Fluid-Dependent Single-Frequency Bioelectrical Impedance Fat Mass Estimates Compared to Digital Imaging and Dual X-ray Absorptiometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
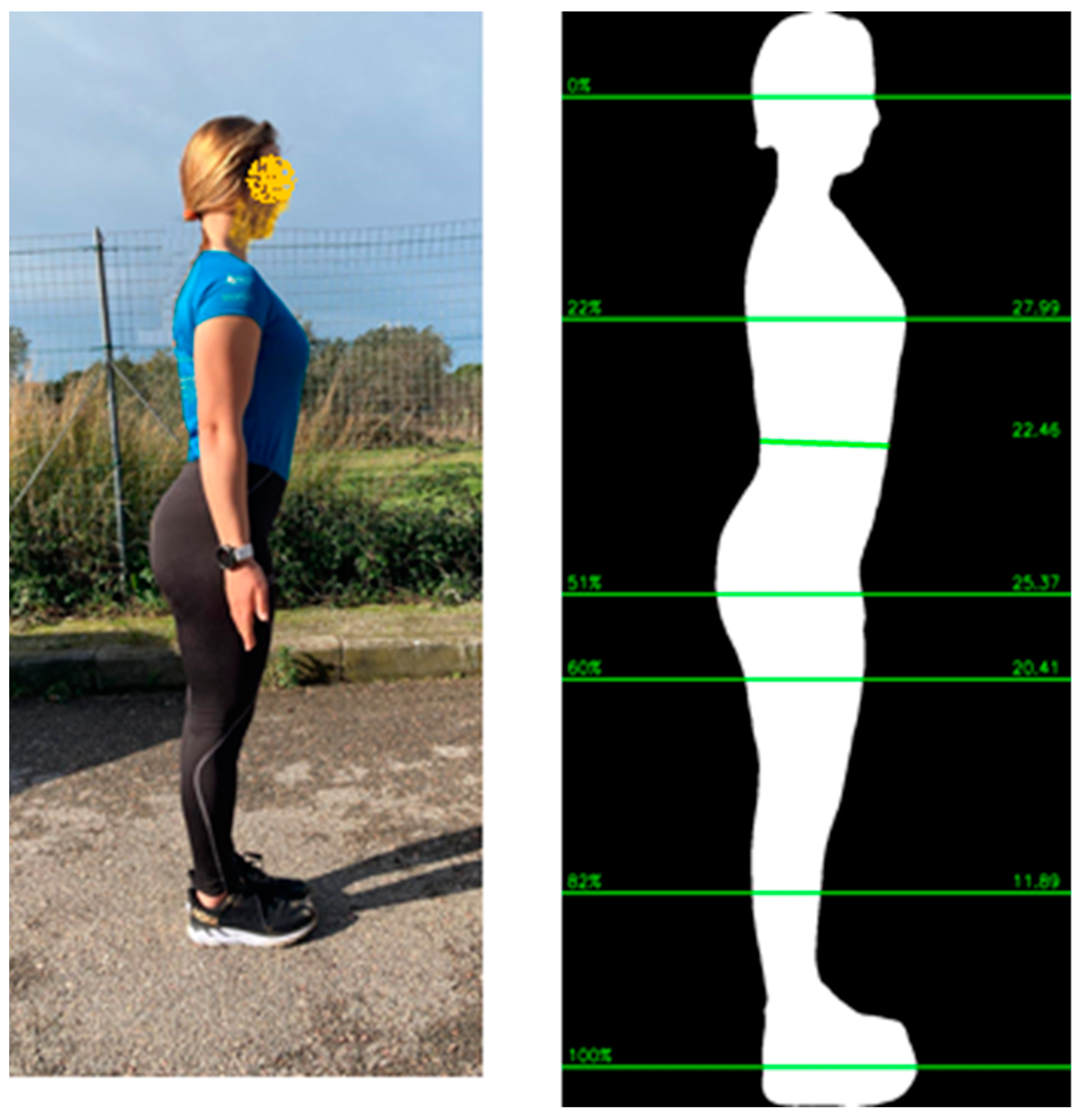
2.2. Body Composition Assessment
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight—Fact Sheet. 2021. Available online: https://www.who.int/newsroom/fact-sheets/detail/obesity-and-overweight (accessed on 15 September 2023).
