The Mediterranean Diet for Adolescents with Type 1 Diabetes: A Prospective Interventional Study
Highlights
- The Mediterranean diet (MED) intervention significantly improved glycemic control, evidenced by an increase in time-in-range (TIR) from 52% to 63% (p=0.047).
- Adherence to the MED showed a marked improvement, reflected in an increase in the I-MEDAS score from 8 to 13 (p<0.001).
- The intervention enhanced monounsaturated fat intake, reduced diastolic blood pressure (p=0.028), and demonstrated a trend toward lower LDL cholesterol levels (p=0.059).
- These findings highlight the feasibility and clinical utility of the Mediterranean diet as a structured dietary intervention for adolescents with type 1 diabetes, with potential benefits in glycemic regulation, cardiovascular risk factors, and dietary adherence.
Abstract
:1. Introduction
2. Materials and Methods
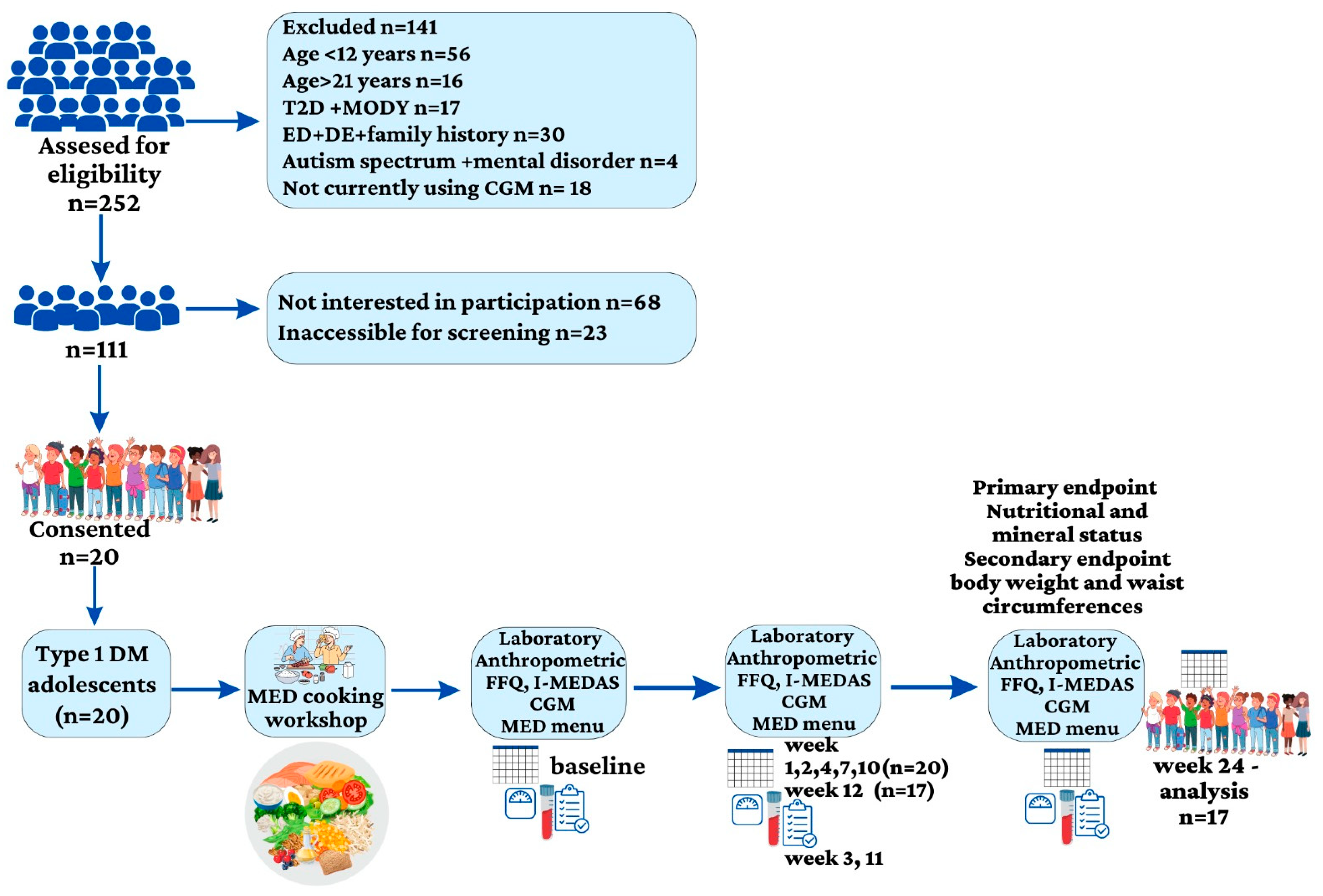
2.1. Participants and Study Design
2.2. Diet Intervention
2.3. Mediterranean Diet
2.4. Assessment of Nutritional Composition and the Mediterranean Diet Screener Score
2.5. Medical History and Anthropometric Measurements
2.6. Insulin and Glycemic Parameters
2.7. Biochemical Parameters
2.8. Trial Outcomes
2.9. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. Food Frequency Questionnaire
3.2.1. Median Percentages of Micronutrients According to DRI
3.2.2. Median Intakes of Selected Nutrients before and after the MED Intervention
3.3. I-MEDAS
3.4. Weight Loss, Waist Circumference, and Blood Pressure
3.5. Glycemic Parameters
3.6. Blood Laboratory Measurements
3.7. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 Diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Wood, J.R.; Miller, K.M.; Maahs, D.M.; Beck, R.W.; DiMeglio, L.A.; Libman, I.M.; Quinn, M.; Tamborlane, W.V.; Woerner, S.E.; T1D Exchange Clinic Network. Most Youth with Type 1 Diabetes in the T1D Exchange Clinic Registry Do Not Meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care 2013, 36, 2035–2037. [Google Scholar] [CrossRef]
- Khadilkar, A.; Oza, C. Glycaemic Control in Youth and Young Adults: Challenges and Solutions. Diabetes Metab. Syndr. Obes. 2022, 15, 121–129. [Google Scholar] [CrossRef]
- Annan, S.F.; Higgins, L.A.; Jelleryd, E.; Hannon, T.; Rose, S.; Salis, S.; Baptista, J.; Chinchilla, P.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional Management in Children and Adolescents with Diabetes. Pediatr. Diabetes 2022, 23, 1297–1321. [Google Scholar] [CrossRef]
- Boucher, J.L. Mediterranean Eating Pattern. Diabetes Spectr. 2017, 30, 72–76. [Google Scholar] [CrossRef]
