Retinol Levels and Severity of Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
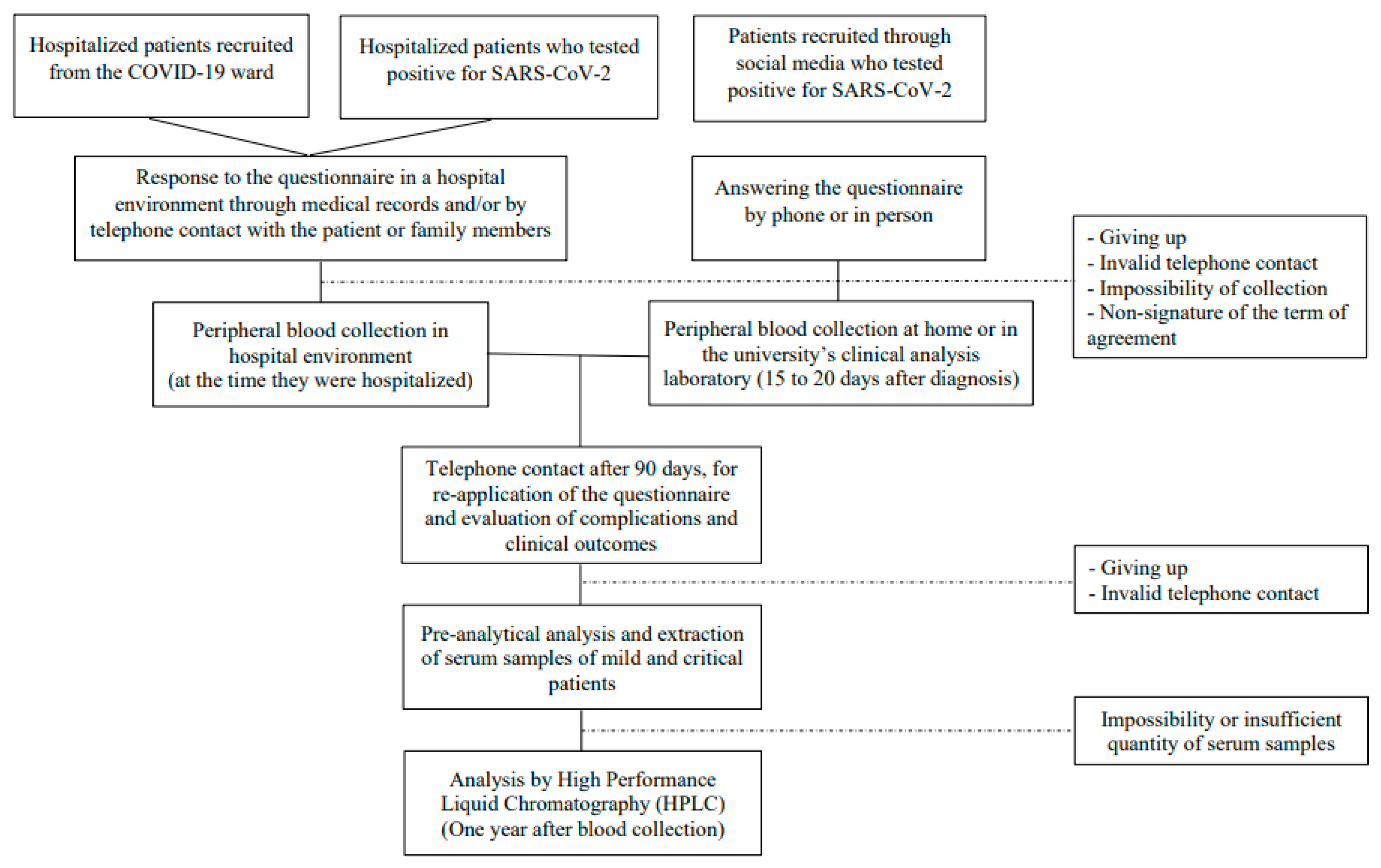
2.1. Study Population
2.2. Data Collection and Characterization of the Population
2.3. Measurement of Vitamin A
2.4. Statistical Analysis