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for eight countries. BMJ Glob. Health 2021, 6, e006351. [Google Scholar] [CrossRef] [PubMed]
- Wellens, R.I.; Roche, A.F.; Khamis, H.J.; Jackson, A.S.; Pollock, M.L.; Siervogel, R.M. Relationships between the body mass index and body composition. Obes. Res. 1996, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Frankenfield, D.C.; Rowe, W.A.; Cooney, R.N.; Smith, J.S.; Becker, D. Limits of body mass index to detect obesity and predict body composition. Nutrition 2001, 17, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Okorodudu, D.O.; Jumean, M.F.; Montori, V.M.; Romero-Corral, A.; Somers, V.K.; Erwin, P.J.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.J.; Hunger, J.M.; Nguyen-Cuu, J.; Wells, C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. Int. J. Obes. 2016, 40, 883–886. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Correia, M.I.T.D.; Heymsfield, S.B. A requiem for BMI in the clinical setting. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 314–321. [Google Scholar] [CrossRef]
- Frühbeck, G.; Kiortsis, D.N.; Catalán, V. Precision medicine: Diagnosis and management of obesity. Lancet Diabetes Endocrinol. 2018, 6, 164–166. [Google Scholar] [CrossRef]
- Salmón-Gómez, L.; Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Relevance of body composition in phenotyping the obesities. Rev. Endocr. Metab. Disord. 2023, 24, 809–823. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The utility of body composition assessment in nutrition and clinical practice: An overview of current methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Sommer, I.; Teufer, B.; Szelag, M.; Nussbaumer-Streit, B.; Titscher, V.; Klerings, I.; Gartlehner, G. The performance of anthropo-metric tools to determine obesity: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12699. [Google Scholar] [CrossRef] [PubMed]