- Mańkiewicz-Żurawska, I.; Jarosz-Chobot, P. Nutrition of Children and Adolescents with Type 1 Diabetes in the Recommendations of the Mediterranean Diet. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 74–80. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, Youth and the Mediterranean Diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in Children and Adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef]
- Zhong, V.W.; Lamichhane, A.P.; Crandell, J.L.; Couch, S.C.; Liese, A.D.; The, N.S.; Tzeel, B.A.; Dabelea, D.; Lawrence, J.M.; Marcovina, S.M.; et al. Association of Adherence to a Mediterranean Diet with Glycemic Control and Cardiovascular Risk Factors in Youth with Type I Diabetes: The SEARCH Nutrition Ancillary Study. Eur. J. Clin. Nutr. 2016, 70, 802–807. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean Diet and Health: A Comprehensive Overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Dominguez-Riscart, J.; Buero-Fernandez, N.; Garcia-Zarzuela, A.; Morales-Perez, C.; Garcia-Ojanguren, A.; Lechuga-Sancho, A.M. Adherence to Mediterranean Diet Is Associated with Better Glycemic Control in Children with Type 1 Diabetes: A Cross-Sectional Study. Front. Nutr. 2022, 9, 813989. [Google Scholar] [CrossRef]
- Antoniotti, V.; Spadaccini, D.; Ricotti, R.; Carrera, D.; Savastio, S.; Goncalves Correia, F.P.; Caputo, M.; Pozzi, E.; Bellone, S.; Rabbone, I.; et al. Adherence to the Mediterranean Diet Is Associated with Better Metabolic Features in Youths with Type 1 Diabetes. Nutrients 2022, 14, 596. [Google Scholar] [CrossRef]
- Cadario, F.; Prodam, F.; Pasqualicchio, S.; Bellone, S.; Bonsignori, I.; Demarchi, I.; Monzani, A.; Bona, G. Lipid Profile and Nutritional Intake in Children and Adolescents with Type 1 Diabetes Improve after a Structured Dietician Training to a Mediterranean-Style Diet. J. Endocrinol. Investig. 2012, 35, 160–168. [Google Scholar] [CrossRef]
- Chiang, J.L.; Maahs, D.M.; Garvey, K.C.; Hood, K.K.; Laffel, L.M.; Weinzimer, S.A.; Wolfsdorf, J.I.; Schatz, D. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care 2018, 41, 2026–2044. [Google Scholar] [CrossRef]
- Potter, J.D. Book Review Eat, Drink, and Be Healthy: The Harvard Medical School Guide to Healthy Eating by Walter C. Willett. 299 Pp. New York, Simon & Schuster, 2001. $25. 0-684-86337-5. New Engl. J. Med. 2002, 346, 633. [Google Scholar] [CrossRef]
- Shai, I.; Rosner, B.A.; Shahar, D.R.; Vardi, H.; Azrad, A.B.; Kanfi, A.; Schwarzfuchs, D.; Fraser, D. Dietary Evaluation and Attenuation of Relative Risk: Multiple Comparisons between Blood and Urinary Biomarkers, Food Frequency, and 24-Hour Recall Questionnaires: The DEARR Study. J. Nutr. 2005, 135, 573–579. [Google Scholar] [CrossRef]
- Ministry of Health Public Health Services Nutrition Division. Tzameret-Israeli National Nutrient Database 2015; Ministry of Health Public Health Services Nutrition Division: Jerusalem, Isreal, 2015.
