Long-Term Prognosis in Relation to Vitamin D Status in Pediatric Solid Tumor Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Samples and Sample Collection
2.3. Biochemical Analyses
2.4. Statistical Analysis
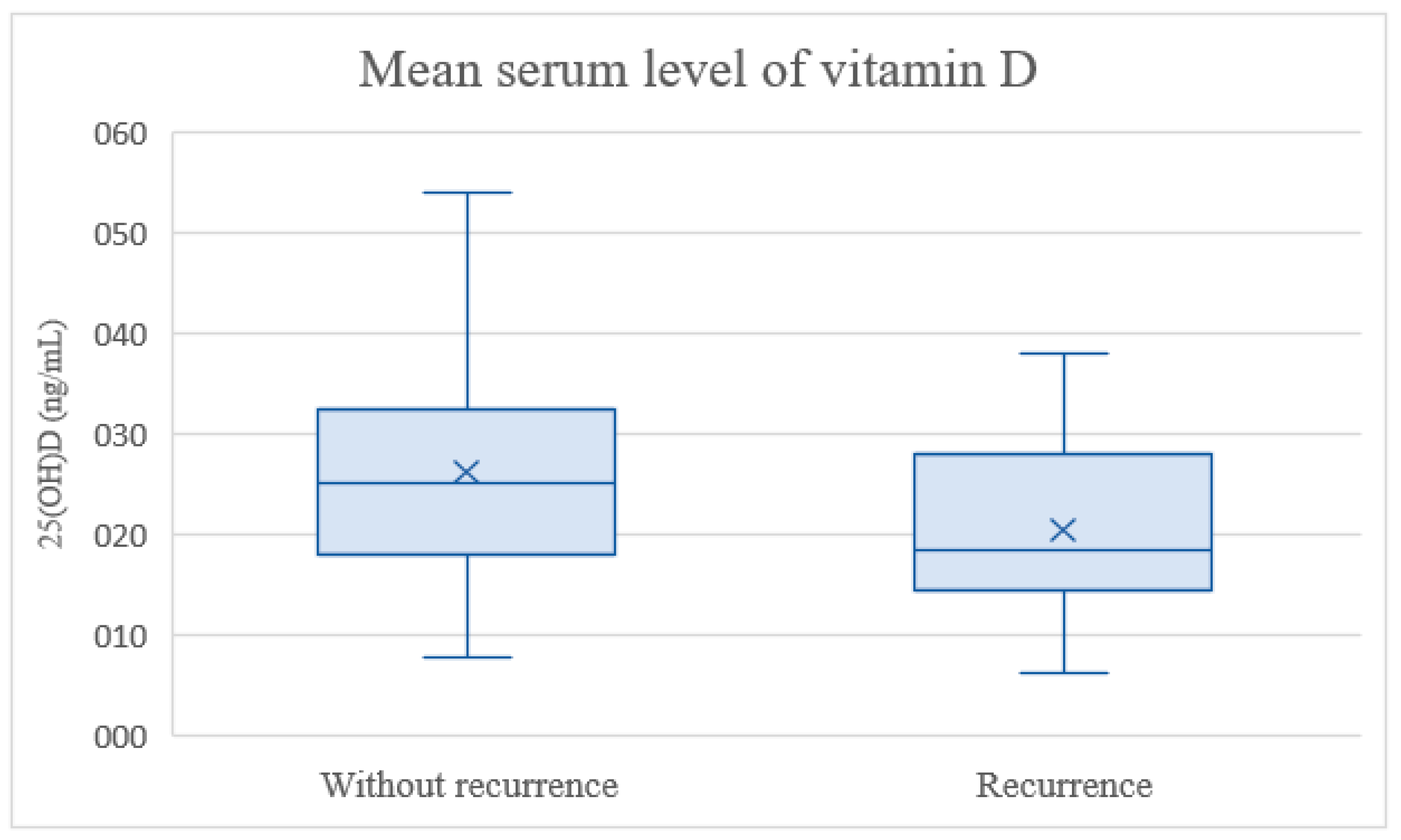
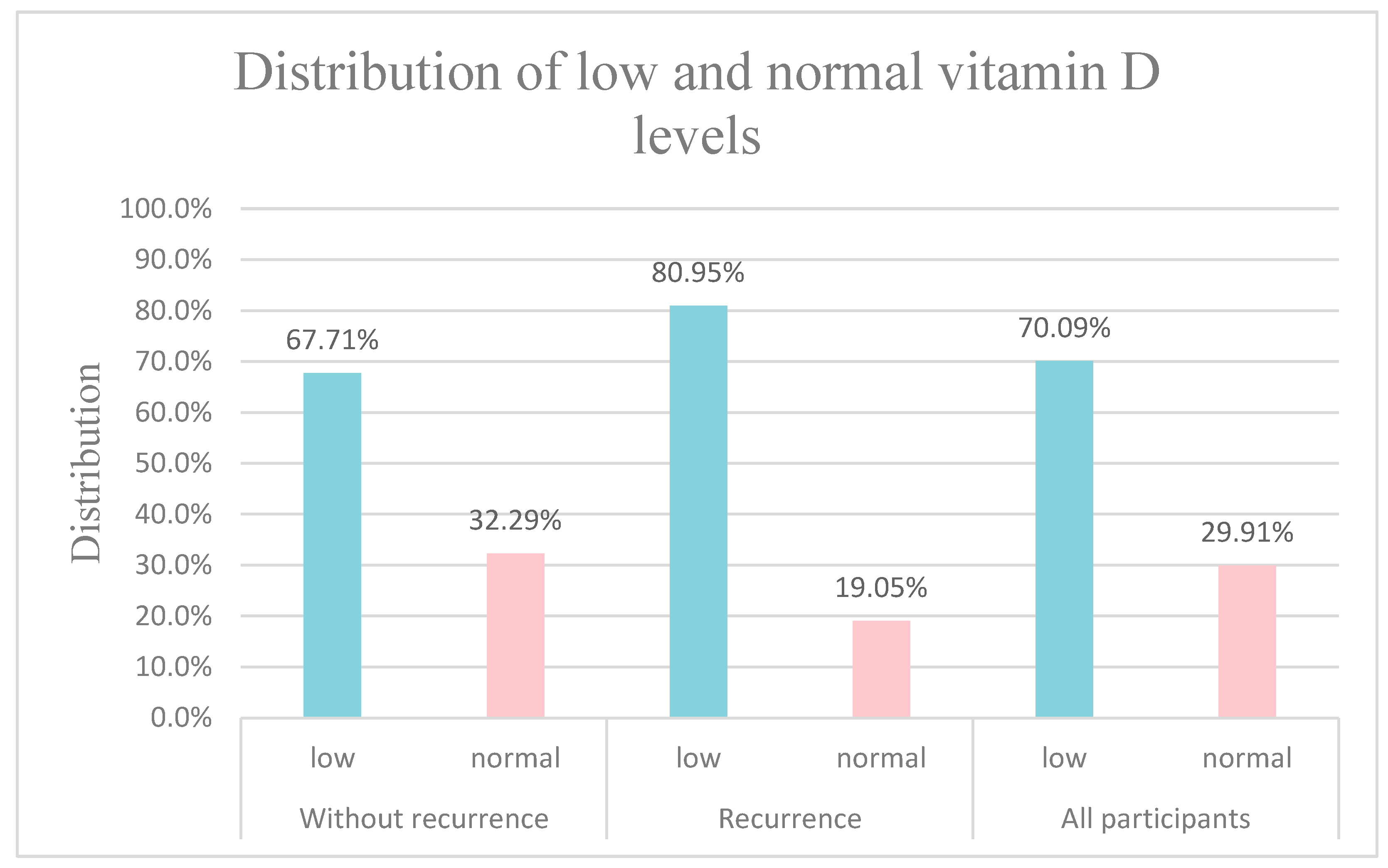
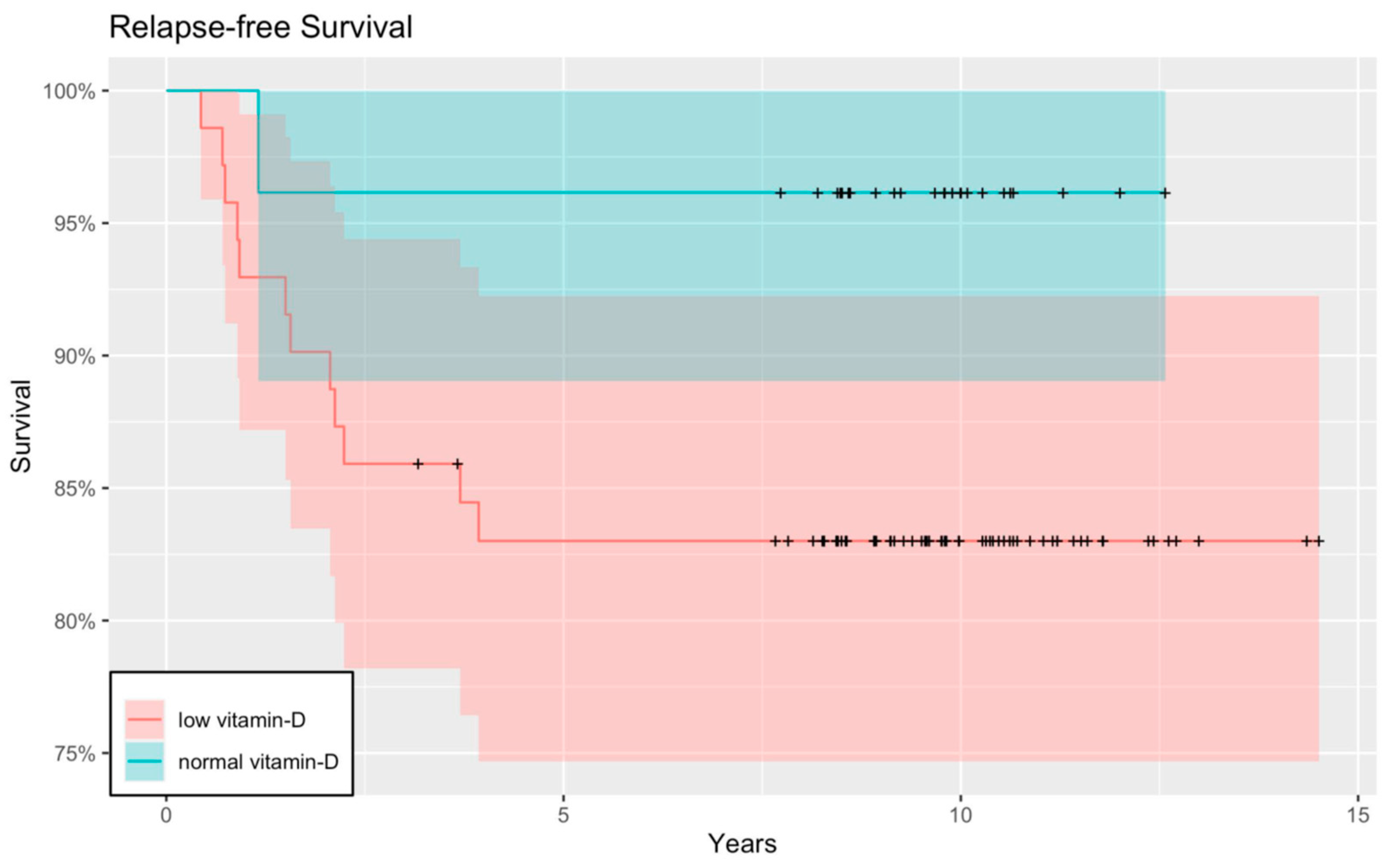
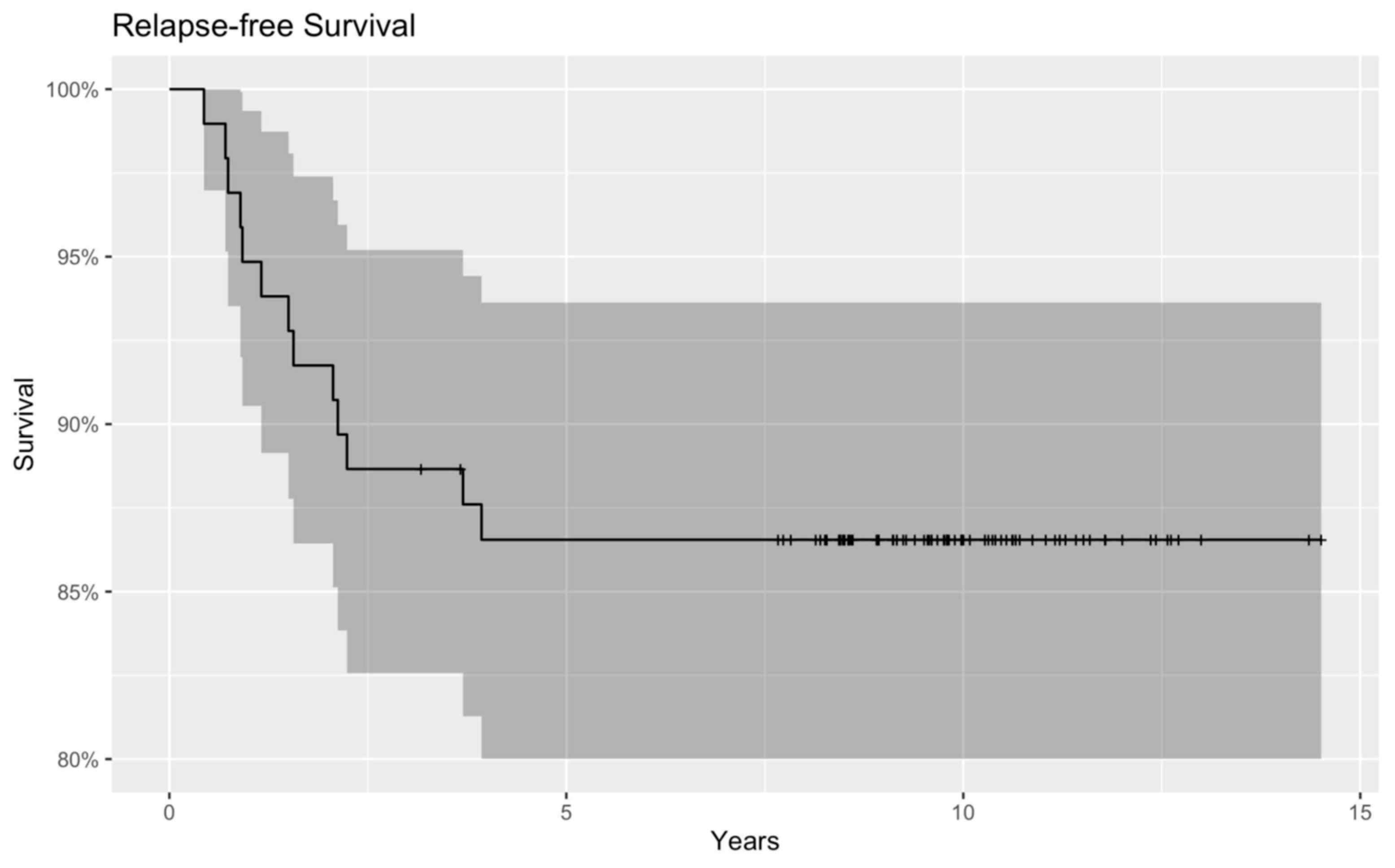
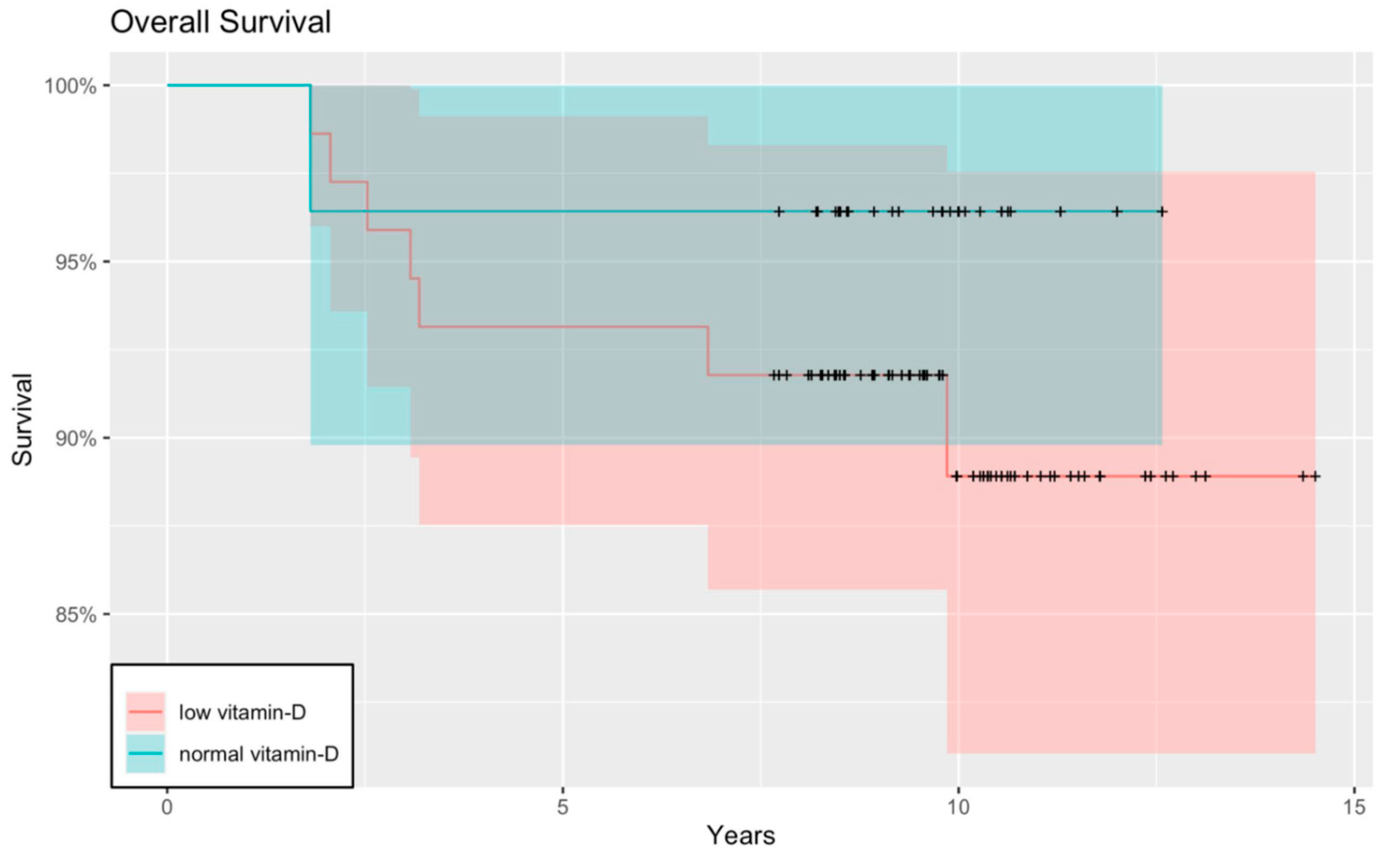
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Implications on Practice and Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations (in order of appearance):
| RFS | relapse-free survival |
| OS | overall survival |
| SE | standard error |
| VDD | Vitamin D deficiency |
| 1,25(OH)2D | 1,25-dihydroxy-cholecalciferol |
| 25(OH)D | 25-hydroxy Vitamin D |
| SU | Semmelweis University |
| BMI | body mass index |
| ATRT | atypical teratoid rhabdoid tumor |
| PNET | primitive neuroectodermal tumor |
| CR | complete remission |
| PR | partial remission |
| SD | stable disease |
| EX | exitus letalis |
| ECLIA | electrochemiluminescence immunoassay |
| HL | Hodgkin’s lymphoma |
| CDK | cyclin-dependent kinase |
| NF-κB | nuclear factor-kappa B |
| IκB | inhibitor of nuclear factor kappa B |
| PD-L1 | programmed death-ligand 1 |
| ICIs | immune checkpoint inhibitors |
| VDR | Vitamin D receptor |
| FCR | fear of cancer recurrence |
| CCSS | Childhood Cancer Survivor Study |
References
- Antonucci, R.; Locci, C.; Clemente, M.G.; Chicconi, E.; Antonucci, L. Vitamin D deficiency in childhood: Old lessons and current challenges. J. Pediatr. Endocrinol. Metab. 2018, 31, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B. A brief history of vitamin d and cancer prevention. Ann. Epidemiol. 2009, 19, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Meeker, S.; Seamons, A.; Maggio-Price, L.; Paik, J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 2016, 22, 933–948. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Johnson, C.R.; Dudenkov, D.V.; Mara, K.C.; Fischer, P.R.; Maxson, J.A.; Thacher, T.D. Serum 25-Hydroxyvitamin D and Subsequent Cancer Incidence and Mortality: A Population-Based Retrospective Cohort Study. Mayo Clin. Proc. 2021, 96, 2157–2167. [Google Scholar] [CrossRef]
- Khammissa, R.A.G.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The Biological Activities of Vitamin D and Its Receptor in Relation to Calcium and Bone Homeostasis, Cancer, Immune and Cardiovascular Systems, Skin Biology, and Oral Health. BioMed Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef]
- Seamans, K.M.; Cashman, K.D. Existing and potentially novel functional markers of vitamin D status: A systematic review. Am. J. Clin. Nutr. 2009, 89, 1997s–2008s. [Google Scholar] [CrossRef]
- Cediel, G.; Pacheco-Acosta, J.; CastiUo-Durdn, C. Vitamin D deficiency in pediatric clinical practice. Arch. Argent. Pediatr. 2018, 116, e75–e81. [Google Scholar] [CrossRef]
- Revuelta Iniesta, R.; Rush, R.; Paciarotti, I.; Rhatigan, E.B.; Brougham, F.H.M.; McKenzie, J.M.; Wilson, D.C. Systematic review and meta-analysis: Prevalence and possible causes of vitamin D deficiency and insufficiency in pediatric cancer patients. Clin. Nutr. 2016, 35, 95–108. [Google Scholar] [CrossRef]
- Zgaga, L.; Theodoratou, E.; Farrington, S.M.; Din, F.V.N.; Ooi, L.Y.; Glodzik, D.; Johnston, S.; Tenesa, A.; Campbell, H.; Dunlop, M.G. Plasma Vitamin D Concentration Influences Survival Outcome after a Diagnosis of Colorectal Cancer. J. Clin. Oncol. 2014, 32, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Q.; Yin, H.; Wu, J.Z.; Chen, R.Z.; Xia, Y.; Wang, L.; Zhu, H.Y.; Fan, L.; Li, J.Y.; Liang, J.H.; et al. 25-Hydroxy vitamin D deficiency predicts inferior prognosis in Hodgkin lymphoma. Leuk. Res. 2021, 105, 106580. [Google Scholar] [CrossRef] [PubMed]