- Laine, C.; Wee, C.C. Overweight and obesity: Clinical challenges. Ann. Int. Med. 2023, 176, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Report of the Council on Science and Public Health. American Medical Association. Is Obesity a Disease? CSAPH Report 3-A-13. Available online: https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/about-ama/councils/Council%20Re-390ports/council-on-science-public-health/a13csaph3.pdf (accessed on 15 September 2023).
- Mestre, L.M.; Lartey, S.T.; Ejima, K.; Mehta, T.; Keith, S.; Maki, K.; Allison, D.B. Body mass index, obesity, and mortality—Part I. Associations and limitations. Nutr. Today 2023, 58, 92–99. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Vega Diaz, N.; Talluri, A.; Nescolarde, L. Classification of hydration in clinical conditions: Indirect and direct approaches using bioimpedance. Nutrients 2019, 11, 809. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Sullivan, K.; Hornikel, B.; Holmes, C.J.; Metoyer, C.J.; Esco, M.R. Accuracy of a mobile 2D imaging system for body volume and subsequent composition estimates in a three-compartment model. Med. Sci. Sports Exerc. 2021, 53, 1003–1009. [Google Scholar] [CrossRef]
- Nana, A.; Staynor, J.M.D.; Arlai, S.; El-Sallam, A.; Dhungel, N.; Smith, M.K. Agreement of anthropometric and body composi-tion measures predicted from 2D smartphone images and body impedance scales with criterion methods. Obes. Res. Clin. Pract. 2022, 16, 37–43. [Google Scholar] [CrossRef]