- Abu-Saad, K.; Endevelt, R.; Goldsmith, R.; Shimony, T.; Nitsan, L.; Shahar, D.R.; Keinan-Boker, L.; Ziv, A.; Kalter-Leibovici, O. Adaptation and Predictive Utility of a Mediterranean Diet Screener Score. Clin. Nutr. 2019, 38, 2928–2935. [Google Scholar] [CrossRef]
- Galilea-Zabalza, I.; Buil-Cosiales, P.; Salas-Salvadó, J.; Toledo, E.; Ortega-Azorín, C.; Díez-Espino, J.; Vázquez-Ruiz, Z.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; et al. Mediterranean Diet and Quality of Life: Baseline Cross-Sectional Analysis of the PREDIMED-PLUS Trial. PLoS ONE 2018, 13, e0198974. [Google Scholar] [CrossRef]
- Baker-Smith, C.M.; Flinn, S.K.; Flynn, J.T.; Kaelber, D.C.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; et al. Diagnosis, Evaluation, and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2018, 142, e20182096. [Google Scholar] [CrossRef]
- Vanderwall, C.; Eickhoff, J.; Randall Clark, R.; Carrel, A.L. BMI Z-Score in Obese Children Is a Poor Predictor of Adiposity Changes over Time. BMC Pediatr. 2018, 18, 187. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013; ISBN 9781134742707. [Google Scholar]
- Agregán, R.; Popova, T.; López-Pedrouso, M.; Cantalapiedra, J.; Lorenzo, J.M.; Franco, D. Fatty Acids. In Food Lipids; Elsevier: Amsterdam, The Netherlands, 2022; pp. 257–286. [Google Scholar]
- Mayneris-Perxachs, J.; Sala-Vila, A.; Chisaguano, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Lamuela-Raventós, R.; et al. Effects of 1-Year Intervention with a Mediterranean Diet on Plasma Fatty Acid Composition and Metabolic Syndrome in a Population at High Cardiovascular Risk. PLoS ONE 2014, 9, e85202. [Google Scholar] [CrossRef] [PubMed]
- Levran, N.; Levek, N.; Sher, B.; Gruber, N.; Afek, A.; Monsonego-Ornan, E.; Pinhas-Hamiel, O. The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes. Nutrients 2023, 15, 1418. [Google Scholar] [CrossRef]
- Nitta, A.; Imai, S.; Kajiayama, S.; Matsuda, M.; Miyawaki, T.; Matsumoto, S.; Kajiyama, S.; Hashimoto, Y.; Ozasa, N.; Fukui, M. Impact of Dietitian-Led Nutrition Therapy of Food Order on 5-Year Glycemic Control in Outpatients with Type 2 Diabetes at Primary Care Clinic: Retrospective Cohort Study. Nutrients 2022, 14, 2865. [Google Scholar] [CrossRef]
- Alman, A.C.; Talton, J.W.; Wadwa, R.P.; Urbina, E.M.; Dolan, L.M.; Daniels, S.R.; Hamman, R.F.; D’Agostino, R.B.; Marcovina, S.M.; Mayer-Davis, E.J.; et al. Cardiovascular Health in Adolescents with Type 1 Diabetes: The SEARCH CVD Study. Pediatr. Diabetes 2014, 15, 502–510. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, S.; Su, Y.; Ying, C.; Luo, H. Effect of Fruit on Glucose Control in Diabetes Mellitus: A Meta-Analysis of Nineteen Randomized Controlled Trials. Front. Endocrinol. 2023, 14, 1174545. [Google Scholar] [CrossRef]
- Ola, M.S.; Al-Dosari, D.; Alhomida, A.S. Role of Oxidative Stress in Diabetic Retinopathy and the Beneficial Effects of Flavonoids. Curr. Pharm. Des. 2018, 24, 2180–2187. [Google Scholar] [CrossRef]