- Yokosawa, E.B.; Arthur, A.E.; Rentschler, K.M.; Wolf, G.T.; Rozek, L.S.; Mondul, A.M. Vitamin D intake and survival and recurrence in head and neck cancer patients. Laryngoscope 2018, 128, E371–E376. [Google Scholar] [CrossRef]
- Jackmann, N.; Mäkitie, O.; Harila-Saari, A.; Gustafsson, J.; Nezirevic Dernroth, D.; Frisk, P. Vitamin D status in children with leukemia, its predictors, and association with outcome. Pediatr. Blood Cancer 2020, 67, e28163. [Google Scholar] [CrossRef]
- Juhász, O.; Jakab, Z.; Szabó, A.; Garami, M. Examining the Vitamin D Status of Children with Solid Tumors. J. Am. Coll. Nutr. 2020, 39, 128–134. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Choudhary, A.; Chou, J.; Heller, G.; Sklar, C. Prevalence of vitamin D insufficiency in survivors of childhood cancer. Pediatr. Blood Cancer 2013, 60, 1237–1239. [Google Scholar] [CrossRef]
- Helou, M.; Ning, Y.; Yang, S.; Irvine, P.; Bachmann, L.M.; Godder, K.; Massey, G. Vitamin d deficiency in children with cancer. J. Pediatr. Hematol. Oncol. 2014, 36, 212–217. [Google Scholar] [CrossRef]
- Genc, D.B.; Vural, S.; Yagar, G. The Incidence of and Factors Associated with Vitamin D Deficiency in Newly Diagnosed Children with Cancer. Nutr. Cancer 2016, 68, 756–761. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, M.; Fan, D.; Hong, Y.; Zhao, M.; Ding, R.; Cheng, Y.; Duan, S. Association between vitamin D supplementation and cancer incidence and mortality: A trial sequential meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 63, 8428–8442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Heist, R.S.; Liu, G.; Asomaning, K.; Neuberg, D.S.; Hollis, B.W.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Su, L.; et al. Circulating 25-Hydroxyvitamin D Levels Predict Survival in Early-Stage Non–Small-Cell Lung Cancer Patients. J. Clin. Oncol. 2007, 25, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Mondul, A.M.; Layne, T.M.; Yu, K.; Huang, J.; Stolzenberg-Solomon, R.Z.; Ziegler, R.G.; Purdue, M.P.; Huang, W.