- Majmudar, M.D.; Chandra, S.; Yakkala, K.; Kennedy, S.; Agrawal, A.; Sippel, M.; Ramu, P.; Chaudhr, A.; Smith, B.; Criminisi, A.; et al. Smartphone camera-based assessment of adiposity: A validation study. NPJ Digit. Med. 2022, 5, 79. [Google Scholar] [CrossRef]
- Farina, G.L.; Orlandi, C.; Lukaski, H.; Nescolarde, L. Digital single-image smartphone assessment of total body fat and abdominal fat using machine learning. Sensors 2022, 22, 8365. [Google Scholar] [CrossRef]
- Waki, M.; Kral, J.G.; Mazariegos, M.; Wang, J.; Pierson, R.N., Jr.; Heymsfield, S.B. Relative expansion of extracellular fluid in obese vs. nonobese women. Am. J. Physiol. 1991, 261 Pt 1, E199–E203. [Google Scholar] [CrossRef] [PubMed]
- Segal, K.R.; Gutin, B.; Presta, E.; Wang, J.; Van Itallie, T.B. Estimation of human body composition by electrical impedance methods: A comparative study. J. Appl. Physiol. 1985, 58, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Hodgdon, J.A.; Fitzgerald, P.I. Validity of impedance predictions at various levels of fatness. Hum. Biol. 1987, 59, 281–298. [Google Scholar] [PubMed]
- Kyle, U.G.; Genton, L.; Karsegard, L.; Slosman, D.O.; Pichard, C. Single prediction equation for bioelectrical impedance analysis in adults aged 20—94 years. Nutrition 2001, 17, 248–253. [Google Scholar] [CrossRef]
- Sun, S.S.; Chumlea, W.C.; Heymsfield, S.B.; Lukaski, H.C.; Schoeller, D.; Friedl, K.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Hubbard, V.S. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am. J. Clin. Nutr. 2003, 77, 331–340. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Piccoli, A. Bioelectrical impedance vector analysis for assessment of hydration in physiological states and clinical conditions. In Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease; Preedy, V.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 287–305. [Google Scholar]