- Leyvraz, M.; Chatelan, A.; da Costa, B.R.; Taffé, P.; Paradis, G.; Bovet, P.; Bochud, M.; Chiolero, A. Sodium Intake and Blood Pressure in Children and Adolescents: A Systematic Review and Meta-Analysis of Experimental and Observational Studies. Int. J. Epidemiol. 2018, 47, 1796–1810. [Google Scholar] [CrossRef]
- Huang, L.; Trieu, K.; Yoshimura, S.; Neal, B.; Woodward, M.; Campbell, N.R.C.; Li, Q.; Lackland, D.T.; Leung, A.A.; Anderson, C.A.M.; et al. Effect of Dose and Duration of Reduction in Dietary Sodium on Blood Pressure Levels: Systematic Review and Meta-Analysis of Randomised Trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef]
- Viroli, G.; Gonçalves, C.; Pinho, O.; Silva-Santos, T.; Padrão, P.; Moreira, P. High Adherence to Mediterranean Diet Is Not Associated with an Improved Sodium and Potassium Intake. Nutrients 2021, 13, 4151. [Google Scholar] [CrossRef]
- Vasara, E.; Marakis, G.; Breda, J.; Skepastianos, P.; Hassapidou, M.; Kafatos, A.; Rodopaios, N.; Koulouri, A.A.; Cappuccio, F.P. Sodium and Potassium Intake in Healthy Adults in Thessaloniki Greater Metropolitan Area-The Salt Intake in Northern Greece (SING) Study. Nutrients 2017, 9, 417. [Google Scholar] [CrossRef]
- Catherine, R.; Benjemin, C.; Robert, C.; Katherine, T.; Thomes, Z. Moderen Nutrition in Health Ad Disease, 11th ed.; Lipponcott Williams & Wilkins: Baltimore, MD, USA, 2012. [Google Scholar]
- Wu, Y.; Lu, H.; Yang, H.; Li, C.; Sang, Q.; Liu, X.; Liu, Y.; Wang, Y.; Sun, Z. Zinc Stimulates Glucose Consumption by Modulating the Insulin Signaling Pathway in L6 Myotubes: Essential Roles of Akt-GLUT4, GSK3β and MTOR-S6K1. J. Nutr. Biochem. 2016, 34, 126–135. [Google Scholar] [CrossRef]
- Little, P.J.; Bhattacharya, R.; Moreyra, A.E.; Korichneva, I.L. Zinc and Cardiovascular Disease. Nutrition 2010, 26, 1050–1057. [Google Scholar] [CrossRef]
- Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998; ISBN 0309064112.
- Eshak, E.S.; Iso, H.; Maruyama, K.; Muraki, I.; Tamakoshi, A. Associations between Dietary Intakes of Iron, Copper and Zinc with Risk of Type 2 Diabetes Mellitus: A Large Population-Based Prospective Cohort Study. Clin. Nutr. 2018, 37, 667–674. [Google Scholar] [CrossRef]
- Squitti, R.; Negrouk, V.; Perera, M.; Llabre, M.M.; Ricordi, C.; Rongioletti, M.C.A.; Mendez, A.J. Serum Copper Profile in Patients with Type 1 Diabetes in Comparison to Other Metals. J. Trace Elem. Med. Biol. 2019, 56, 156–161. [Google Scholar] [CrossRef]
- Wolff, S.P.; Dean, R.T. Glucose Autoxidation and Protein Modification. The Potential Role of ‘Autoxidative Glycosylation’ in Diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef]
- Oost, L.J.; van Heck, J.I.P.; Tack, C.J.; de Baaij, J.H.F. The Association between Hypomagnesemia and Poor Glycaemic Control in Type 1 Diabetes Is Limited to Insulin Resistant Individuals. Sci. Rep. 2022, 12, 6433. [Google Scholar] [CrossRef]
