Nutritional Intervention in Cushing’s Disease: The Ketogenic Diet’s Effects on Metabolic Comorbidities and Adrenal Steroids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Assays
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on Diagnosis and Management of Cushing’s Disease: A Guideline Update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Barbot, M.; Zilio, M.; Scaroni, C. Cushing’s Syndrome: Overview of Clinical Presentation, Diagnostic Tools and Complications. Best. Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101380. [Google Scholar] [CrossRef]
- Guarnotta, V.; Prinzi, A.; Pitrone, M.; Pizzolanti, G.; Giordano, C. Circulating Irisin Levels as a Marker of Osteosarcopenic-Obesity in Cushing’s Disease. Diabetes Metab. Syndr. Obes. 2020, 13, 1565–1574. [Google Scholar] [CrossRef]
- Nieman, L.K. Hypertension and Cardiovascular Mortality in Patients with Cushing Syndrome. Endocrinol. Metab. Clin. N. Am. 2019, 48, 717–725. [Google Scholar] [CrossRef]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.-E.; Maki, K.C. Review of Current Evidence and Clinical Recommendations on the Effects of Low-Carbohydrate and Very-Low-Carbohydrate (Including Ketogenic) Diets for the Management of Body Weight and Other Cardiometabolic Risk Factors: A Scientific Statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond Weight Loss: A Review of the Therapeutic Uses of Very-Low-Carbohydrate (Ketogenic) Diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Benelli, M.; Brancaleoni, M.; Dainelli, G.; Merlini, D.; Negri, R. Middle and Long-Term Impact of a Very Low-Carbohydrate Ketogenic Diet on Cardiometabolic Factors: A Multi-Center, Cross-Sectional, Clinical Study. High. Blood Press. Cardiovasc. Prev. 2015, 22, 389–394. [Google Scholar] [CrossRef]
- Merra, G.; Miranda, R.; Barrucco, S.; Gualtieri, P.; Mazza, M.; Moriconi, E.; Marchetti, M.; Chang, T.F.M.; De Lorenzo, A.; Di Renzo, L. Very-Low-Calorie Ketogenic Diet with Aminoacid Supplement versus Very Low Restricted-Calorie Diet for Preserving Muscle Mass during Weight Loss: A Pilot Double-Blind Study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2613–2621. [Google Scholar]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Vargas, L.; Tamayo, A.; Buehn, R.; Peacock, C.A. A High Protein Diet Has No Harmful Effects: A One-Year Crossover Study in Resistance-Trained Males. J. Nutr. Metab. 2016, 2016, 9104792. [Google Scholar] [CrossRef]