-Y.; Abnet, C.C.; et al. Prediagnostic Serum Vitamin D, Vitamin D Binding Protein Isoforms, and Cancer Survival. JNCI Cancer Spectr. 2022, 6, pkac019. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Meyerhardt, J.A.; Wu, K.; Feskanich, D.; Hollis, B.W.; Giovannucci, E.L.; Fuchs, C.S. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J. Clin. Oncol. 2008, 26, 2984–2991. [Google Scholar] [CrossRef]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njølstad, I.; Løchen, M.L.; März, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar] [CrossRef]
- Borchmann, S.; Cirillo, M.; Goergen, H.; Meder, L.; Sasse, S.; Kreissl, S.; Bröckelmann, P.J.; von Tresckow, B.; Fuchs, M.; Ullrich, R.T.; et al. Pretreatment Vitamin D Deficiency Is Associated with Impaired Progression-Free and Overall Survival in Hodgkin Lymphoma. J. Clin. Oncol. 2019, 37, 3528–3537. [Google Scholar] [CrossRef]
- Bienertová-Vašků, J.; Drábová, K.; Zlámal, F.; Tomandl, J.; Kýr, M.; Šplíchal, Z.; Štěrba, J. Pre-treatment VD levels and VDR receptors as potential predictors of occurrence and overall survival in paediatric patients with solid tumours—A single institution pilot study. Tumor Biol. 2016, 37, 9209–9219. [Google Scholar] [CrossRef]
- Moukayed, M.; Grant, W.B. Molecular link between vitamin D and cancer prevention. Nutrients 2013, 5, 3993–4021. [Google Scholar] [CrossRef]
- Jensen, S.S.; Madsen, M.W.; Lukas, J.; Binderup, L.; Bartek, J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol. Endocrinol. 2001, 15, 1370–1380. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Feldman, D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Desmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2011, 441, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Dimitrov, V.; Bouttier, M.; Boukhaled, G.; Salehi-Tabar, R.; Avramescu, R.G.; Memari, B.; Hasaj, B.; Lukacs, G.L.; Krawczyk, C.M.; White, J.H. Hormonal vitamin D up-regulates tissue-specific PD-L1 and PD-L2 surface glycoprotein expression in humans but not mice. J. Biol. Chem. 2017, 292, 20657–20668. [Google Scholar] [CrossRef]