- Pérez-Segura, P.; de Dios, O.; Herrero, L.; Vales-Villamarín, C.; Aragón-Gómez, I.; Gavela-Pérez, T.; Garcés, C.; Soriano-Guillén, L. Children with Type 1 Diabetes Have Elevated High-Sensitivity C-Reactive Protein Compared with a Control Group. BMJ Open Diabetes Res. Care 2020, 8, e001424. [Google Scholar] [CrossRef]
- Grandjean, A.C. Dietary Intake Data Collection: Challenges and Limitations. Nutr. Rev. 2012, 70 (Suppl. S2), S101–S104. [Google Scholar] [CrossRef]
| Baseline | After 6 Months | Delta | p-Value | |
|---|---|---|---|---|
| %Protein from calories | 17 (16–20) | 18 (16–20) | 0.0 (−1.1–2.3) | 0.552 |
| %Fat from calories | 35 (32–37) | 38 (34–40) | 2 (−1–5) | 0.092 |
| %Carbohydrate from calories | 42 (39–46) | 39 (35–46) | −2.7 (−8.3–1.5) | 0.058 |
| Percent DRI | ||||
| Fiber | 100 (72–113) | 111 (77–133) | 0.5 (−8.7–47.2) | 0.501 |
| Calcium | 120 (75–160) | 131 (76–149) | 1.5 (−25.7–16) | 0.816 |
| Iron | 75 (59–189) | 84 (60–129) | 0.0 (−35–15) | 0.868 |
| Magnesium | 143 (94–189) | 161 (116–195) | 21 (0–43) | 0.068 |
| Phosphorus | 241 (140–271) | 221 (149–278) | 0 (−27–31) | 0.981 |
| Potassium | 92 (71–107) | 87 (69–107) | −0.5 (−8.5–16.7) | 0.887 |
| Sodium | 250 (194–332) | 251 (186–326) | 0.5 (−37.0–25.7) | 0.653 |
| Zinc | 142 (115–159) | 131 (109–167) | 0 (−8–20) | 0.795 |
| Copper | 189 (145–262) | 180 (144–245) | 0 (−13–49) | 0.756 |
| Vitamin C | 302 (225–405) | 283 (217–358) | 1 (−107–111) | 0.984 |
| Thiamin B1 | 127 (110–169) | 130 (114–166) | 0 (−4–29) | 0.408 |
| Riboflavin B2 | 217 (161–261) | 225 (167–266) | 0 (−23–56) | 0.463 |
| Niacin B3 | 164 (122–211) | 168 (135–224) | 5 (−11–42) | 0.309 |
| Folate B9 | 105 (79–137) | 105 (86–122) | 0 (−17–32) | 0.981 |
| Pyridoxine B6 | 205 (154(259) | 200 (160–273) | 0 (−30–30) | 0.868 |
| Vitamin A | 134 (88–196) | 131 (110–166) | 0 (−17–41) | 0.687 |
| Vitamin E | 85 (60–121) | 93 (69–119) | 8 (−9–25) | 0.266 |
| Vitamin D | 141 (98–246) | 210 (83–254) | 17 (−3–51) | 0.103 |
| Baseline | After 6 Months | Delta | p-Value | |
|---|---|---|---|---|
| Total calories, kcal | 2077.8 (1840.7–2661.9) | 2050.9 (1770.4–2827.5) | 0.0 (−306.3–268.3) | 0.943 |
| Energy percent ultra-process | 17.7 (13.5–21.8) | 15.2 (7.9–21.4) | 0.0 (−9.4–1.3) | 0.255 |
| Protein, g | 97.1 (81.7–120.9) | 97.9 (79.1–125.8) | 0.7 (−11.3–8.2) | 0.906 |
| Fat, g | 85.7 (71.8–104.8) | 89.6 (69.5–104.8) | 1.1 (−8.7–15.7) | 0.554 |
| Carbohydrate, g | 222.0 (188.8–306.0) | 197.8 (159.6–278.3) | 0.0 (−63.4–25.3) | 0.356 |
| Total sugars, g | 100.9 (64.1–136.6) | 88.7 (70.9–106.9) | −0.2 (−18.4–12.1) | 0.523 |
| Sugar alcohols, g | 0.28 (0.1–0.7) | 0.2 (0.1–1.7) | 0.0 (−0.2–0.2) | 0.975 |
| Fructose, g | 19.4 (11.1–25.5) | 19.2 (12.4–25.0) | 0.0 (−5.4–4.0) | 0.653 |
| Fiber, g | 27.3 (19.9–41.3) | 28.5 (23.5–42.1) | 0.0 (−2.2–8.1) | 0.795 |