- Guarnotta, V.; Emanuele, F.; Amodei, R.; Giordano, C. Very Low-Calorie Ketogenic Diet: A Potential Application in the Treatment of Hypercortisolism Comorbidities. Nutrients 2022, 14, 2388. [Google Scholar] [CrossRef]
- Lemmens, S.G.; Born, J.M.; Martens, E.A.; Martens, M.J.; Westerterp-Plantenga, M.S. Influence of Consumption of a High-Protein vs. High-Carbohydrate Meal on the Physiological Cortisol and Psychological Mood Response in Men and Women. PLoS ONE 2011, 6, e16826. [Google Scholar] [CrossRef]
- Galvão-Teles, A.; Graves, L.; Burke, C.W.; Fotherby, K.; Fraser, R. Free Cortisol in Obesity; Effect of Fasting. Acta Endocrinol. 1976, 81, 321–329. [Google Scholar] [CrossRef]
- Edelstein, C.K.; Roy-Byrne, P.; Fawzy, F.I.; Dornfeld, L. Effects of Weight Loss on the Dexamethasone Suppression Test. Am. J. Psychiatry 1983, 140, 338–341. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Mann, T.; Vinas, D.; Hunger, J.M.; DeJager, J.; Taylor, S.E. Low Calorie Dieting Increases Cortisol. Psychosom. Med. 2010, 72, 357–364. [Google Scholar] [CrossRef]
- Peeters, F.; Nicholson, N.A.; Berkhof, J. Cortisol Responses to Daily Events in Major Depressive Disorder. Psychosom. Med. 2003, 65, 836–841. [Google Scholar] [CrossRef]
- Martens, M.J.I.; Rutters, F.; Lemmens, S.G.T.; Born, J.M.; Westerterp-Plantenga, M.S. Effects of Single Macronutrients on Serum Cortisol Concentrations in Normal Weight Men. Physiol. Behav. 2010, 101, 563–567. [Google Scholar] [CrossRef]
- Bray, G.A.; Most, M.; Rood, J.; Redmann, S.; Smith, S.R. Hormonal Responses to a Fast-Food Meal Compared with Nutritionally Comparable Meals of Different Composition. Ann. Nutr. Metab. 2007, 51, 163–171. [Google Scholar] [CrossRef]
- Berger, M. Influence of Weight Loss on the Dexamethasone Suppression Test. Arch. Gen. Psychiatry 1983, 40, 585. [Google Scholar] [CrossRef]
- Dugandzic, M.K.; Pierre-Michel, E.-C.; Kalayjian, T. Ketogenic Diet Initially Masks Symptoms of Hypercortisolism in Cushing’s Disease. Metabolites 2022, 12, 1033. [Google Scholar] [CrossRef]
- Abbasi, J. Interest in the Ketogenic Diet Grows for Weight Loss and Type 2 Diabetes. JAMA 2018, 319, 215. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of Fats and Carbohydrate Intake with Cardiovascular Disease and Mortality in 18 Countries from Five Continents (PURE): A Prospective Cohort Study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Barbot, M.; Guarnotta, V.; Zilio, M.; Ceccato, F.; Ciresi, A.; Daniele, A.; Pizzolanti, G.; Campello, E.; Frigo, A.C.; Giordano, C.; et al. Effects of Pasireotide Treatment on Coagulative Profile: A Prospective Study in Patients with Cushing’s Disease. Endocrine 2018, 62, 207–214. [Google Scholar] [CrossRef]