- Stucci, L.S.; D’Oronzo, S.; Tucci, M.; Macerollo, A.; Ribero, S.; Spagnolo, F.; Marra, E.; Picasso, V.; Orgiano, L.; Marconcini, R.; et al. Vitamin D in melanoma: Controversies and potential role in combination with immune check-point inhibitors. Cancer Treat. Rev. 2018, 69, 21–28. [Google Scholar] [CrossRef]
- Zinser, G.M.; Suckow, M.; Welsh, J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J. Steroid Biochem. Mol. Biol. 2005, 97, 153–164. [Google Scholar] [CrossRef]
- Kallay, E.; Pietschmann, P.; Toyokuni, S.; Bajna, E.; Hahn, P.; Mazzucco, K.; Bieglmayer, C.; Kato, S.; Cross, H.S. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis 2001, 22, 1429–1435. [Google Scholar] [CrossRef]
- Hendrickson, W.K.; Flavin, R.; Kasperzyk, J.L.; Fiorentino, M.; Fang, F.; Lis, R.; Fiore, C.; Penney, K.L.; Ma, J.; Kantoff, P.W.; et al. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J. Clin. Oncol. 2011, 29, 2378–2385. [Google Scholar] [CrossRef]
- Ditsch, N.; Toth, B.; Mayr, D.; Lenhard, M.; Gallwas, J.; Weissenbacher, T.; Dannecker, C.; Friese, K.; Jeschke, U. The association between vitamin D receptor expression and prolonged overall survival in breast cancer. J. Histochem. Cytochem. 2012, 60, 121–129. [Google Scholar] [CrossRef]
- Juhász, O.; Jákob, N.; Rajnai, H.; Imrei, M.; Garami, M. Immunohistochemical Detection of the Presence of Vitamin D Receptor in Childhood Solid Tumors. Cancers 2022, 14, 3295. [Google Scholar] [CrossRef]
- Anderson, M.G.; Nakane, M.; Ruan, X.; Kroeger, P.E.; Wu-Wong, J.R. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother. Pharmacol. 2006, 57, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.N.; Vrooman, L.; Apfelbaum, E.M.; Whitley, K.; Bechard, L.; Lehmann, L.E. 25-hydroxy vitamin D deficiency following pediatric hematopoietic stem cell transplant. Biol. Blood Marrow Transplant. 2011, 17, 749–753. [Google Scholar] [CrossRef]