| Calcium, mg | 1283.1 (775.2–1639.8) | 1308.4 (899.1–1692.1) | 31.9 (−234.7–204.3) | 0.554 |
| Iron, mg | 11.9 (10.3–17.9) | 11.9 (10.4–17.6) | 0.0 (−3.1–2.3) | 0.981 |
| Magnesium, mg | 479.2 (327.9–631.4) | 552.8 (398.3–691.4) | 66.0 (0.0–128.8) | 0.084 |
| Phosphorus, mg | 1768.9 (1335.6–2038.1) | 1724.4 (1410.9–2023.7) | 0.00 (−144.6–356.1) | 0.586 |
| Potassium, mg | 4304.0 (3338.1–5069.6) | 4110.3 (3248.1–5044.1) | −13.7 (−414.7–789.2) | 0.906 |
| Sodium, mg | 3757.9 (2914.5–4994.4) | 3771.1 (2800.2–4900.8) | −2.7 (−551.4–387.2) | 0.687 |
| Zinc, mg | 12.1 (9.9–14.1) | 11.9 (8.8–15.6) | 0.0 (−1.4–1.7) | 0.906 |
| Copper, mg | 1.9 (1.4–2.6) | 1.9 (1.4–2.5) | 0.0 (−0.1–0.4) | 0.723 |
| Selenium, mcg | 132.9 (118.4–181.1) | 148.9 (105.0–183.0) | 0.0 (−17.2–26.7) | 0.687 |
| Choline, mg | 503.2 (345.7–682.0) | 517.6 (339.1–717.9) | 0.0 (−145.4–107.3) | 0.906 |
| Vitamin A, mcg | 2081.0 (1281.1–2597.6) | 1934.1 (1202.8–2304.4) | 5.19 (−406.6–233.9) | 0.723 |
| Vitamin C, mg | 228.4 (162.9–304.1) | 212.7 (148.1–274.1) | 0.0 (−32.8–33.7) | 0.981 |
| Thiamin B1, mg | 1.3 (1.1–1.9) | 1.4 (1.2–1.8) | 0.0 (−0.4–0.2) | 0.687 |
| Riboflavin B2, mg | 2.2 (1.7–3.3) | 2.5 (2.0–3.0) | 0.0 (−0.3–0.5) | 0.653 |
| Niacin B3, mg | 23.0 (18.8–29.8) | 25.1 (18.4–32.8) | 0.0 (−2.3–6.1) | 0.523 |
| Vitamin B6, mg | 2.6 (1.9–3.3) | 2.5 (1.9–3.3) | 0.0 (−0.7–0.4) | 0.831 |
| Folate B9, mcg | 422.3 (316.3–551.2) | 423.4 (331.6–488.6) | 0.0 (−127.8–127.5) | 0.943 |
| Vitamin B12 mcg | 5.8 (3.8–7.0) | 6.1 (4.1–7.2) | 0.0 (−0.8–0.5) | 0.981 |
| Vitamin D, mcg | 7.0 (4.8–12.3) | 9.8 (4.1–12.6) | 0.5 (−1.2–2.6) | 0.246 |
| Vitamin K, mcg | 224.5 (184.3–354.4) | 218 (155.6–317.0) | −4.0 (−56.5–35.5) | 0.356 |
| Vitamin E, mcg | 12.7 (9.0–17.1) | 13.2 (10.3–17.2) | 0.0 (−3.6–3.7) | 0.460 |
| Cholesterol, mg | 395.1 (262.6–559.3) | 346.0 (242.3–617.1) | 0.0 (−99.2–45.4) | 0.831 |
| Saturated fat, mg | 27.9 (20.7–31.6) | 27.1 (19.7–30.9) | −1.1 (−3.1–0.7) | 0.287 |
| Monounsaturated fat, g | 34.1 (27.0–42.4) | 39.3 (29.7–43.2) | 2.3 (0.0–11.4) | 0.149 |
| Polyunsaturated fat, g | 17.7 (15.0–24.2) | 18.2 (13.1–25.4) | 0.0 (−3.3–3.4) | 0.906 |
| Docosahexaenoic acid (DHA), g | 0.1018 (0.0433–0.1614) | 0.1555 (0.0474–0.1994) | 0.0184 (0.0785) | 0.035 |
| Palmitoleic acid, g | 1.0639 (0.8908–1.3945) | 1.0873 (0.7696–1.6299) | −0.0069 (−0.1928–0.2098) | 0.943 |
| Parinaric acid, g | 0.0196 (0.004–0.0278) | 0.0198 (0.0052–0.0556) | −0.0454 (−0.0832; −0.0056) | 0.049 |
| Eicosapentaenoic acid (EPA), g | 0.0266 (0.0081–0.0419) | 0.0364 (0.0158–0.0736) | 0.0048 (0.0000–0.0323) | 0.035 |
| Erucic acid, g | 0.0431 (0.0051–0.0604) | 0.0435 (0.0134–0.1145) | 0.0042 (0.0000–0.0526) | 0.031 |
| Docosapentaenoic acid (DPA), g | 0.0220 (0.0117–0.0392) | 0.0312 (0.0137–0.0476) | 0.0015 (−0.0001–0.0174) | 0.049 |
| Baseline | Six Months | p-Value | ||
|---|---|---|---|---|