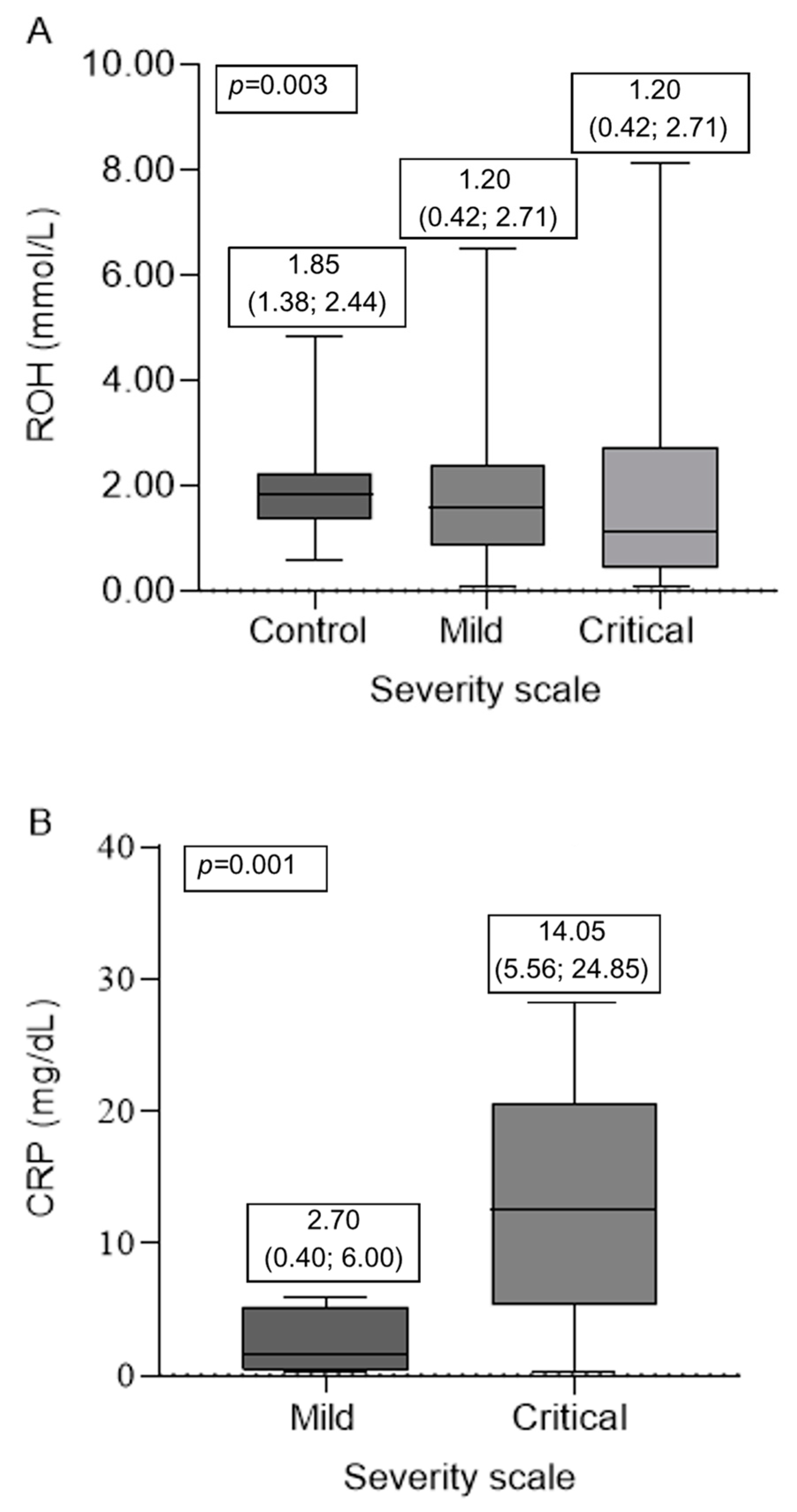
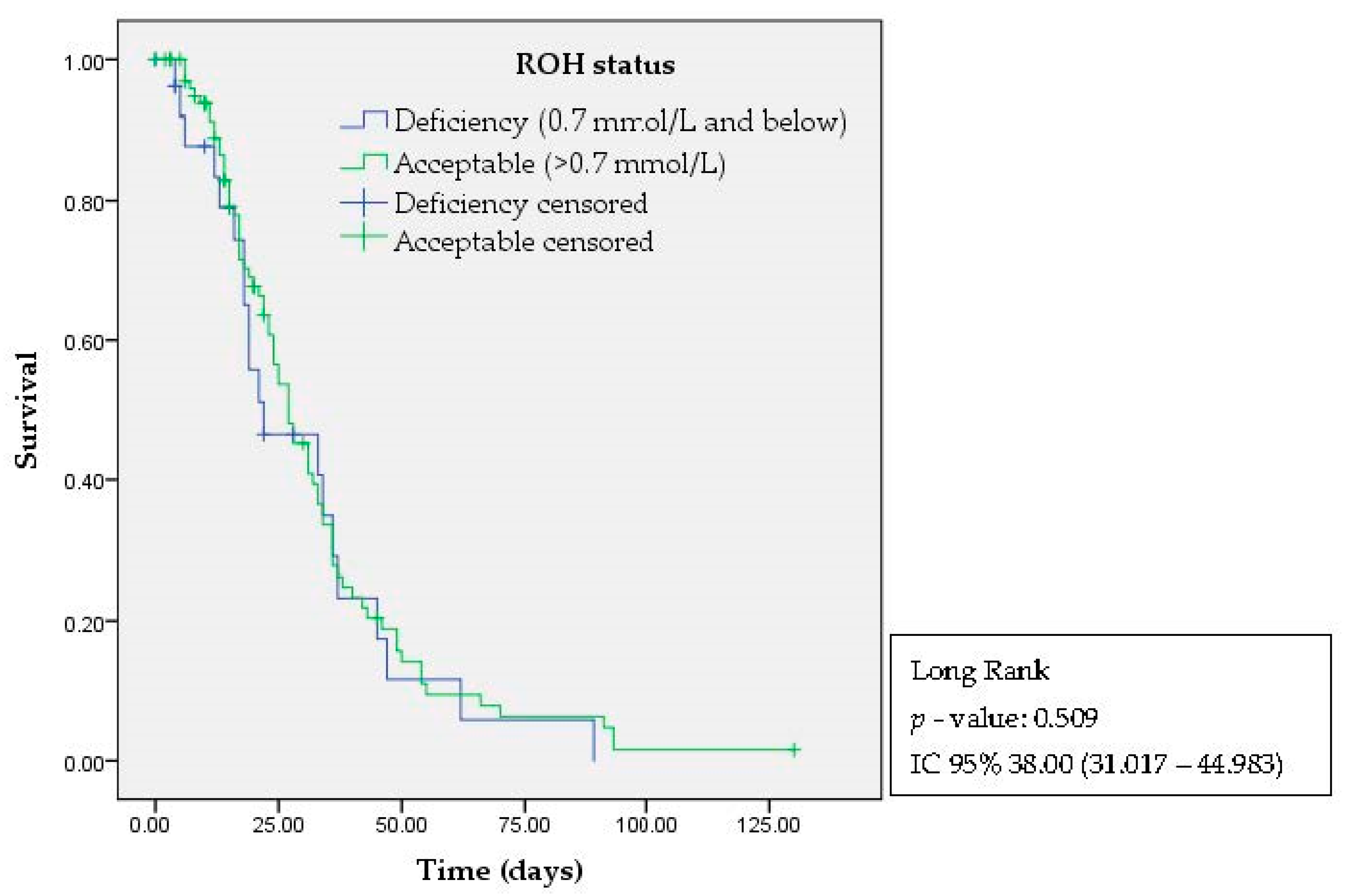
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan American Health Organization/World Health Organization. Epidemiological Update: Coronavirus Disease (COVID-19). 9 November 2020; PAHO/WHO: Washington, DC, USA, 2020.
- Organization World Health. Coronavirus Disease (COVID-19): What Are the Symptoms of COVID-19? Available online: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19 (accessed on 1 November 2022).
