Leucine Supplementation Exacerbates Morbidity in Male but Not Female Mice with Colorectal Cancer-Induced Cachexia
Abstract
:1. Introduction
2. Methods
2.1. Animals and Housing
2.2. Leucine Supplementation
2.3. Euthanasia and Tissue Collection
2.4. Histology
2.5. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
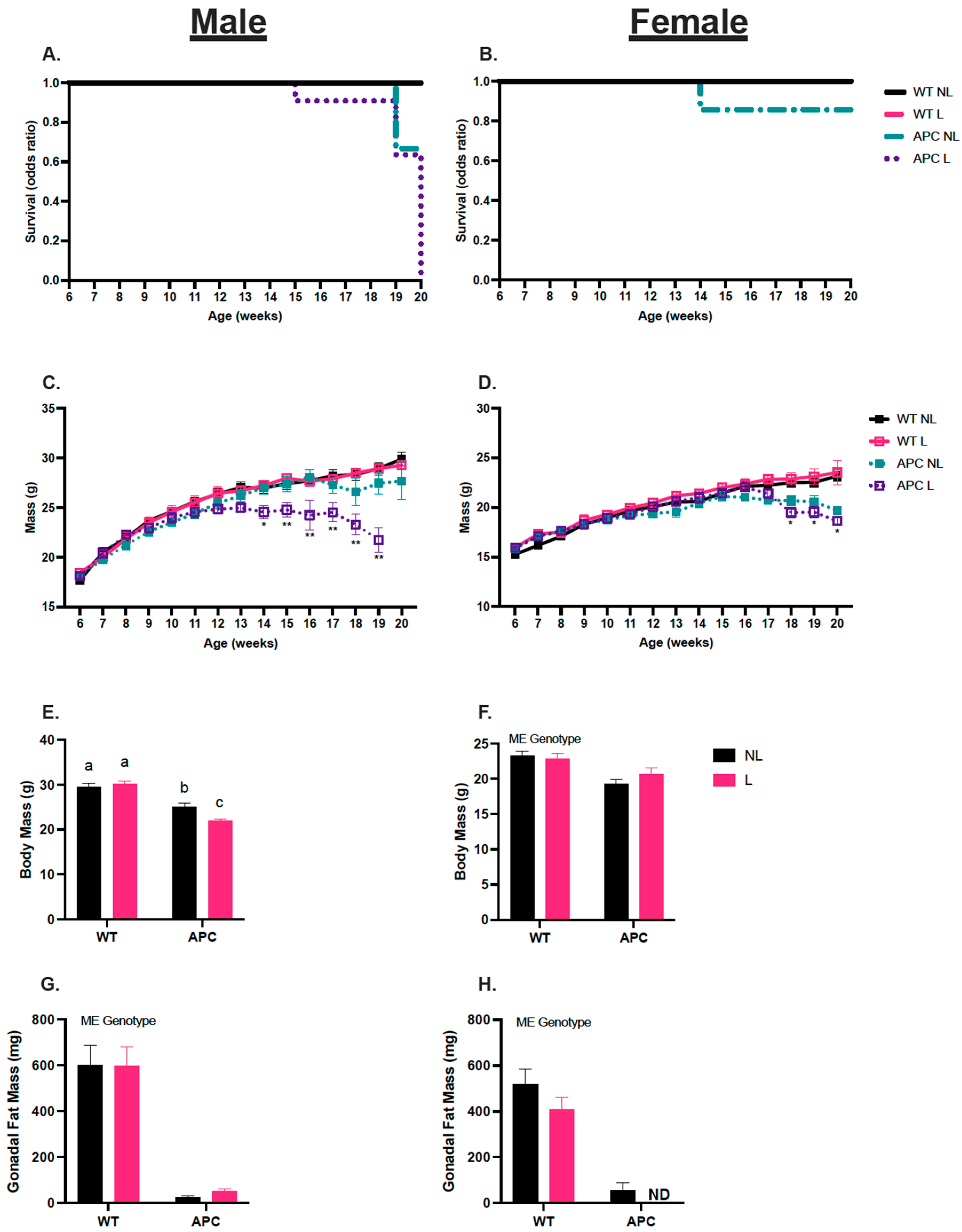
3.1. Leucine Negatively Affects Survival in Male APCMin/+ Mice
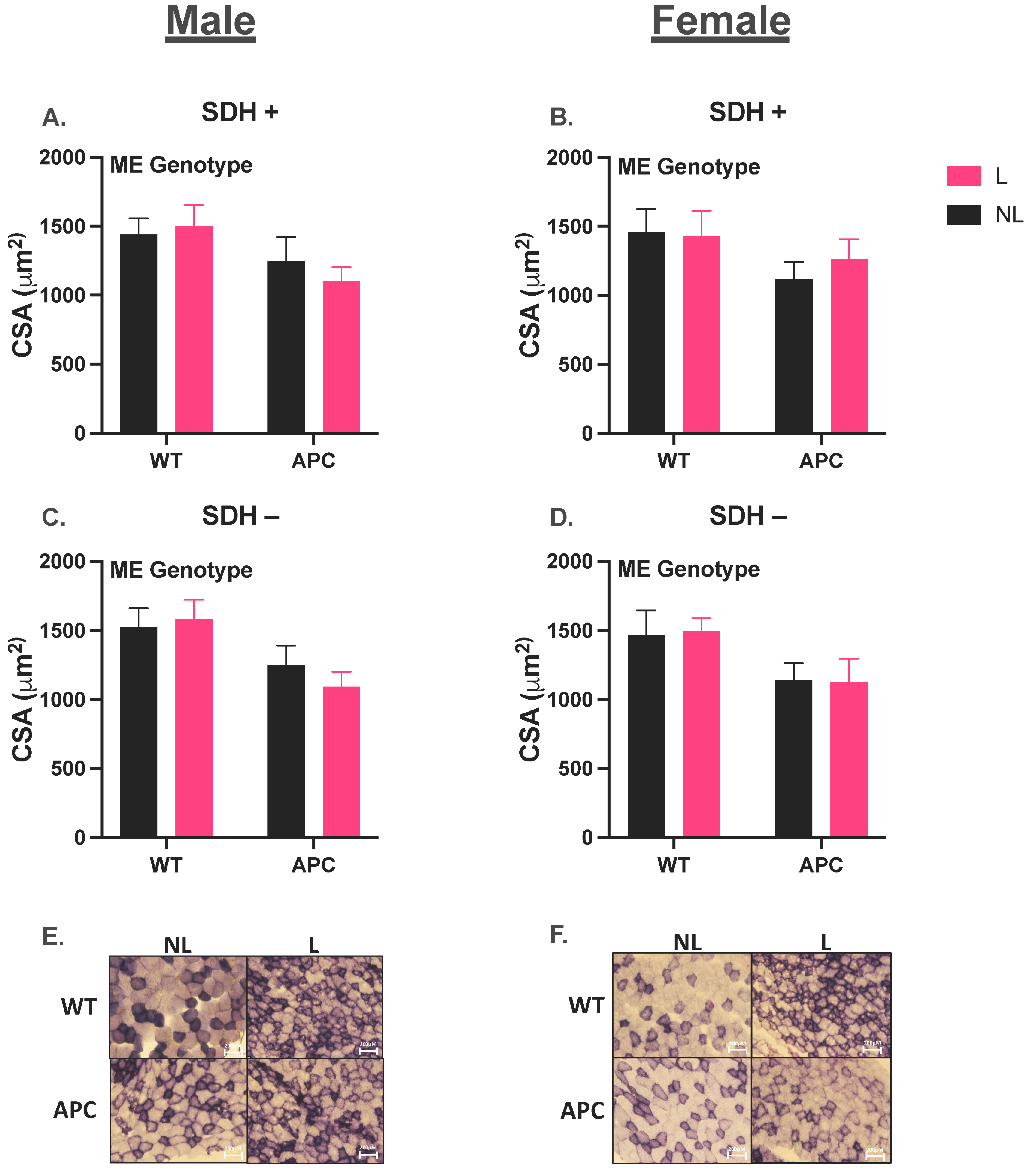
3.2. Validation of Cancer Cachectic Phenotype
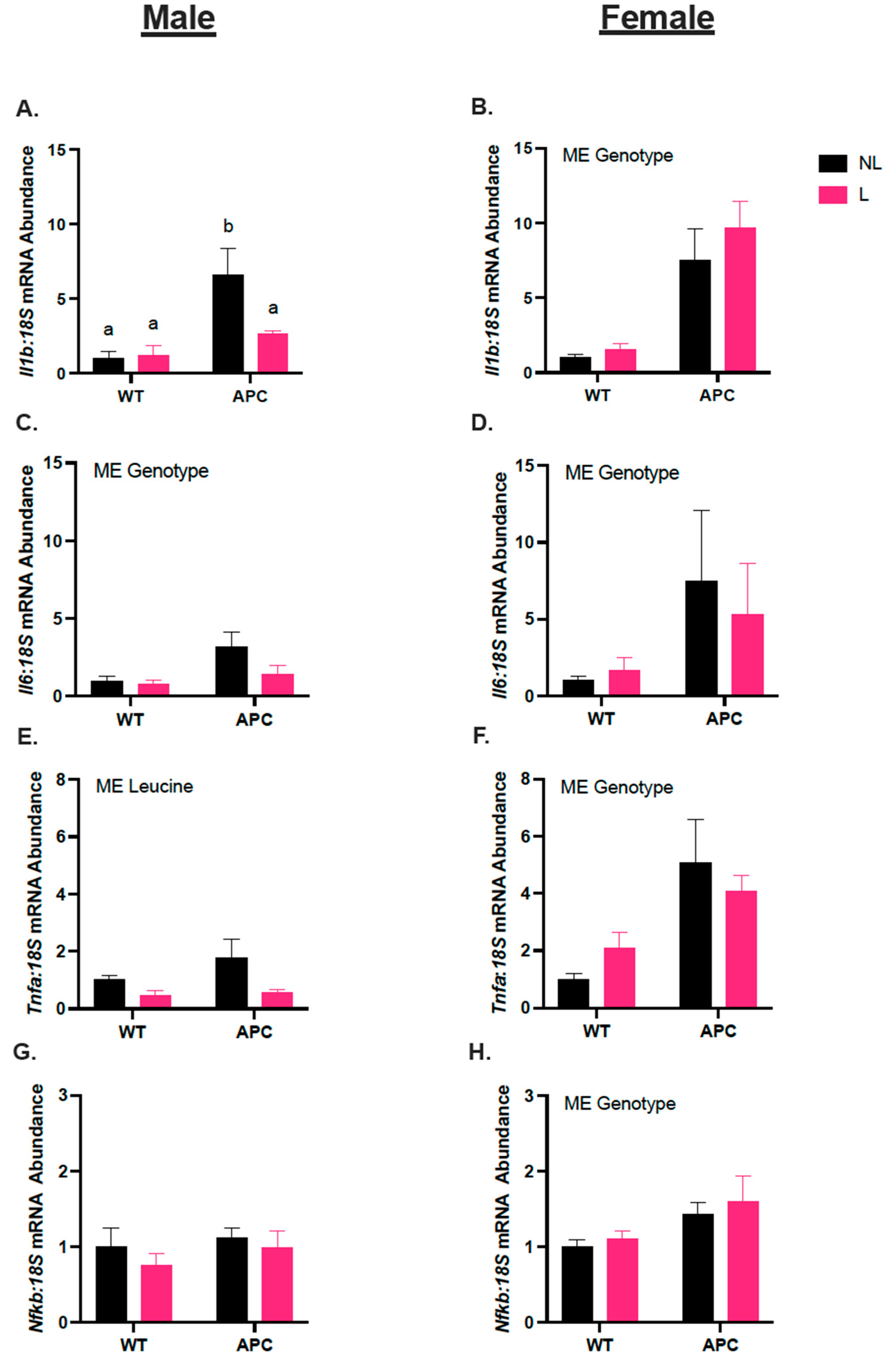
3.3. Leucine Alters Inflammatory Gene Expression Only in Male Mice
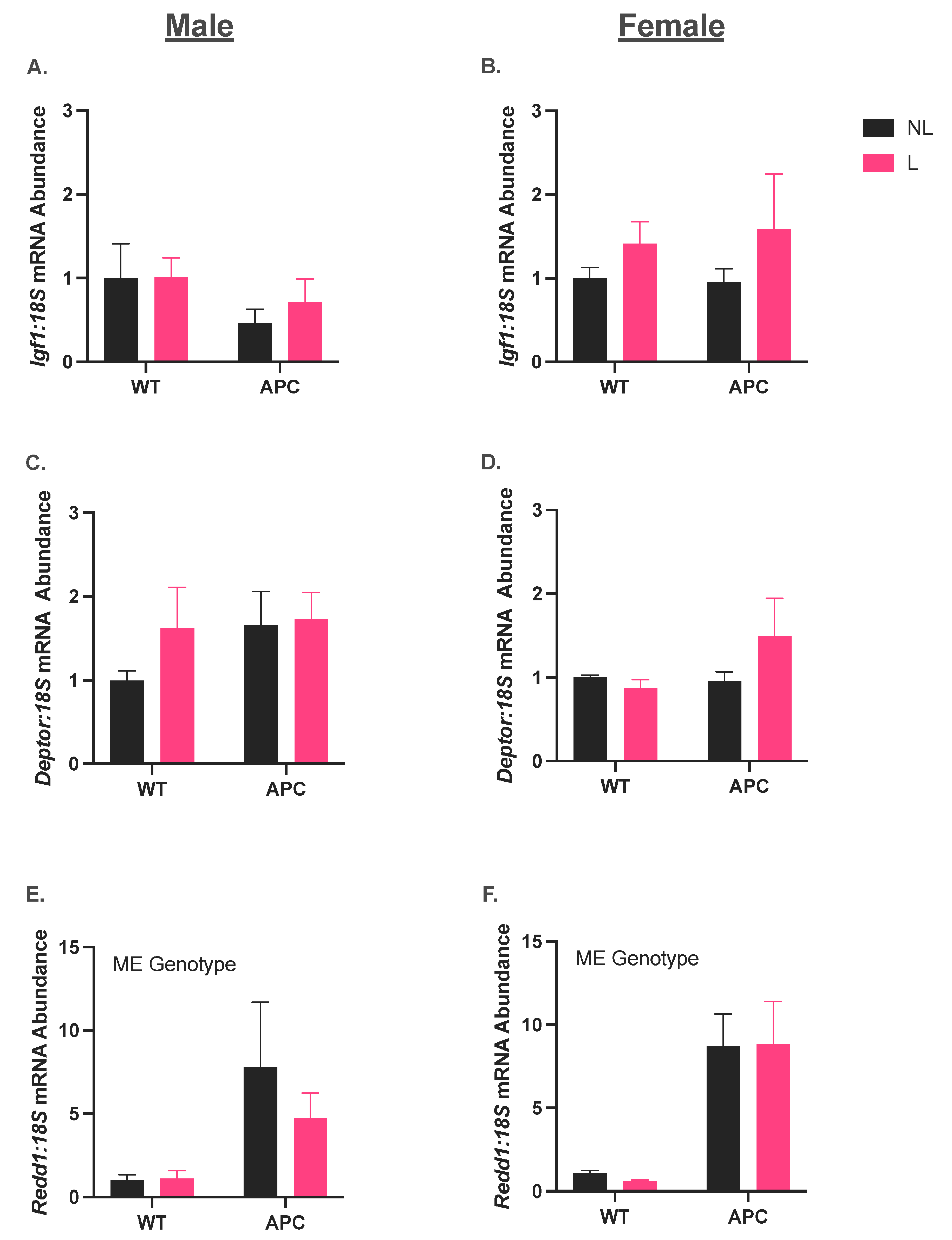
3.4. Leucine Supplementation Did Not Affect the Induction of Anabolic Suppressor Gene Expression in CC
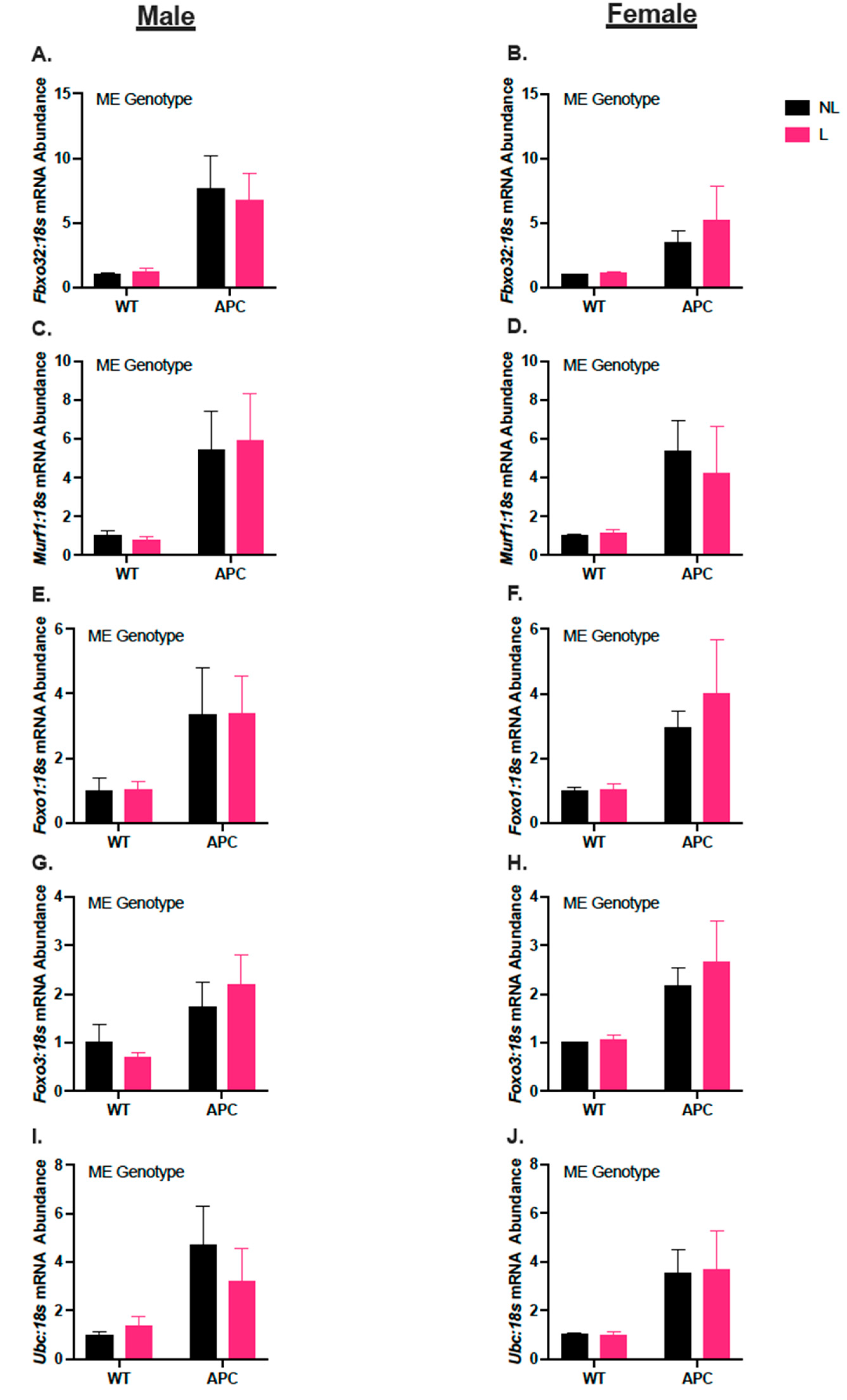
3.5. Leucine Supplementation Did Not Affect Induction of Gene Expression in Markers for Protein Degradation in ApcMin/+
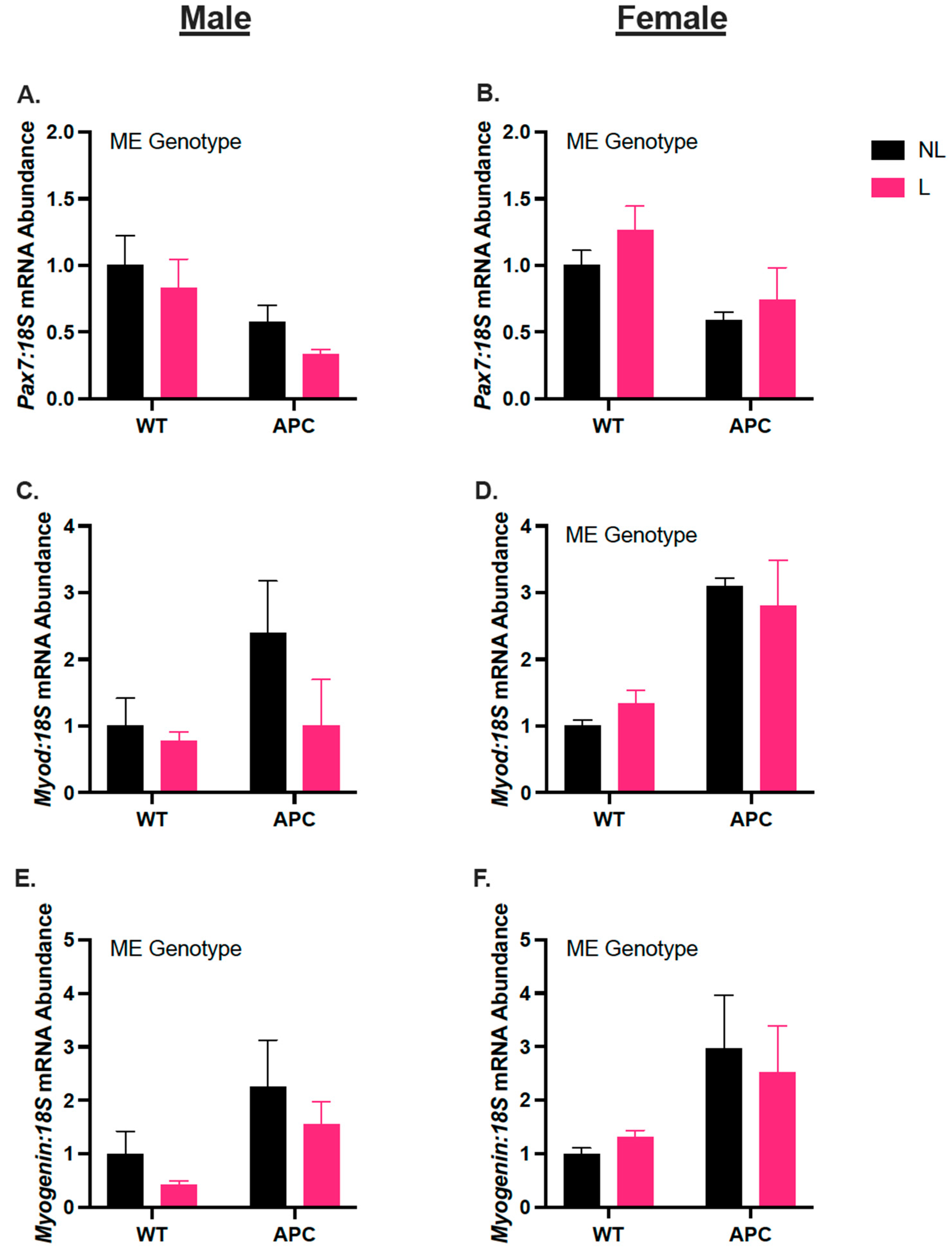
3.6. Biological Sex Differences in Gene Expression of Myogenic Regulators, Independent of Leucine Supplementation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 17 September 2023).
- National Cancer Institute. Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 17 September 2023).
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Nonmuscle Tissues Contribution to Cancer Cachexia. Mediat. Inflamm. 2015, 2015, 182872. [Google Scholar] [CrossRef]