| Total Score | 8 (7–11) | 13 (12–14) Delta: 5 (2–6) | <0.001 | |
| The criterion for a positive score | ||||
| Preference for olive oil | Yes | Y = 1 (5%) N = 19 (95%) | Y = 18 (90%) N = 2 (90%) | <0.001 |
| Poultry more than red/processed meat | Yes | Y = 12 (60%) N = 8 (40%) | Y = 19 (95%) N = 1 (5%) | 0.016 |
| Non-starchy vegetables | 2+ servings/d | Y = 16 (80%) N = 4 (20%) | Y = 19 (95%) N = 1 (5%) | 0.25 |
| Fruits- without juice | 3+ servings/d | Y = 4 (20%) N = 16 (80%) | Y = 6 (30%) N = 14 (70%) | 0.625 |
| Butter/Margarine | <1 serving/d | Y = 9 (45%) N = 11 (55%) | Y = 16 (80%) N = 4 (20%) | 0.039 |
| Sweet soft drinks | <1 serving/d | Y = 13 (65%) N = 7 (35%) | Y = 18 (90%) N = 2 (10%) | 0.063 |
| Whole grains | 3 + servings/d | Y = 7 (35%) N = 13 (65%) | Y = 13 (65%) N = 7 (35%) | 0.07 |
| Red and ultra-processed meat | <7 servings/wk | Y = 15 (75%) N = 5 (25%) | Y = 15 (75%) N = 5 (25%) | * |
| Alcohol | 7+ servings/wk | Y = 20 (100%) N = 0 (0%) | Y = 20 (100%) N = 0 (0%) | * |
| Non sweetened dairy | 2 + servings/d | Y = 16 (80%) N = 4 (20%) | Y = 17 (85%) N = 3 (15%) | 0.999 |
| Legumes | 3+ servings/d | Y = 1 (5%) N = 19 (95%) | Y = 8 (40%) N = 12 (60%) | 0.016 |
| Fish (fresh& preserved) | 3 + servings/wk | Y = 10 (55%) N = 10 (50%) | Y = 17 (85%) N = 3 (15%) | 0.016 |
| Nuts | 3 + servings/wk | Y = 3 (15%) N = 17 (85%) | Y = 12 (60%) N = 8 (40%) | 0.004 |
| Hummus/tahini salad | 3 + servings/wk | Y = 7 (35%) N = 13 (65%) | Y = 6 (30%) N = 14 (70%) | 0.999 |
| Desserts | <3 servings/wk | Y = 7 (35%) N = 13 (65%) | Y = 15 (75%) N = 5 (25%) | 0.008 |
| Savory pastries | ≤2 servings/wk | Y = 16 (80%) N = 4 (20%) | Y = 19 (95%) N = 1 (5%) | 0.375 |
| Salty snacks | ≤3 servings/wk | Y = 16 (80%) N = 4 (20%) | Y = 16 (80%) N = 4 (20%) | * |
| Before | After | Change | p-Value | ||
|---|---|---|---|---|---|
| Anthropometric measurements | BMI z-score | 1.1 (0.6–1.3) | 1.2 (0.5–1.5) | 0.0 (−0.1–0.1) | 0.316 |
| Waist circumference percentile | 71.0 (35.5–79.0) | 59.5 (26.5–77.0) | −1.0 (−5.5–1.7) | 0.161 | |
| Glucose variables | TIR 70–180 mg/dL | 52 (38–60) | 63 (47–71) | 7 (−1–14) | 0.047 |
| Total daily dose unit/kg | 0.76 (0.64–0.97) | 0.72 (0.61–0.89) | −0.04 (−0.13–0.00) | 0.067 | |
| Blood pressure percentiles | Systolic % | 64 (38–94) | 60 (30–72) | −1 (−28–14) | 0.349 |
| Diastolic% | 73 (68–88) | 69 (50–79) | −8 (−21–5) | 0.028 | |
| Blood tests | HbA1c % | 7.5 (6.8–8.5) | 7.1 (6.7–7.9) | 0.0 (−0.6–0.3) | 0.453 |
| Cholesterol mg/dL | 176 (160–186) | 171 (156–189) | 0 (−22–7) | 0.293 | |
| LDL cholesterol mg/dL | 114 (105–134) | 104 (96–124) | −3.5 (−24–1.5) | 0.059 | |
| HDL cholesterol mg/dL | 64 (58–69) | 62 (50–74) | −2 (−8–1) | 0.195 | |
| Triglyceride mg/dL | 68 (61–95) | 72 (51–87) | 0 (−16–9) | 0.877 | |
| CRP <0.20–5.00 mg/L | 1.9 (1.0–5.3) | 2.1 (0.5–6.9) | 0.0 (−0.3–0.6) | 0.744 | |