- Perticone, M.; Maio, R.; Sciacqua, A.; Suraci, E.; Pinto, A.; Pujia, R.; Zito, R.; Gigliotti, S.; Sesti, G.; Perticone, F. Ketogenic Diet-Induced Weight Loss Is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules 2019, 24, 2499. [Google Scholar] [CrossRef]
- Dubuc, G.R.; Phinney, S.D.; Stern, J.S.; Havel, P.J. Changes of Serum Leptin and Endocrine and Metabolic Parameters after 7 Days of Energy Restriction in Men and Women. Metabolism 1998, 47, 429–434. [Google Scholar] [CrossRef]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11beta-Hydroxysteroid Dehydrogenase Type 1: A Tissue-Specific Regulator of Glucocorticoid Response. Endocr. Rev. 2004, 25, 831–866. [Google Scholar] [CrossRef]
- Purnell, J.Q.; Kahn, S.E.; Samuels, M.H.; Brandon, D.; Loriaux, D.L.; Brunzell, J.D. Enhanced Cortisol Production Rates, Free Cortisol, and 11beta-HSD-1 Expression Correlate with Visceral Fat and Insulin Resistance in Men: Effect of Weight Loss. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E351–E357. [Google Scholar] [CrossRef]
- Stimson, R.H.; Johnstone, A.M.; Homer, N.Z.M.; Wake, D.J.; Morton, N.M.; Andrew, R.; Lobley, G.E.; Walker, B.R. Dietary Macronutrient Content Alters Cortisol Metabolism Independently of Body Weight Changes in Obese Men. J. Clin. Endocrinol. Metab. 2007, 92, 4480–4484. [Google Scholar] [CrossRef]
- Stimson, R.H.; Mohd-Shukri, N.A.; Bolton, J.L.; Andrew, R.; Reynolds, R.M.; Walker, B.R. The Postprandial Rise in Plasma Cortisol in Men Is Mediated by Macronutrient-Specific Stimulation of Adrenal and Extra-Adrenal Cortisol Production. J. Clin. Endocrinol. Metab. 2014, 99, 160–168. [Google Scholar] [CrossRef]
- Stomby, A.; Simonyte, K.; Mellberg, C.; Ryberg, M.; Stimson, R.H.; Larsson, C.; Lindahl, B.; Andrew, R.; Walker, B.R.; Olsson, T. Diet-Induced Weight Loss Has Chronic Tissue-Specific Effects on Glucocorticoid Metabolism in Overweight Postmenopausal Women. Int. J. Obes. 2015, 39, 814–819. [Google Scholar] [CrossRef]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Heintze, U.; Janke, J.; Luft, F.C.; Sharma, A.M. Regulation of 11beta-HSD Genes in Human Adipose Tissue: Influence of Central Obesity and Weight Loss. Obes. Res. 2004, 12, 9–17. [Google Scholar] [CrossRef]