- Haehling, S.; Anker, M.S.; Anker, S.D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: Facts and numbers update 2016. J. Cachexia Sarcopenia Muscle 2016, 7, 507–509. [Google Scholar] [CrossRef]
- Bruggeman, A.R.; Kamal, A.H.; Leblanc, T.W.; Ma, J.D.; Baracos, V.E.; Roeland, E.J. Cancer Cachexia: Beyond Weight Loss. J. Oncol. Pract. 2016, 12, 1163–1171. [Google Scholar] [CrossRef]
- Malla, J.; Zahra, A.; Venugopal, S.; Selvamani, T.Y.; Shoukrie, S.I.; Selvaraj, R.; Dhanoa, R.K.; Hamouda, R.K.; Mostafa, J. What Role Do Inflammatory Cytokines Play in Cancer Cachexia? Cureus 2022, 14, e26798. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- De Vos-Geelen, J.; Fearon, K.C.; Schols, A.M. The energy balance in cancer cachexia revisited. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Lee, D.E.; Rosa-Caldwell, M.E.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Huseman, K.; Sataranatarajan, K.; Van Remmen, H.; Washington, T.A.; et al. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2018, 9, 987–1002. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J. The role of cytokines in cancer cachexia. Med. Res. Rev. 1999, 19, 223–248. [Google Scholar] [CrossRef]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef]
- Laviano, A.; Di Lazzaro Giraldi, G.; Koverech, A. Does nutrition support have a role in managing cancer cachexia? Curr. Opin. Support. Palliat. Care 2016, 10, 288–292. [Google Scholar] [CrossRef]