| Urea 17–45 mg/dL | 28.0 (22.2–33.7) | 26.0 (20.0–33.7) | 0.0 (−7.0–2.0) | 0.307 | |
| Creatinine 0.62–1.10 mg/dL | 0.70 (0.57–0.79) | 0.73 (0.54–0.85) | 0.01 (−0.01–0.08) | 0.025 | |
| Zinc 50.0–150.0 mcg/dL | 119.0 (96.5–140.0) | 131.5 (110.5–150.7) | 8.5 (0.0–30.7) | 0.031 | |
| Calcium 8.1–10.4 mg/dL | 9.8 (0.6–10.0) | 9.8 (9.5–10.1) | 0.0 (−0.2–0.2) | 1.000 | |
| Phosphorus 2.00–4.00 mg/dL | 4.2 (3.4–4.3) | 4.1 (3.5–4.3) | −0.05 (−0.37–0.20) | 0.477 | |
| Potassium 3.5–5.1 mmol/L | 4.2 (4.1–4.4) | 4.4 (4.2–4.6) | 0.1 (−0.1–0.4) | 0.044 | |
| Magnesium 1.90–2.70 mg/dL | 1.9 (1.8–2.0) | 1.9 (1.8–2.0) | 0.0 (0.0–0.1) | 0.424 | |
| Vitamin C 4.6–14.9 mg/L | 11.7 (9.0–12.9) | 12.0 (9.0–13.4) | 0.2 (−0.8–0.9) | 0.321 | |
| Vitamin B1 66.5–200.0 nmol/L | 144.8 (121.0–171.0) | 141.5 (118.3–158.0) | −0.2 (−11.4–4.8) | 0.472 | |
| Folic acid 5.9–24.0 ng/mL | 8.7 (6.7–12.3) | 10.5 (6.2–13.5) | 0.0 (−2.3–3.9) | 0.679 | |
| R | p-Value | |
|---|---|---|
| Delta food energy | −0.473 | 0.035 |
| Delta energy from ultra-processed | 0.741 | <0.0001 |
| Delta total fat | 0.566 | 0.009 |
| Delta carbohydrates | 0.659 | 0.002 |
| Delta calcium | 0.771 | <0.0001 |
| Delta zinc | 0.641 | 0.002 |
| Delta copper | 0.78 | <0.0001 |
| Delta vitamin C | 0.576 | 0.008 |
| Delta thiamin | 0.891 | <0.001 |
| Correlation with delta polyunsaturated fat (g) | ||
| Delta energy from ultra-processed food | 0.7 | 0.001 |
| Delta carbohydrate | 0.893 | <0.0001 |
| Delta total fiber | 0.765 | <0.0001 |
| Delta fructose | 0.671 | 0.001 |
| Delta magnesium | 0.805 | <0.0001 |
| Delta zinc | 0.63 | 0.003 |
| Delta copper | 0.784 | <0.0001 |
| Delta folate | 0.768 | <0.0001 |
| Delta thiamine | 0.712 | <0.0001 |
| Delta vitamin C | 0.637 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levran, N.; Levek, N.; Sher, B.; Mauda-Yitzhak, E.; Gruber, N.; Afek, A.; Monsonego-Ornan, E.; Pinhas-Hamiel, O. The Mediterranean Diet for Adolescents with Type 1 Diabetes: A Prospective Interventional Study. Nutrients 2023, 15, 4577. https://doi.org/10.3390/nu15214577
Levran N, Levek N, Sher B, Mauda-Yitzhak E, Gruber N, Afek A, Monsonego-Ornan E, Pinhas-Hamiel O. The Mediterranean Diet for Adolescents with Type 1 Diabetes: A Prospective Interventional Study. Nutrients. 2023; 15(21):4577. https://doi.org/10.3390/nu15214577
Chicago/Turabian StyleLevran, Neriya, Noah Levek, Bruria Sher, Elinor Mauda-Yitzhak, Noah Gruber, Arnon Afek, Efrat Monsonego-Ornan, and Orit Pinhas-Hamiel. 2023. "The Mediterranean Diet for Adolescents with Type 1 Diabetes: A Prospective Interventional Study" Nutrients 15, no. 21: 4577. https://doi.org/10.3390/nu15214577
APA StyleLevran, N., Levek, N., Sher, B., Mauda-Yitzhak, E., Gruber, N., Afek, A., Monsonego-Ornan, E., & Pinhas-Hamiel, O. (2023). The Mediterranean Diet for Adolescents with Type 1 Diabetes: A Prospective Interventional Study. Nutrients, 15(21), 4577. https://doi.org/10.3390/nu15214577