- Woods, C.P.; Corrigan, M.; Gathercole, L.; Taylor, A.; Hughes, B.; Gaoatswe, G.; Manolopoulos, K.; Hogan, A.E.; O’Connell, J.; Stewart, P.M.; et al. Tissue Specific Regulation of Glucocorticoids in Severe Obesity and the Response to Significant Weight Loss Following Bariatric Surgery (BARICORT). J. Clin. Endocrinol. Metab. 2015, 100, 1434–1444. [Google Scholar] [CrossRef]
- Nakamura, Y.; Walker, B.R.; Ikuta, T. Systematic Review and Meta-Analysis Reveals Acutely Elevated Plasma Cortisol Following Fasting but Not Less Severe Calorie Restriction. Stress 2016, 19, 151–157. [Google Scholar] [CrossRef]
- Yang, Z.; Mi, J.; Wang, Y.; Xue, L.; Liu, J.; Fan, M.; Zhang, D.; Wang, L.; Qian, H.; Li, Y. Effects of Low-Carbohydrate Diet and Ketogenic Diet on Glucose and Lipid Metabolism in Type 2 Diabetic Mice. Nutrition 2021, 89, 111230. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Q.; Tominaga, T.; Liu, C.; Suzuki, K. An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice. Nutrients 2018, 10, 1696. [Google Scholar] [CrossRef]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569. [Google Scholar] [CrossRef]
- Mavropoulos, J.C.; Yancy, W.S.; Hepburn, J.; Westman, E.C. The Effects of a Low-Carbohydrate, Ketogenic Diet on the Polycystic Ovary Syndrome: A Pilot Study. Nutr. Metab. 2005, 2, 35. [Google Scholar] [CrossRef]
- Gower, B.A.; Chandler-Laney, P.C.; Ovalle, F.; Goree, L.L.; Azziz, R.; Desmond, R.A.; Granger, W.M.; Goss, A.M.; Bates, G.W. Favourable Metabolic Effects of a Eucaloric Lower-Carbohydrate Diet in Women with PCOS. Clin. Endocrinol. 2013, 79, 550–557. [Google Scholar] [CrossRef]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018, 3, 280. [Google Scholar] [CrossRef]
- Palgi, A.; Read, J.L.; Greenberg, I.; Hoefer, M.A.; Bistrian, B.R.; Blackburn, G.L. Multidisciplinary Treatment of Obesity with a Protein-Sparing Modified Fast: Results in 668 Outpatients. Am. J. Public Health 1985, 75, 1190–1194. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium–Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [Google Scholar] [CrossRef]
- Monda, V.; Polito, R.; Lovino, A.; Finaldi, A.; Valenzano, A.; Nigro, E.; Corso, G.; Sessa, F.; Asmundo, A.; Di Nunno, N.; et al. Short-Term Physiological Effects of a Very Low-Calorie Ketogenic Diet: Effects on Adiponectin Levels and Inflammatory States. Int. J. Mol. Sci. 2020, 21, 3228. [Google Scholar] [CrossRef]