- Osterwalder, U.; Herzog, B. Sun protection factors: World wide confusion. Br. J. Dermatol. 2009, 161 (Suppl. 3), 13–24. [Google Scholar] [CrossRef] [PubMed]
- Atteveld, J.E.v.; Pluijm, S.M.F.; Ness, K.K.; Hudson, M.M.; Chemaitilly, W.; Kaste, S.C.; Robison, L.L.; Neggers, S.J.C.M.M.; Yasui, Y.; Heuvel-Eibrink, M.M.v.d.; et al. Prediction of Low and Very Low Bone Mineral Density Among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2019, 37, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.A. Vitamin D status and bone biomarkers in childhood cancer. Pediatr. Blood Cancer 2008, 50, 479–482, discussion 486. [Google Scholar] [CrossRef] [PubMed]
- Clarisse, D.; Offner, F.; De Bosscher, K. Latest perspectives on glucocorticoid-induced apoptosis and resistance in lymphoid malignancies. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188430. [Google Scholar] [CrossRef]
- Vallero, S.G.; Lijoi, S.; Bertin, D.; Pittana, L.S.; Bellini, S.; Rossi, F.; Peretta, P.; Basso, M.E.; Fagioli, F. End-of-life care in pediatric neuro-oncology. Pediatr. Blood Cancer 2014, 61, 2004–2011. [Google Scholar] [CrossRef]
- Godschalk, M.; Levy, J.R.; Downs, R.W., Jr. Glucocorticoids decrease vitamin D receptor number and gene expression in human osteosarcoma cells. J. Bone Miner. Res. 1992, 7, 21–27. [Google Scholar] [CrossRef]
- Kittivisuit, S.; Sripornsawan, P.; Songthawee, N.; Chavananon, S.; Yam-Ubon, U.; McNeil, E.B.; Jaruratanasirikul, S.; Chotsampancharoen, T. Vitamin D Deficiency in Childhood Cancer Survivors: Results from Southern Thailand. Nutrients 2023, 15, 1328. [Google Scholar] [CrossRef]