- McCreery, E.; Costello, J. Providing nutritional support for patients with cancer cachexia. Int. J. Palliat. Nurs. 2013, 19, 32–37. [Google Scholar] [CrossRef]
- van de Worp, W.; Schols, A.; Theys, J.; van Helvoort, A.; Langen, R.C.J. Nutritional Interventions in Cancer Cachexia: Evidence and Perspectives From Experimental Models. Front. Nutr. 2020, 7, 601329. [Google Scholar] [CrossRef]
- Beaudry, A.G.; Law, M.L. Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients 2022, 14, 2824. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, K.C.; Hamilton-Reeves, J.M.; Baracos, V.E. Current Therapeutic Targets in Cancer Cachexia: A Pathophysiologic Approach. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389942. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.R.; Chiocchetti, G.M.E.; Oroy, L.; Vieira, W.F.; Busanello, E.N.B.; Marques, A.C.; Salgado, C.M.; de Oliveira, A.L.R.; Vieira, A.S.; Suarez, P.S.; et al. Leucine-Rich Diet Improved Muscle Function in Cachectic Walker 256 Tumour-Bearing Wistar Rats. Cells 2021, 10, 3272. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Gomes-Marcondes, M.C.C. L-leucine dietary supplementation modulates muscle protein degradation and increases pro-inflammatory cytokines in tumour-bearing rats. Cytokine 2017, 96, 253–260. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Ventrucci, G.; Gomes-Marcondes, M.C.C. A leucine-rich diet modulates the mTOR cell signalling pathway in the gastrocnemius muscle under different Walker-256 tumour growth conditions. BMC Cancer 2019, 19, 349. [Google Scholar] [CrossRef]
- Lee, H.W.; Baker, E.; Lee, K.M.; Persinger, A.M.; Hawkins, W.; Puppa, M. Effects of low-dose leucine supplementation on gastrocnemius muscle mitochondrial content and protein turnover in tumor-bearing mice. Appl. Physiol. Nutr. Metab. 2019, 44, 997–1004. [Google Scholar] [CrossRef]
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metab. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Kakizoe, T.; Ohtani, M.; Sato, S.; Sugimura, T.; Fukushima, S. L-isoleucine and L-leucine: Tumor promoters of bladder cancer in rats. Science 1986, 231, 843–845. [Google Scholar] [CrossRef]
- Xie, X.L.; Wei, M.; Yunoki, T.; Kakehashi, A.; Yamano, S.; Kato, M.; Wanibuchi, H. Long-term treatment with L-isoleucine or L-leucine in AIN-93G diet has promoting effects on rat bladder carcinogenesis. Food Chem. Toxicol. 2012, 50, 3934–3940. [Google Scholar] [CrossRef] [PubMed]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, R.N.; Counts, B.R.; Carson, J.A. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr. Opin. Support. Palliat. Care 2018, 12, 394–403. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex. Differ. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Counts, B.R.; Fix, D.K.; Hetzler, K.L.; Carson, J.A. The Effect of Estradiol Administration on Muscle Mass Loss and Cachexia Progression in Female. Front. Endocrinol. 2019, 10, 720. [Google Scholar] [CrossRef]