| Patients with Cushing’s Disease (N = 15) | Controls (N = 15) | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Clinical parameters | |||
| Age (years) | 47.2 ± 10.6 | 50.8 ± 11.7 | 0.535 |
| BMI (Kg/m2) | 35.7 ± 4.5 | 32.8 ± 2.14 | 0.175 |
| Waist circumference (cm) | 114.4 ± 12.7 | 96.6 ± 14.2 | 0.035 |
| Systolic blood pressure (mmHg) | 125.8 ± 15.6 | 122.3 ± 8.6 | 0.453 |
| Diastolic blood pressure (mmHg) | 88.2 ± 7.8 | 83.2 ± 4.8 | 0.057 |
| Hormonal parameters | |||
| Fasting serum cortisol (mcg/dL) | 13.2 ± 4.11 | 11.2 ± 3.44 | 0.443 |
| Fasting serum ACTH (ng/L) | 77.8 ± 40.8 | 27.1 ± 19.5 | 0.043 |
| Urinary free cortisol (mcg/24 h) | 56.8 ± 30.9 | 33.4 ± 10.7 | <0.001 |
| Salivary cortisol | 6.6 ± 2.76 | 0.76 ± 0.49 | <0.001 |
| Cortisol after low-dose suppression test (mcg/dL) | 4.14 ± 3.55 | 0.99 ± 0.66 | 0.002 |
| Androstenedione (mcg/L) | 2.86 ± 2.31 | 0.71 ± 0.34 | <0.001 |
| DHEAS (mcg/dL) | 1743 ± 1110 | 624.5 ± 324.5 | 0.008 |
| Cortisone (mcg/L) | 15.1 ± 3.9 | 18.6 ± 6.5 | 0.624 |
| 17OHP (mcg/L) | 0.53 ± 0.22 | 0.32 ± 0.21 | 0.339 |
| Metabolic parameters | |||
| Glycemia (mmol/L) | 4.91 ± 0.83 | 4.44 ± 0.34 | 0.239 |
| Insulinemia (microU/mL) | 9.48 ± 8.44 | 7.46 ± 3.83 | 0.639 |
| HbA1c (%) | 5.96 ± 0.96 | 5.14 ± 0.32 | 0.045 |
| Total cholesterol (mmol/L) | 4.96 ± 0.6 | 4.97 ± 0.6 | 0.637 |
| HDL cholesterol (mmol/L) | 1.36 ± 0.28 | 1.38 ± 0.53 | 0.958 |
| Triglycerides (mmol/L) | 1.2 ± 0.5 | 0.82 ± 0.37 | 0.116 |
| LDL cholesterol (mmol/L) | 2.63 ± 0.58 | 2.8 ± 0.44 | 0.931 |
| GOT (U/L) | 17.5 ± 5.06 | 22.2 ± 10.8 | 0.131 |
| GPT (U/L) | 22.2 ± 10.8 | 22.8 ± 6.96 | 0.919 |
| Alkaline phosphatase (U/L) | 71.7 ± 21.6 | 72.2 ± 19.6 | 0.975 |
| GammaGT (U/L) | 15.1 ± 8.01 | 15 ± 12.1 | 0.445 |
| Creatinine (mg/dL) | 0.72 ± 0.08 | 0.71 ± 0.13 | 0.854 |
| Na (mmol/L) | 140.2 ± 2.16 | 139.8 ± 1.78 | 0.758 |
| K (mmol/L) | 4.4 ± 0.42 | 4.2 ± 0.44 | 0.408 |
| Calcium (mg/dL) | 9.36 ± 0.25 | 9.31 ± 0.26 | 0.717 |
| Phosphorus (mg/dL) | 3.28 ± 0.29 | 3.56 ± 0.23 | 0.395 |
| Vitamin D (mcg/L) | 21.4 ± 5.22 | 25.6 ± 5.09 | 0.033 |
| PTH (ng/L) | 52.7 ± 17.5 | 43 ± 11.8 | 0.351 |
| C-reactive protein (mg/L) | 2.44 ± 1.95 | 0.79 ± 0.75 | 0.145 |
| Dio | 0.97 ± 0.66 | 2.91 ± 2.77 | <0.001 |
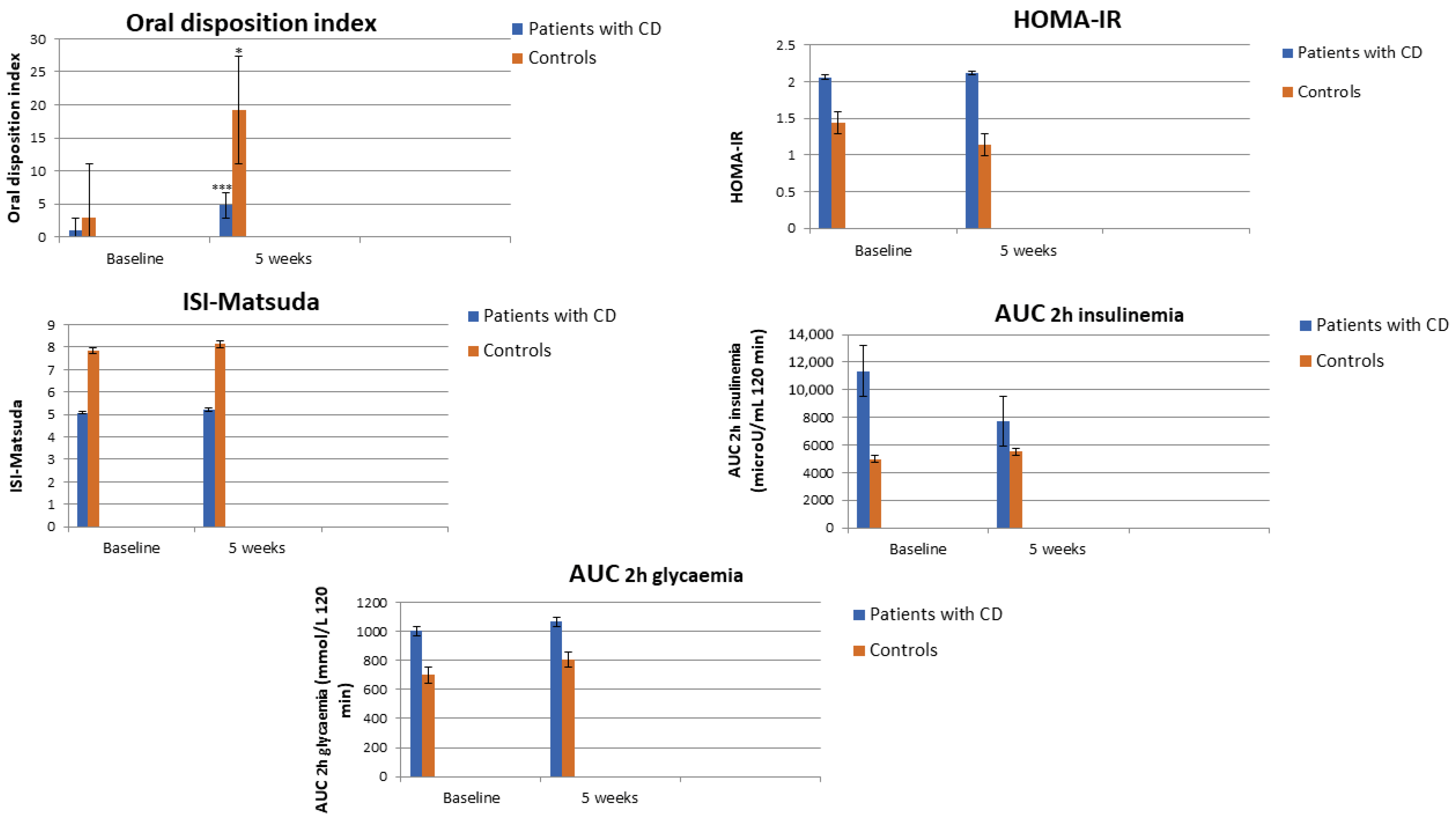