- Rosen, G.P.; Beebe, K.L.; Shaibi, G.Q. Vitamin D levels differ by cancer diagnosis and decline over time in survivors of childhood cancer. Pediatr. Blood Cancer 2013, 60, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Wroot, H.; Afzal, A.R.; Forbes, C.; Russell, K.B.; Trepanier, L.; Patton, M.; Fidler-Benaoudia, M.; Reynolds, K.; Schulte, F. Fear of cancer recurrence among survivors of childhood cancer. Psychooncology 2020, 29, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Haupt, R.; Spinetta, J.J.; Ban, I.; Barr, R.D.; Beck, J.D.; Byrne, J.; Calaminus, G.; Coenen, E.; Chesler, M.; D’Angio, G.J.; et al. Long term survivors of childhood cancer: Cure and care: The Erice Statement. Eur. J. Cancer 2007, 43, 1778–1780. [Google Scholar] [CrossRef] [PubMed]
- Mertens, A.C.; Liu, Q.; Neglia, J.P.; Wasilewski, K.; Leisenring, W.; Armstrong, G.T.; Robison, L.L.; Yasui, Y. Cause-Specific Late Mortality among 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2008, 100, 1368–1379. [Google Scholar] [CrossRef]
- Wasilewski-Masker, K.; Liu, Q.; Yasui, Y.; Leisenring, W.; Meacham, L.R.; Hammond, S.; Meadows, A.T.; Robison, L.L.; Mertens, A.C. Late recurrence in pediatric cancer: A report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2009, 101, 1709–1720. [Google Scholar] [CrossRef]
- Effinger, K.E.; Haardörfer, R.; Marchak, J.G.; Escoffery, C.; Landier, W.; Kommajosula, A.; Hendershot, E.; Sadak, K.T.; Eshelman-Kent, D.; Kinahan, K.; et al. Current pediatric cancer survivorship practices: A report from the Children’s Oncology Group. J. Cancer Surviv. 2023, 17, 1139–1148. [Google Scholar] [CrossRef]
- Meadows, A.T. Pediatric cancer survivorship: Research and clinical care. J. Clin. Oncol. 2006, 24, 5160–5165. [Google Scholar] [CrossRef]
- Frost, P. The Problem of Vitamin D Scarcity: Cultural and Genetic Solutions by Indigenous Arctic and Tropical Peoples. Nutrients 2022, 14, 4071. [Google Scholar] [CrossRef]

| Number of Patients | CR | PR | SD | EX | |
|---|---|---|---|---|---|
| Relapsed * | 21 | 8 | 2 | 2 | 9 |
| Not relapsed | 96 | 90 | - | 6 | - |
| Neuroblastoma | 35 | 30 | - | 3 | 2 |
| Ewing sarcoma | 18 | 16 | - | - | 2 |