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef] [PubMed]
- Taribagil, P.; Creer, D.; Tahir, H. Long COVID syndrome. BMJ Case Rep. 2021, 14, e241485. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The inflammation link and the role of nutrition in potential mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Bakdash, G.; Vogelpoel, L.T.C.; van Capel, T.M.M.; Kapsenberg, M.L.; de Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.C. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Jovic, T.H.; Ali, S.R.; Ibrahim, N.; Jessop, Z.M.; Tarassoli, S.P.; Dobbs, T.D.; Holford, P.; Thornton, C.A.; Whitaker, I.S. Could vitamins help in the fight against COVID-19? Nutrients 2020, 12, 2550. [Google Scholar] [CrossRef]
- Benn, C.S. Combining vitamin A and vaccines: Convenience or conflict? Dan. Med. J. 2012, 59, 1–28. [Google Scholar]
- Jørgensen, M.J.; Hein-Kristensen, L.; Hempel, C.; Ravn, H.; Wiese, L.; Kurtzhals, J.A.L.; Benn, C.S. The effect of vitamin A supplementation and diphtheria-tetanus-pertussis vaccination on parasitaemia in an experimental murine malaria model. Scand. J. Infect. Dis. 2011, 43, 296–303. [Google Scholar] [CrossRef]
- Voloudakis, G.; Hoffman, G.; Venkatesh, S.; Lee, K.M.; Dobrindt, K.; Vicari, J.M.; Zhang, W.; Beckmann, N.D.; Jiang, S.; Hoagland, D.; et al. IL10RB as a key regulator of COVID-19 host susceptibility and severity. medRxiv 2021. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, J.; Cha, Y.; Kolitz, S.; Funt, J.; Chong, R.E.; Barrett, S.; Kusko, R.; Zeskind, B.; Kaufman, H. Advanced bioinformatics rapidly identifies existing therapeutics for patients with coronavirus disease-2019 (COVID-19). J. Transl. Med. 2020, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sato, S.; Sotoyama, Y.; Orba, Y.; Sawa, H.; Yamauchi, H.; Sasaki, M.; Takaoka, A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021, 22, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Geng, T.; Harrison, A.G.; Wang, P. Differential Roles of RIG-I-like Receptors in SARS-CoV-2 Infection. Mil. Med. Res. 2021, 8, 1–3. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, F.; Hu, D.; Ge, W.; Tian, M.; Wang, W.; Pan, P.; Wu, K.; Wu, J. SARS-CoV-2 nucleocapsid protein interacts with rig-i and represses rig-mediated ifn-β production. Viruses 2021, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Shin, O.S. SARS-CoV-2 Nucleocapsid Protein Targets RIG-I-Like Receptor Pathways to Inhibit the Induction of Interferon Response. Cells 2021, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Pain, A. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes; Food and Nutrition Board, Institute of Medicine, National Academy Press: Washington, DC, USA, 2000.
- Padovani, R.M.; Amaya-Farfán, J.; Colugnati, F.A.B.; Domene, S.M.Á. Dietary reference intakes: Application of tables in nutritional studies. Rev. Nutr. 2006, 19, 741–760. [Google Scholar] [CrossRef]
- Tepasse, P.R.; Vollenberg, R.; Fobker, M.; Kabar, I.; Schmidt, H.; Meier, J.A.; Nowacki, T.; Hüsing-Kabar, A. Vitamin a plasma levels in covid-19 patients: A prospective multicenter study and hypothesis. Nutrients 2021, 13, 2173. [Google Scholar] [CrossRef]
- National Institutes of Health. COVID-19 Treatment Guidelines: COVID-19 Treatment Guidelines: Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 1 November 2022).
- de Oliveira Fernandes Miranda, C.T.; Duarte, V.H.R.; Cruz, M.S.D.M.; Duarte, M.K.R.N.; de Araújo, J.N.G.; Santos, A.M.Q.S.D.; Oliveira, J.M.D.; Paiva, M.S.M.O.; Rezende, A.A.; Hirata, M.H.; et al. Association of Serum Alpha-Tocopherol and Retinol with the Extent of Coronary Lesions in Coronary Artery Disease. J. Nutr. Metab. 2018, 2018, 6104169. [Google Scholar] [CrossRef]
- Ortega, R.M.; López-Sobaler, A.M.; Elena Quintas, M.; Martínez, R.M.; Andrés, P. The influence of smoking on vitamin c status during the third trimester of pregnancy and on vitamin c levels in maternal milk. J. Am. Coll. Nutr. 1998, 17, 379–384. [Google Scholar] [CrossRef]
- Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005 WHO Global Database on Vitamin A Deficiency. 2009. Available online: https://apps.who.int/iris/handle/10665/44110 (accessed on 1 January 2020).