| AUC 2h insulinemia (uU/mL 120 min) | 11,370 ± 5592 | 4977 ± 1636.5 | 0.040 |
| AUC 2h glycemia (mmol/L 120 min) | 1004.1 ± 330.3 | 700.6 ± 111.9 | 0.022 |
| HOMA-IR | 2.06 ± 1.95 | 1.44 ± 0.71 | 0.521 |
| ISI-Matsuda | 5.08 ± 3.66 | 7.85 ± 3.73 | 0.049 |
| Patients with Cushing’s Disease (N = 15) | |||||
|---|---|---|---|---|---|
| Baseline Mean (± SD) | Week 1 Mean (± SD) | Week 3 Mean (± SD) | Week 5 Mean (± SD) | p | |
| Clinical parameters | |||||
| BMI (Kg/m2) | 35.7 ± 6.54 | 34.4 ± 6.15 | 33.2 ± 5.91 | 32.3 ± 5.53 | 0.002 |
| Waist circumference (cm) | 114.4 ± 12.7 | 111.6 ± 11.1 | 109 ± 10.7 | 107.8 ± 8.16 | 0.024 |
| Systolic blood pressure (mmHg) | 125.8 ± 15.6 | 120.4 ± 11.8 | 118.4 ± 10.3 | 113.8 ± 9.6 | 0.015 |
| Diastolic blood pressure (mmHg) | 88.2 ± 7.8 | 85.4 ± 5.8 | 84.2 ± 6.1 | 82.1 ± 5.1 | 0.005 |
| Hormonal parameters | |||||
| Fasting serum cortisol (mcg/dL) | 11.4 ± 2.22 | 12.7 ± 3.33 | 14.2 ± 3.08 | 14.7 ± 2.53 | 0.122 |
| Fasting serum ACTH (ng/L) | 77.8 ± 40.4 | 60.3 ± 29.5 | 58.2 ± 27.3 | 41.8 ± 26.9 | 0.026 |
| Urinary free cortisol (mcg/24 h) | 56.8 ± 30.9 | 67.9 ± 43.3 | 55.6 ± 43.7 | 52.3 ± 37.6 | 0.472 |
| Late-night salivary cortisol | 6.67 ± 4.76 | 7.38 ± 3.32 | 4.76 ± 3.73 | 2.67 ± 2.33 | 0.160 |
| Cortisol after low dose suppression test (mcg/dL) | 4.14 ± 3.55 | 3.89 ± 3.03 | 3.88 ± 3.01 | 3.06 ± 2.85 | 0.139 |
| 17OHP (mcg/L) | 0.53 ± 0.22 | 0.63 ± 0.21 | 0.85 ± 0.18 | 0.76 ± 0.14 | 0.897 |
| Androstenedione (mcg/L) | 2.86 ± 2.31 | 2.5 ± 2.11 | 2.36 ± 1.47 | 3.05 ± 1.98 | 0.558 |
| DHEAS (mcg/L) | 1743 ± 1110 | 1892.5 ± 165.6 | 1871.7 ± 333.5 | 1656.7 ± 323.5 | 0.125 |
| Cortisone (mcgd/L) | 15.1 ± 3.9 | 16.6 ± 4.01 | 17.8 ± 3.85 | 19.8 ± 4.51 | 0.025 |
| Metabolic parameters | |||||
| Glycemia (mmol/L) | 4.91 ± 0.83 | 4.88 ± 1.11 | 4.94 ± 0.55 | 4.46 ± 0.7 | 0.356 |
| Insulinemia (microU/mL) | 6.7 ± 4.02 | 5.8 ± 3.11 | 4.15 ± 3.33 | 5.63 ± 5.02 | 0.241 |
| Total cholesterol (mmol/L) | 4.96 ± 0.6 | 4.92 ± 0.83 | 4.16 ± 0.75 | 4.06 ± 0.81 | 0.006 |
| HDL cholesterol (mmol/L) | 1.36 ± 0.28 | 1.43 ± 0.28 | 1.46 ± 0.35 | 1.63 ± 0.3 | 0.017 |
| Triglycerides (mmol/L) | 1.2 ± 0.5 | 0.9 ± 0.23 | 0.89 ± 0.2 | 0.75 ± 0.15 | 0.040 |
| LDL cholesterol (mmol/L) | 2.63 ± 0.58 | 2.78 ± 0.74 | 2.22 ± 0.75 | 2.25 ± 0.78 | 0.017 |
| GOT (U/L) | 17.5 ± 5.06 | 20.1 ± 11.3 | 16.7 ± 8.99 | 15.2 ± 8.77 | 0.075 |