| Medulloblastoma | 16 | 14 | - | - | 2 |
| Nephroblastoma | 8 | 8 | - | - | - |
| Retinoblastoma | 5 | 3 | - | 2 | - |
| Hepatoblastoma | 4 | 4 | - | - | - |
| Ganglioneuroma | 4 | 4 | - | - | - |
| Teratoma | 3 | 3 | - | - | - |
| ATRT | 3 | 1 | - | 1 | 1 |
| PNET | 2 | 2 | - | - | - |
| Ependymoma | 2 | 2 | - | - | - |
| Papillary thyroid Carcinoma | 2 | 1 | 1 | - | - |
| Astrocytoma | 2 | 2 | - | - | - |
| Cerebral neuroblastoma | 1 | 1 | - | - | - |
| Anaplastic astocytoma | 1 | 1 | - | - | - |
| Medullar thyroid carcinoma | 1 | 1 | - | - | - |
| Testicular tumor | 1 | 1 | - | - | - |
| Rhabdoid renal tumor | 1 | 1 | - | - | - |
| Ethmoid sinus tumor | 1 | 1 | - | - | - |
| Adrenal cortex carcinoma | 1 | 1 | - | - | - |
| Tumor of the peripheral nerves and autonomous nervous system | 1 | 1 | - | - | - |
| Glioneural tumor | 1 | - | - | 1 | - |
| Optic glioma | 1 | - | - | 1 | - |
| Choroid plexus carcinoma | 1 | - | - | - | 1 |
| Plexus papilloma carcinoma | 1 | - | - | - | 1 |
| Pineoblastoma | 1 | - | 1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kárász, N.; Juhász, O.; Imrei, M.; Garami, M. Long-Term Prognosis in Relation to Vitamin D Status in Pediatric Solid Tumor Patients. Nutrients 2023, 15, 4571. https://doi.org/10.3390/nu15214571
Kárász N, Juhász O, Imrei M, Garami M. Long-Term Prognosis in Relation to Vitamin D Status in Pediatric Solid Tumor Patients. Nutrients. 2023; 15(21):4571. https://doi.org/10.3390/nu15214571
Chicago/Turabian StyleKárász, Nóra, Orsolya Juhász, Marcell Imrei, and Miklós Garami. 2023. "Long-Term Prognosis in Relation to Vitamin D Status in Pediatric Solid Tumor Patients" Nutrients 15, no. 21: 4571. https://doi.org/10.3390/nu15214571
APA StyleKárász, N., Juhász, O., Imrei, M., & Garami, M. (2023). Long-Term Prognosis in Relation to Vitamin D Status in Pediatric Solid Tumor Patients. Nutrients, 15(21), 4571. https://doi.org/10.3390/nu15214571