- Lim, S.; Deaver, J.W.; Rosa-Caldwell, M.E.; Haynie, W.S.; Morena da Silva, F.; Cabrera, A.R.; Schrems, E.R.; Saling, L.W.; Jansen, L.T.; Dunlap, K.R.; et al. Development of metabolic and contractile alterations in development of cancer cachexia in female tumor-bearing mice. J. Appl. Physiol. 2022, 132, 58–72. [Google Scholar] [CrossRef]
- Zhong, X.; Zimmers, T.A. Sex Differences in Cancer Cachexia. Curr. Osteoporos. Rep. 2020, 18, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Morena da Silva, F.; Lim, S.; Cabrera, A.R.; Schrems, E.R.; Jones, R.G.; Rosa-Caldwell, M.E.; Washington, T.A.; Murach, K.A.; Greene, N.P. The time-course of cancer cachexia onset reveals biphasic transcriptional disruptions in female skeletal muscle distinct from males. BMC Genom. 2023, 24, 374. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.R.; Deaver, J.W.; Lim, S.; Morena da Silva, F.; Schrems, E.R.; Saling, L.W.; Tsitkanou, S.; Rosa-Caldwell, M.E.; Wiggs, M.P.; Washington, T.A.; et al. Females Display Relatively Preserved Muscle Quality Compared to Males During the Onset and Early Stages of C26-induced Cancer-Cachexia. J. Appl. Physiol. 