| GPT (U/L) | 22.2 ± 10.8 | 24.6 ± 15.7 | 24.6 ± 12.3 | 27 ± 11.5 | 0.166 |
| Alkaline phosphatase (U/L) | 71.7 ± 21 | 68 ± 19 | 64.2 ± 21.2 | 57.2 ± 20.2 | 0.008 |
| GammaGT (U/L) | 15.2 ± 8.01 | 16.5 ± 6.24 | 14.2 ± 3.86 | 14.5 ± 5.01 | 0.431 |
| Creatinine (mg/dL) | 0.72 ± 0.08 | 0.82 ± 0.13 | 0.78 ± 0.13 | 0.78 ± 0.12 | 0.055 |
| Na (mmol/L) | 140.2 ± 2.16 | 139.6 ± 1.67 | 139.6 ± 1.14 | 139.8 ± 1.30 | 0.824 |
| K (mmol/L) | 4.44 ± 0.42 | 4.32 ± 0.31 | 4.12 ± 0.27 | 4.08 ± 0.35 | 0.063 |
| Calcium (mg/dL) | 9.36 ± 0.25 | 9.68 ± 0.23 | 9.58 ± 0.24 | 9.34 ± 0.36 | 0.246 |
| Phosphorus (mg/dL) | 3.28 ± 0.29 | 3.36 ± 0.34 | 3.32 ± 0.24 | 3.14 ± 0.27 | 0.952 |
| Vitamin D (mcg/L) | 21.4 ± 5.223. | 27.2 ± 6.53 | 30.2 ± 7.69 | 32 ± 14.03 | 0.015 |
| PTH (ng/L) | 52.7 ± 17.5 | 44.7 ± 14.7 | 50.5 ± 21.8 | 38.5 ± 27.7 | 0.188 |
| C-reactive protein (mg/L) | 2.44 ± 1.92 | 2.34 ± 2.28 | 1.13 ± 1.02 | 1.39 ± 1.35 | 0.682 |
| Controls (N = 15) | |||||
|---|---|---|---|---|---|
| Baseline Mean (± SD) | Week 1 Mean (± SD) | Week 3 Mean (± SD) | Week 5 Mean (± SD) | p | |
| Clinical parameters | |||||
| BMI (Kg/m2) | 32.8 ± 2.14 | 27.4 ± 3.14 | 26.3 ± 2.91 | 25.5 ± 2.33 | 0.003 |
| Waist circumference (cm) | 96.6 ± 14.2 | 95.1 ± 14.8 | 93.2 ± 13.5 | 92 ± 13.3 | 0.002 |
| Systolic blood pressure (mmHg) | 122.3 ± 8.6 | 119.4 ± 6.7 | 115.3 ± 5.6 | 114.7 ± 6.3 | 0.025 |
| Diastolic blood pressure (mmHg) | 83.2 ± 4.8 | 81.5 ± 3.6 | 80.7 ± 4.2 | 78.2 ± 4.6 | 0.007 |
| Hormonal parameters | |||||
| Fasting serum cortisol (mcg/dL) | 11.2 ± 3.44 | 18.8 ± 1.83 | 17.9 ± 2.11 | 15.7 ± 2.05 | 0.180 |
| Urinary free cortisol (mcg/24 h) | 33.4 ± 10.7 | 29.5 ± 15.7 | 32.6 ± 17.8 | 27.9 ± 14.3 | 0.552 |
| Salivary cortisol | 0.76 ± 0.49 | 0.85 ± 0.59 | 0.48 ± 0.15 | 0.52 ± 0.27 | 0.818 |
| 17OHP (mcg/L) | 0.32 ± 0.21 | 0.34 ± 0.28 | 0.45 ± 0.27 | 0.41 ± 0.22 | 0.768 |
| Androstenedione (mcg/dL) | 0.71 ± 0.34 | 1.21 ± 0.75 | 1.35 ± 1.05 | 1.15 ± 0.95 | 0.567 |
| DHEAS (mcg/L) | 624.5 ± 324.5 | 557.1 ± 194.5 | 587.9 ± 234.5 | 598.4 ± 205.5 | 0.768 |
| Cortisone (mcg/L) | 18.6 ± 6.5 | 20.1 ± 5.6 | 19.5 ± 4.9 | 18.6 ± 7.3 | 0.645 |
| Metabolic parameters | |||||
| Glycemia (mmol/L) | 4.44 ± 0.34 | 4.03 ± 0.98 | 4.22 ± 0.71 | 3.83 ± 0.16 | 0.074 |
| Insulinemia (microU/mL) | 7.46 ± 3.83 | 10.1 ± 9.51 | 4.15 ± 3.32 | 5.65 ± 5.05 | 0.241 |
| HbA1c (%) | 5.14 ± 0.32 | 5.25 ± 0.63 | 5.25 ± 0.49 | 5.11 ± 0.71 | 0.290 |