- McBride, G.B. A Proposal for Strength-of-Agreement Criteria for Lin’s Concordance Correlation Coefficient; 2005 NIWA Client Report: HAM2005-062; NIWA: Singapore, 2005. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Marini, E.; Campa, F.; Buffa, R.; Stagi, S.; Matias, C.N.; Toselli, S.; Sardinha, L.B.; Silva, A.M. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin. Nutr. 2020, 39, 447–454. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Ross, R.; Heymsfield, S.B. Does adipose tissue affect bioelectrical impedance in obese men and women? J. Appl. Physiol. 1998, 84, 257–262. [Google Scholar] [CrossRef]
- Marken Lichtenbelt, W.D.; Fogelholm, M. Increased extracellular water compartment, relative to intracellular water compart-ment, after weight reduction. J. Appl. Physiol. 1999, 87, 294–298. [Google Scholar] [CrossRef]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Woodard, H.Q.; White, D.R. The composition of body tissues. Br. J. Radiol. 1986, 59, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Nescolarde, L.; Roca, E.; Bogónez-Franco, P.; Hernández-Hermoso, J.; Bayes-Genis, A.; Ara, J. Relationship between bioimpe-dance vector displacement and renal function after a marathon in non-elite runners. Front. Physiol. 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Scharfetter, H.; Brunner, P.; Mayer, M.; Brandstatter, B.; Hinghofer-Szalkay, H. Fat and hydration monitoring by abdominal bioimpedance analysis: Data interpretation by hierarchical electrical modeling. IEEE Trans. Biomed. Eng. 2005, 52, 975–982. [Google Scholar] [CrossRef]