2023, 135, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Morena da Silva, F.; Rosa-Caldwell, M.E.; Schrems, E.R.; Martinez, L.; Amos, M.G.; Lim, S.; Cabrera, A.R.; Brown, J.L.; Washington, T.A.; Greene, N.P. PGC-1α overexpression is not sufficient to mitigate cancer cachexia in either male or female mice. Appl. Physiol. Nutr. Metab. 2022, 47, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.A.; Brown, L.A.; Lee, D.E.; Brown, J.L.; Baum, J.I.; Greene, N.P.; Washington, T.A. The Akt/mTOR pathway: Data comparing young and aged mice with leucine supplementation at the onset of skeletal muscle regeneration. Data Brief. 2016, 8, 1426–1432. [Google Scholar] [CrossRef]
- Perry, R.A.; Brown, L.A.; Lee, D.E.; Brown, J.L.; Baum, J.I.; Greene, N.P.; Washington, T.A. Differential effects of leucine supplementation in young and aged mice at the onset of skeletal muscle regeneration. Mech. Ageing Dev. 2016, 157, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, M.; Lee, J.; He, C.; Xie, Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1234–E1244. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Hardee, J.P.; Fix, D.K.; Carson, J.A. Skeletal muscle function during the progression of cancer cachexia in the male Apc. J. Appl. Physiol. 2018, 124, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Bermúdez-Silva, F.J.; Elie, M.; Leste-Lasserre, T.; Belluomo, I.; Clark, S.; Duchampt, A.; Mithieux, G.; Cota, D. Leucine supplementation modulates fuel substrates utilization and glucose metabolism in previously obese mice. Obesity 2014, 22, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Reiman, T.; Mourtzakis, M.; Gioulbasanis, I.; Antoun, S. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am. J. Clin. Nutr. 2010, 91, 1133S–1137S. [Google Scholar] [CrossRef] [PubMed]
- Burkart, M.; Schieber, M.; Basu, S.; Shah, P.; Venugopal, P.; Borgia, J.A.; Gordon, L.; Karmali, R. Evaluation of the impact of cachexia on clinical outcomes in aggressive lymphoma. Br. J. Haematol. 2019, 186, 45–53. [Google Scholar] [CrossRef]
- Hetzler, K.L.; Hardee, J.P.; Puppa, M.J.; Narsale, A.A.; Sato, S.; Davis, J.M.; Carson, J.A. Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim. Biophys. Acta 2015, 1852, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, C.; Yin, H.; Yu, J.; Chen, S.; Fang, J.; Guo, F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 2016, 7, 63679–63689. [Google Scholar] [CrossRef]
- Baltgalvis, K.A.; Berger, F.G.; Pena, M.M.; Davis, J.M.; Muga, S.J.; Carson, J.A. Interleukin-6 and cachexia in ApcMin/+ mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R393–R401. [Google Scholar] [CrossRef]
- White, J.P.; Puppa, M.J.; Gao, S.; Sato, S.; Welle, S.L.; Carson, J.A. Muscle mTORC1 suppression by IL-6 during cancer cachexia: A role for AMPK. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1042–E1052. [Google Scholar] [CrossRef] [PubMed]