- Djaharuddin, I.; Munawwarah, S.; Nurulita, A.; Ilyas, M.; Tabri, N.A.; Lihawa, N. Comorbidities and mortality in COVID-19 patients. Gac. Sanit. 2021, 35, S530–S532. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male 2021, 23, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Levin, A.T.; Hanage, W.P.; Owusu-Boaitey, N.; Cochran, K.B.; Walsh, S.P.; Meyerowitz-Katz, G. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020, 35, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cheng, X.; Feng, X.; Wan, H.; Chen, S.; Xiong, M. Clinical Symptom Differences between Mild and Severe COVID-19 Patients in China: A Meta-Analysis. Front. Public Health 2021, 8, 561264. [Google Scholar] [CrossRef]
- Doty, R.L. Age-Related Deficits in Taste and Smell. Otolaryngol. Clin. N. Am. 2018, 51, 815–825. [Google Scholar] [CrossRef]
- Bernstein, I.A.; Roxbury, C.R.; Lin, S.Y.; Rowan, N.R. The association of frailty with olfactory and gustatory dysfunction in older adults: A nationally representative sample. Int. Forum Allergy Rhinol. 2021, 11, 866–876. [Google Scholar] [CrossRef]
- Sanna, F.; Loy, F.; Piras, R.; Moat, A.; Masala, C. Age-Related Cognitive Decline and the Olfactory Identification Deficit Are Associated to Increased Level of Depression. Front. Neurosci. 2021, 15, 76. [Google Scholar] [CrossRef]
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef]
- Islam, M.F.; Cotler, J.; Jason, L.A. Post-viral fatigue and COVID-19: Lessons from past epidemics. Fatigue 2020, 8, 61–69. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M. Features of 20,133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, G.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Ghahramani, S.; Tabrizi, R.; Lankarani, K.B.; Kashani, S.M.A.; Rezaei, S.; Zeidi, N.; Akbari, M.; Heydari, S.T.; Akbari, H.; Nowrouzi-Sohrabi, P.; et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: A systematic review and meta-analysis. Eur. J. Med. Res. 2020, 25, 30. [Google Scholar] [CrossRef]
- Sarohan, A.R.; Kızıl, M.; İnkaya, A.Ç.; Mahmud, S.; Akram, M.; Cen, O. A novel hypothesis for COVID-19 pathogenesis: Retinol depletion and retinoid signaling disorder. Cell. Signal. 2021, 87, 110121. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Le, G.C.; Misset, B.; et al. Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Voelkle, M.; Gregoriano, C.; Neyer, P.; Koch, D.; Kutz, A.; Bernasconi, L.; Conen, A.; Mueller, B.; Schuetz, P. Prevalence of Micronutrient Deficiencies in Patients Hospitalized with COVID-19: An Observational Cohort Study. Nutrients 2022, 14, 1862. [Google Scholar] [CrossRef]
- Tomasa-Irriguible, T.M.; Bielsa-Berrocal, L.; Bordejé-Laguna, L.; Tural-Llàcher, C.; Barallat, J.; Manresa-Domínguez, J.M.; Torán-Monserrat, P. Low levels of few micronutrients may impact COVID-19 disease progression: An observational study on the first wave. Metabolites 2021, 11, 565. [Google Scholar] [CrossRef]
- Žarković, N.; Jastrząb, A.; Jarocka-Karpowicz, I.; Orehovec, B.; Baršić, B.; Tarle, M.; Kmet, M.; Lukšić, I.; Łuczaj, W.; Skrzydlewska, E. The Impact of Severe COVID-19 on Plasma Antioxidants. Molecules 2022, 27, 5323. [Google Scholar] [CrossRef]