- Organ, L.W.; Bradham, G.B.; Gore, D.T.; Lozier, S.L. Segmental bioelectrical impedance analysis: Theory and application of a new technique. J. Appl. Physiol. 1994, 77, 98–112. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Siders, W.A. Validity and accuracy of regional bioelectrical impedance devices to determine whole-body fatness. Nutrition 2003, 19, 851–857. [Google Scholar] [CrossRef]
- Chamney, P.W.; Wabel, P.; Moissl, U.M.; Muller, M.J.; Bosy-Westphal, A.; Korth, O.; Fuller, N.J. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am. J. Clin. Nutr. 2007, 85, 80–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Höglund, P.; Clyne, N. Comparison of DEXA and bioimpedance for body composition measurements in nondialysis patients with CKD. J. Ren. Nutr. 2019, 29, 33–38. [Google Scholar] [CrossRef]


| Females | Males | Rugby and Non-Rugby Males | ||
|---|---|---|---|---|
| BMI > 25 kg/m² | ||||
| Total | Total | Rugby | Non-Rugby | |
| N | 69 | 119 | 54 | 40 |
| Age, y | 37 ± 13 | 28 ± 9 | 26 ± 3 | 36 ± 15 |
| (19–65) | (19–68) | (20–34) | (19–68) | |
| Height, cm | 162.7 ± 6.3 | 182.5 ± 8.7 | 186.2 ± 7.2 | 179.7 ± 9.2 |
| (150.0–178.0) | (160.0–202.0) | (173.0–202.0) | (163.0–198.0) | |
| Weight, kg | 67.8 ± 14.5 | 93.2 ± 13.3 | 104.8 ± 11.4 | 91.4 ± 12.2 |
| (41.8–103.6) | (61.1–125.6) | (79.4–123.0) | (69.5–124.6) | |
| BMI, kg/m2 | 25.6 ± 5.4 | 27.6 ± 4.0 | 30.4 ± 3.3 | 28.7 ± 3.0 |
| (16.1–37.2) | (19.5–37.0) | (25.2–36.6) | (25.1–37.1) | |
| DXA Fat, kg | 24.3 ± 11.6 | 18.8 ± 7.7 | 19.3 ± 6.5 | 23.3 ± 6.9 |
| (6.4–52.3) | (5.6–35.2) | (9.0–35.2) | (10.3–35.1) | |
| DXA Fat, % | 34.2 ± 10.2 | 19.8 ± 6.7 | 18.2 ± 4.7 | 25.4 ± 6.2 |