- Mehl, K.A.; Davis, J.M.; Berger, F.G.; Carson, J.A. Myofiber degeneration/regeneration is induced in the cachectic ApcMin/+ mouse. J. Appl. Physiol. 2005, 99, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Laird, B.J.; Mcmillan, D.; Skipworth, R.J.E.; Fallon, M.T.; Paval, D.R.; Mcneish, I.; Gallagher, I.J. The Emerging Role of Interleukin 1β (IL-1β) in Cancer Cachexia. Inflammation 2021, 44, 1223–1228. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, H.; Zhou, Y.; Tang, X.; Yu, B.; Li, J. Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer 2007, 7, 45. [Google Scholar] [CrossRef]
- Dinarello, C.A. An Expanding Role for Interleukin-1 Blockade from Gout to Cancer. Mol. Med. 2014, 20, S43–S58. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Murphy, E.A.; Carson, J.A. The Impact of Immune Cells on the Skeletal Muscle Microenvironment During Cancer Cachexia. Front. Physiol. 2020, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Baazim, H.; Antonio-Herrera, L.; Bergthaler, A. The interplay of immunology and cachexia in infection and cancer. Nat. Rev. Immunol. 2022, 22, 309–321. [Google Scholar] [CrossRef]
- White, J.P.; Baynes, J.W.; Welle, S.L.; Kostek, M.C.; Matesic, L.E.; Sato, S.; Carson, J.A. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS ONE 2011, 6, e24650. [Google Scholar] [CrossRef]
- He, W.A.; Berardi, E.; Cardillo, V.M.; Acharyya, S.; