.S. Synergistic Inhibitory Effect of Lactobacillus Cell Lysates and Butyrate on Poly I:C-Induced IL-8 Production in Human Intestinal Epithelial Cells. Probiotics Antimicrob. Proteins, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Kim, M.Y.; Hyun, I.K.; An, S.; Kim, D.; Kim, K.H.; Kang, S.S. In vitro anti-inflammatory and antibiofilm activities of bacterial lysates from lactobacilli against oral pathogenic bacteria. Food Funct. 2022, 13, 12755–12765. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef]
- Lombardi, F.; Palumbo, P.; Mattei, A.; Augello, F.R.; Cifone, M.G.; Giuliani, M.; Cinque, B. Soluble Fraction from Lysates of Selected Probiotic Strains Differently Influences Re-Epithelialization of HaCaT Scratched Monolayer through a Mechanism Involving Nitric Oxide Synthase 2. Biomolecules 2019, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, E.J.; Lee, H.J. Ameliorative Effects of Lactobacillus plantarum HAC01 Lysate on 3T3-L1 Adipocyte Differentiation via AMPK Activation and MAPK Inhibition. Int. J. Mol. Sci. 2022, 23, 5901. [Google Scholar] [CrossRef]
- Mulcahy, M.E.; Leech, J.M.; Renauld, J.C.; Mills, K.H.; McLoughlin, R.M. Interleukin-22 regulates antimicrobial peptide expression and keratinocyte differentiation to control Staphylococcus aureus colonization of the nasal mucosa. Mucosal Immunol. 2016, 9, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Moore, B.B. Lung Section Staining and Microscopy. Bio-Protocol 2017, 7, e2286. [Google Scholar] [CrossRef]
- Hoffmann, F.M.; Berger, J.L.; Lingel, I.; Laumonnier, Y.; Lewkowich, I.P.; Schmudde, I.; König, P. Distribution and Interaction of Murine Pulmonary Phagocytes in the Naive and Allergic Lung. Front. Immunol. 2018, 9, 1046. [Google Scholar] [CrossRef]
- Ordoñez, C.L.; Khashayar, R.; Wong, H.H.; Ferrando, R.; Wu, R.; Hyde, D.M.; Hotchkiss, J.A.; Zhang, Y.; Novikov, A.; Dolganov, G.; et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am. J. Respir. Criti. Care Med. 2001, 163, 517–523. [Google Scholar] [CrossRef]
- Woznicki, J.A.; Saini, N.; Flood, P.; Rajaram, S.; Lee, C.M.; Stamou, P.; Skowyra, A.; Bustamante-Garrido, M.; Regazzoni, K.; Crawford, N.; et al. TNF-α synergises with IFN-γ to induce caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial cells. Cell Death Dis. 2021, 12, 864. [Google Scholar] [CrossRef]
- Defres, S.; Marwick, C.; Nathwani, D. MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia. Eur. Respir. J. 2009, 34, 1470–1476. [Google Scholar] [CrossRef]
- Josse, J.; Laurent, F.; Diot, A. Staphylococcal Adhesion and Host Cell Invasion: Fibronectin-Binding and Other Mechanisms. Front. Microbiol. 2017, 8, 2433. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Z.; Li, Y.; Shen, P.; Hu, X.; Cao, Y.; Zhang, N. Selenium Deficiency Facilitates Inflammation Following S. aureus Infection by Regulating TLR2-Related Pathways in the Mouse Mammary Gland. Biol. Trace Elem. Res. 2016, 172, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-O.; Yu, C.; Kim, H.; Chung, D.-K. Lactoplantibacillus plantarum KG Lysates Inhibit the Internalization of Staphylococcus aureus by Human Keratinocytes through the Induction of Human Beta-Defensin 3. Appl. Sci. 2022, 12, 12504. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Kim, M.; Kim, Y.E.; Kim, H.; Lee, S.; Lee, Y.; Kim, K.Y. MSF Enhances Human Antimicrobial Peptide β-Defensin (HBD2 and HBD3) Expression and Attenuates Inflammation via the NF-κB and p38 Signaling Pathways. Molecules 2023, 28, 2744. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, N.R.; Gim, M.G.; Lee, J.M.; Lee, S.Y.; Ko, M.Y.; Kim, J.Y.; Han, S.H.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-alpha production in THP-1 cells and endotoxin shock in mice. J. Immunol. 2008, 180, 2553–2561. [Google Scholar] [CrossRef]
- Li, A.L.; Sun, Y.Q.; Du, P.; Meng, X.C.; Guo, L.; Li, S.; Zhang, C. The Effect of Lactobacillus actobacillus Peptidoglycan on Bovine β-Lactoglobulin-Sensitized Mice via TLR2/NF-κB Pathway. Iran. J. Allergy Asthma Immunol. 2017, 16, 147–158. [Google Scholar]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Ozaki, H.; Ishii, K.; Horiuchi, H.; Arai, H.; Kawamoto, T.; Okawa, K.; Iwamatsu, A.; Kita, T. Cutting edge: Combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J. Immunol. 1999, 163, 553–557. [Google Scholar] [CrossRef]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the Role of TNF-α and IFN-γ Activation on the Dynamics of iNOS Gene Expression in LPS Stimulated Macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Son, M.; Sin, J.; Kim, H.; Chung, D.-K. Nanoparticles of Lactiplantibacillus plantarum K8 Reduce Staphylococcus aureus Respiratory Infection and Tumor Necrosis Factor Alpha- and Interferon Gamma-Induced Lung Inflammation. Nutrients 2023, 15, 4728. https://doi.org/10.3390/nu15224728
Hong J, Son M, Sin J, Kim H, Chung D-K. Nanoparticles of Lactiplantibacillus plantarum K8 Reduce Staphylococcus aureus Respiratory Infection and Tumor Necrosis Factor Alpha- and Interferon Gamma-Induced Lung Inflammation. Nutrients. 2023; 15(22):4728. https://doi.org/10.3390/nu15224728
Chicago/Turabian StyleHong, Jonghyo, Minseong Son, Jaeeun Sin, Hangeun Kim, and Dae-Kyun Chung. 2023. "Nanoparticles of Lactiplantibacillus plantarum K8 Reduce Staphylococcus aureus Respiratory Infection and Tumor Necrosis Factor Alpha- and Interferon Gamma-Induced Lung Inflammation" Nutrients 15, no. 22: 4728. https://doi.org/10.3390/nu15224728
APA StyleHong, J., Son, M., Sin, J., Kim, H., & Chung, D.-K. (2023). Nanoparticles of Lactiplantibacillus plantarum K8 Reduce Staphylococcus aureus Respiratory Infection and Tumor Necrosis Factor Alpha- and Interferon Gamma-Induced Lung Inflammation. Nutrients, 15(22), 4728. https://doi.org/10.3390/nu15224728