| (12.1–50.5) | (8.3–36.8) | (9.8–28.9) | (11.4–36.8) | |
| DXA FFM, kg | 43.5 ± 5.6 | 74.4 ± 12.7 | 84.7 ± 6.6 | 68.1 ± 10.1 |
| (31.0–55.8) | (47.4–103.2) | (68.9–103.2) | (50.6–97.2) | |
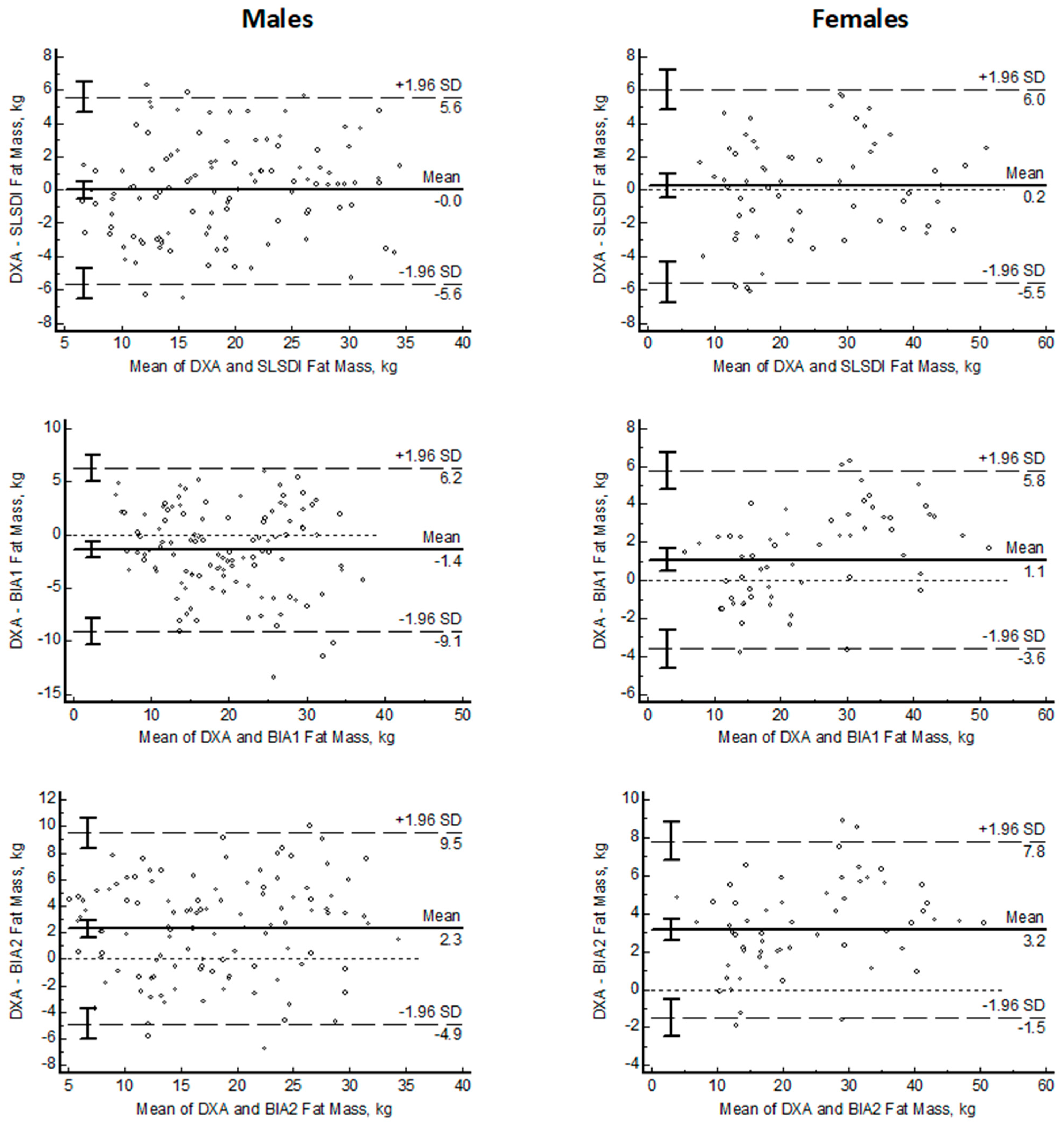
| Males N = 119 | Females N = 69 | ||
|---|---|---|---|
| DXA | Fat mass, kg | 18.8 ± 7.6 | 24.3 ± 11.6 |
| SLSDI | Fat mass, kg | 18.8 ± 7.2 | 24.1 ± 11.3 |
| Bias, kg | −0.1 ± 2.9 | 0.2 ± 3.0 | |
| p | 0.97 | 0.29 | |
| SEE, kg | 2.9 | 3.0 | |
| CCC | 0.93 | 0.96 | |
| MAE, kg | 2.3 ± 1.7 | 2.4 ± 1.6 | |
| MAPE, % | 14.8 ± 13.3 | 13.8 ± 14.3 | |
| LOA 95% CI, kg | 5.6–(−5.6) | 6.0–(−5.5) | |
| BIA1 | Fat mass, kg | 20.3 ± 8.2 | 23.2 ± 10.6 |
| Bias, kg | −1.4 ± 3.9 | 1.1 ± 2.4 | |
| p | 0.0001 | 0.0001 | |
| SEE, kg | 3.7 | 2.3 | |
| CCC | 0.86 | 0.98 | |
| MAE, kg | 3.3 ± 2.5 | 2.2 ± 1.4 | |
| MAPE, % | 20.8 ± 18.3 | 9.9 ± 6.2 | |
| LOA 95% CI, kg | 6.2–(−9.1) | 5.8–(−3.6) | |
| BIA2 | Fat mass, kg | 21.1 ± 7.3 | 21.1 ± 10.9 |
| Bias, kg | 2.3 ± 3.7 | 3.2 ± 2.4 | |
| p | 0.0001 | 0.0001 | |
| SEE, kg | 3.6 | 2.3 | |
| CCC | 0.84 | 0.94 | |
| MAE, kg | 3.6 ± 2.4 | 3.4 ± 2.1 | |
| MAPE, % | 21.3 ± 15.8 | 16.0 ± 13.9 | |
| LOA 95% CI, kg | 9.5–(−4.9) | 7.8–(−1.5) |
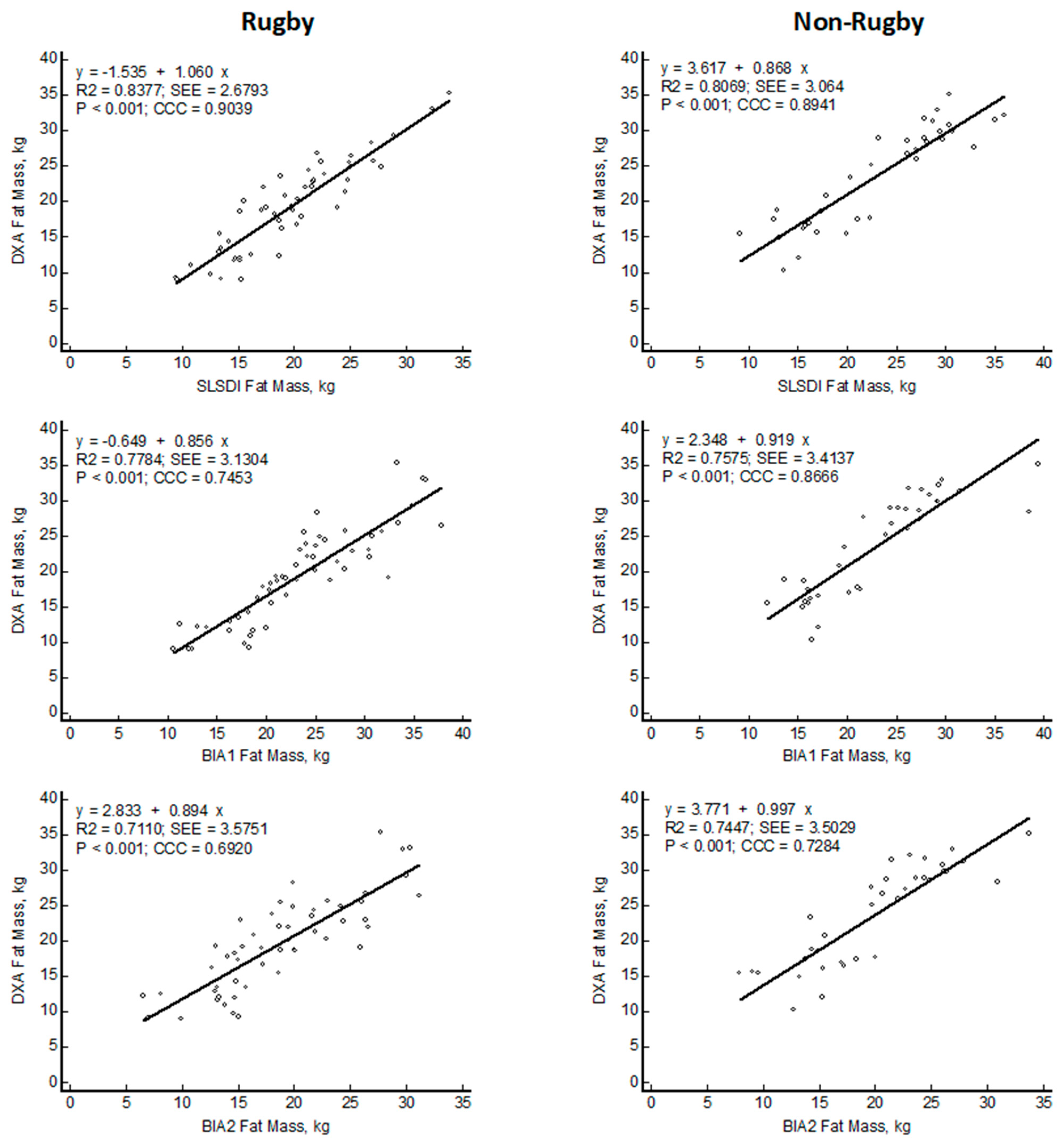
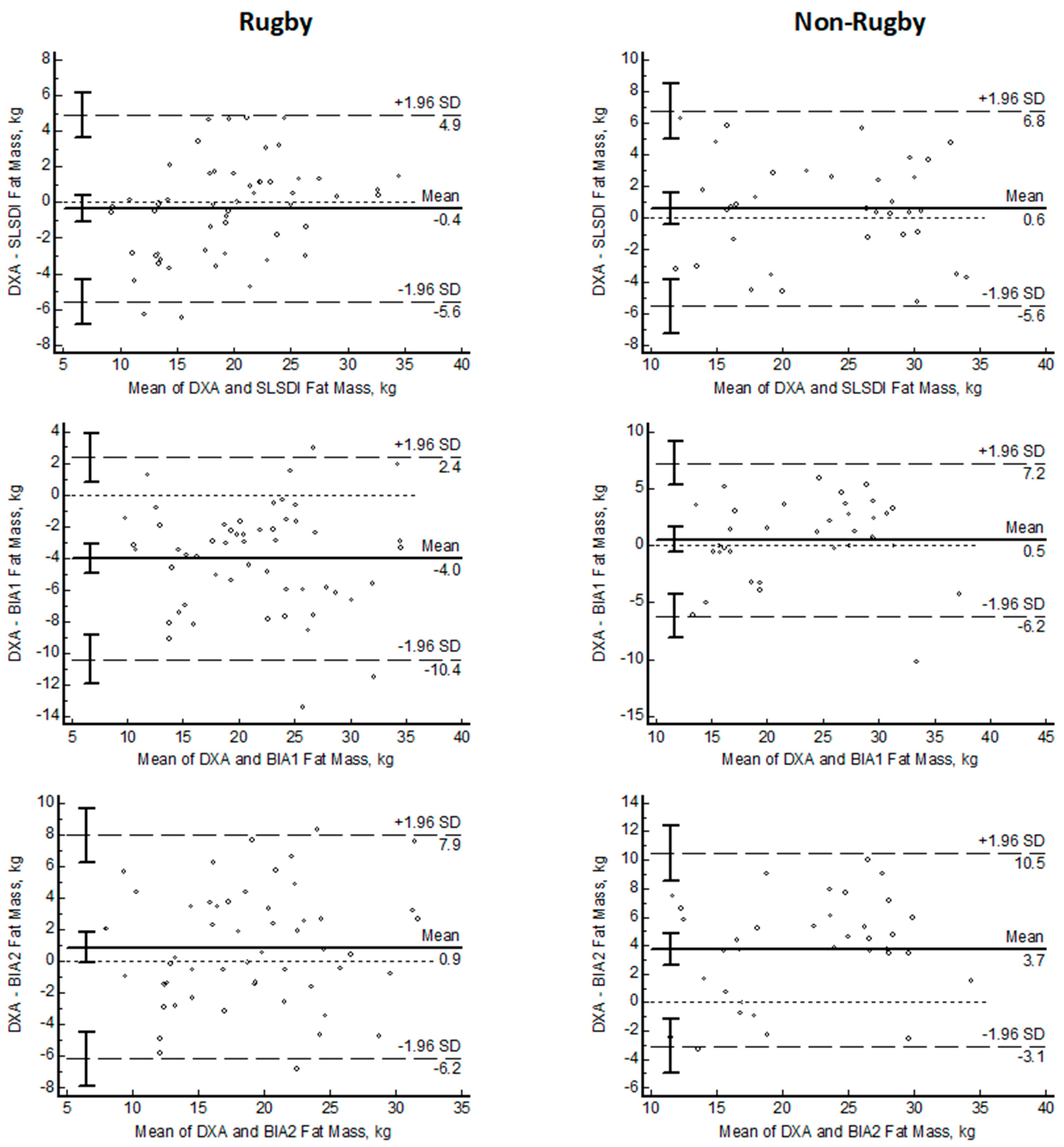
| Rugby N = 54 | Non-Rugby N = 40 | ||
|---|---|---|---|
| DXA | Fat mass, kg | 19.3 ± 6.6 | 23.3 ± 6.8 |
| SLSDI | Fat mass, kg | 19.7 ± 5.7 | 22.7 ± 7.1 |
| Bias, kg | −0.4 ± 2.7 | 0.6 ± 3.2 | |
| p | 0.33 | 0.22 | |
| SEE, kg | 2.6 | 3.1 | |
| CCC | 0.92 | 0.89 | |
| MAE, kg | 2.1 ± 1.7 | 2.6 ± 1.8 | |
| MAPE, % | 13.0 ± 14.2 | 12.8 ± 10.5 | |
| LOA 95% CI, kg | 4.9–(−5.6) | 6.8–(−5.6) | |
| BIA1 | Fat mass, kg | 23.4 ± 6.8 | 22.8 ± 6.5 |