- Sampaio, L.R.; Silva, M.C.M.; Oliveira, A.N.; Souza, C.L.S. Avaliação bioquímica do estado nutricional. In Avaliação Nutricional; Sampaio, L.R., Ed.; EDUFBA: Salvador, Brazil, 2012; pp. 49–72. ISBN 978-85-232-1874-4. [Google Scholar]
| Variables | Controls (n = 46) | Severity Scale | p-Value | |
|---|---|---|---|---|
| Mild (n = 88) | Critical (n = 106) | |||
| Mean (SD) or Median (IQR) or %(n) | ||||
| Age (years) | 54 (44; 62) | 47 (42; 55) | 67 (55; 79) | <0.001 |
| BMI (kg/m2) | 29.50 (26.78; 31.48) | 27.68 (25.26; 30.48) | 28.51 (24.09; 31.69) | 0.241 |
| Sex | 0.973 | |||
| Female | 56.5% (26) | 54.5% (48) | 54.7% (58) | |
| Male | 43.5% (20) | 45.5% (40) | 45.3% (48) | |
| Creatinine (mg/dL) | 0.85 (0.80; 1.00) | 0.93 (0.68; 1.10) | 1.56 (0.90; 3.07) | 0.001 |
| AST (U/L) | 31.00 (26.00; 41.00) | 24.00 (16.00; 52.50) | 51.00 (31.00; 68.00) | <0.001 |
| ALT (U/L) | 25.50 (20.00; 34.00) | 33.50 (25.00; 82.50) | 44.00 (24.00; 84.00) | <0.001 |
| Obesity | 34.8% (16) | 30.7% (27) | 23.6% (25) | 0.308 |
| Hypertension | 80.4% (37) | 37.5% (33) | 63.2% (67) | <0.001 |
| Diabetes | 24.4% (11) | 6.8% (6) | 32.1% (34) | <0.001 |
| Associated with COVID-19 | Mild (n = 88) | Critical (n = 106) | p-Value |
|---|---|---|---|
| Median (IQR) or %(n) | |||
| Time with the disease (days) | 10 (5, 20) | 24 (16, 36) | <0.001 |
| Comorbidities | <0.001 | ||
| None | 33% (29) | 16.0% (17) | |
| 1 to 2 | 55.7% (49) | 30.2% (32) | |
| 2 to 3 | 11.4% (10) | 41.5% (44) | |
| >4 | - | 12.3% (13) | |
| Symptoms | <0.001 | ||
| None | 8.0% (7) | - | |
| 1 to 4 | 26.4% (23) | 67.9% (72) | |
| 5 to 8 | 34.5% (30) | 14.2% (15) | |
| >9 | 31.0% (27) | 17.9% (19) | |
| Survival (90th day) | <0.001 | ||
| No | - | 49.5% (52) | |
| Yes | 100% (88) | 50.5% (53) | |
| Persistent symptoms after 90 days | <0.001 | ||
| No | 39.8% (35) | 92.5% (49) | |
| Yes | 60% (53) | 7.5% (4) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, M.C.d.C.; Araujo, J.K.C.P.; da Silva, A.G.C.L.; da Silva, N.S.; de Araújo, N.K.; Luchessi, A.D.; Ribeiro, K.D.d.S.; Silbiger, V.N. Retinol Levels and Severity of Patients with COVID-19. Nutrients 2023, 15, 4642. https://doi.org/10.3390/nu15214642
Carvalho MCdC, Araujo JKCP, da Silva AGCL, da Silva NS, de Araújo NK, Luchessi AD, Ribeiro KDdS, Silbiger VN. Retinol Levels and Severity of Patients with COVID-19. Nutrients. 2023; 15(21):4642. https://doi.org/10.3390/nu15214642
Chicago/Turabian StyleCarvalho, Maria Clara da Cruz, Júlia Kaline Carvalho Pereira Araujo, Ana Gabriella Costa Lemos da Silva, Nayara Sousa da Silva, Nathalia Kelly de Araújo, Andre Ducati Luchessi, Karla Danielly da Silva Ribeiro, and Vivian Nogueira Silbiger. 2023. "Retinol Levels and Severity of Patients with COVID-19" Nutrients 15, no. 21: 4642. https://doi.org/10.3390/nu15214642
APA StyleCarvalho, M. C. d. C., Araujo, J. K. C. P., da Silva, A. G. C. L., da Silva, N. S., de Araújo, N. K., Luchessi, A. D., Ribeiro, K. D. d. S., & Silbiger, V. N. (2023). Retinol Levels and Severity of Patients with COVID-19. Nutrients, 15(21), 4642. https://doi.org/10.3390/nu15214642