| Total cholesterol (mmol/L) | 4.97 ± 0.6 | 4.48 ± 0.2 | 4.07 ± 0.67 | 4.07 ± 0.75 | 0.026 |
| HDL cholesterol (mmol/L) | 1.38 ± 0.53 | 1.47 ± 0.62 | 1.5 ± 0.69 | 1.6 ± 0.32 | 0.001 |
| Triglycerides (mmol/L) | 0.82 ± 0.37 | 0.89 ± 0.27 | 0.69 ± 0.1 | 0.71 ± 0.22 | 0.145 |
| LDL cholesterol (mmol/L) | 2.8 ± 0.44 | 2.69 ± 0.2 | 2.28 ± 0.1 | 2.25 ± 0.14 | 0.132 |
| GOT (U/L) | 22.2 ± 10.8 | 22.5 ± 3.53 | 22.5 ± 0.71 | 21.5 ± 2.12 | 0.212 |
| GPT (U/L) | 22.8 ± 6.96 | 16 ± 2.82 | 18.5 ± 2.12 | 18 ± 1.97 | 0.267 |
| Alkaline phosphatase (U/L) | 72.2 ± 19.6 | 78.5 ± 27.5 | 69.5 ± 26.1 | 69 ± 25.4 | 0.120 |
| GammaGT (U/L) | 15 ± 12.1 | 12.5 ± 7.07 | 9.5 ± 4.94 | 9.5 ± 3.53 | 0.290 |
| Creatinine (mg/dL) | 0.71 ± 0.13 | 0.74 ± 0.11 | 0.77 ± 0.13 | 0.76 ± 0.15 | 0.501 |
| Na (mmol/L) | 139.8 ± 1.78 | 139.5 ± 2.12 | 138.5 ± 2.09 | 139 ± 2.02 | 0.572 |
| K (mmol/L) | 4.2 ± 0.44 | 4.05 ± 0.21 | 4.15 ± 0.35 | 3.91 ± 0.28 | 0.129 |
| Calcium (mg/dL) | 9.31 ± 0.26 | 9.29 ± 0.12 | 9.45 ± 0.21 | 9.3 ± 0.27 | 0.244 |
| Phosphorus (mg/dL) | 3.56 ± 0.23 | 3.55 ± 0.21 | 3.35 ± 0.51 | 3.81 ± 0.14 | 0.753 |
| Vitamin D (mcg/L) | 24.6 ± 9.09 | 34 ± 8.09 | 37.5 ± 7.78 | 42.5 ± 17.6 | 0.514 |
| PTH (ng/L) | 43 ± 11.8 | 32 ± 2.82 | 37.5 ± 2.12 | 37 ± 1.41 | 0.267 |
| C-reactive protein (mg/L) | 0.79 ± 0.75 | 1.42 ± 1.1 | 1.07 ± 0.65 | 0.76 ± 0.14 | 0.443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnotta, V.; Amodei, R.; Di Gaudio, F.; Giordano, C. Nutritional Intervention in Cushing’s Disease: The Ketogenic Diet’s Effects on Metabolic Comorbidities and Adrenal Steroids. Nutrients 2023, 15, 4647. https://doi.org/10.3390/nu15214647
Guarnotta V, Amodei R, Di Gaudio F, Giordano C. Nutritional Intervention in Cushing’s Disease: The Ketogenic Diet’s Effects on Metabolic Comorbidities and Adrenal Steroids. Nutrients. 2023; 15(21):4647. https://doi.org/10.3390/nu15214647
Chicago/Turabian StyleGuarnotta, Valentina, Roberta Amodei, Francesca Di Gaudio, and Carla Giordano. 2023. "Nutritional Intervention in Cushing’s Disease: The Ketogenic Diet’s Effects on Metabolic Comorbidities and Adrenal Steroids" Nutrients 15, no. 21: 4647. https://doi.org/10.3390/nu15214647
APA StyleGuarnotta, V., Amodei, R., Di Gaudio, F., & Giordano, C. (2023). Nutritional Intervention in Cushing’s Disease: The Ketogenic Diet’s Effects on Metabolic Comorbidities and Adrenal Steroids. Nutrients, 15(21), 4647. https://doi.org/10.3390/nu15214647