| Bias, kg | −4.0 ± 3.3 | 0.5 ± 3.4 | |
| p | 0.0001 | 0.36 | |
| SEE, kg | 3.1 | 3.4 | |
| CCC | 0.78 | 0.87 | |
| MAE, kg | 4.3 ± 2.9 | 2.6 ± 2.2 | |
| MAPE, % | 25.8 ± 21.2 | 12.5 ± 12.4 | |
| LOA 95% CI, kg | 2.4–(−10.4) | 7.2–(−6.2) | |
| BIA2 | Fat mass, kg | 18.5 ± 6.2 | 19.7 ± 5.9 |
| Bias, kg | 0.9 ± 3.6 | 3.7 ± 3.5 | |
| p | 0.08 | 0.0001 | |
| SEE, kg | 3.5 | 3.5 | |
| CCC | 0.85 | 0.73 | |
| MAE, kg | 3.0 ± 2.1 | 4.4 ± 2.5 | |
| MAPE, % | 16.8 ± 13.1 | 19.1 ± 11.0 | |
| LOA 95% CI, kg | 7.9–(−6.2) | 10.5–(−3.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nescolarde, L.; Orlandi, C.; Farina, G.L.; Gori, N.; Lukaski, H. Fluid-Dependent Single-Frequency Bioelectrical Impedance Fat Mass Estimates Compared to Digital Imaging and Dual X-ray Absorptiometry. Nutrients 2023, 15, 4638. https://doi.org/10.3390/nu15214638
Nescolarde L, Orlandi C, Farina GL, Gori N, Lukaski H. Fluid-Dependent Single-Frequency Bioelectrical Impedance Fat Mass Estimates Compared to Digital Imaging and Dual X-ray Absorptiometry. Nutrients. 2023; 15(21):4638. https://doi.org/10.3390/nu15214638
Chicago/Turabian StyleNescolarde, Lexa, Carmine Orlandi, Gian Luca Farina, Niccolo’ Gori, and Henry Lukaski. 2023. "Fluid-Dependent Single-Frequency Bioelectrical Impedance Fat Mass Estimates Compared to Digital Imaging and Dual X-ray Absorptiometry" Nutrients 15, no. 21: 4638. https://doi.org/10.3390/nu15214638
APA StyleNescolarde, L., Orlandi, C., Farina, G. L., Gori, N., & Lukaski, H. (2023). Fluid-Dependent Single-Frequency Bioelectrical Impedance Fat Mass Estimates Compared to Digital Imaging and Dual X-ray Absorptiometry. Nutrients, 15(21), 4638. https://doi.org/10.3390/nu15214638






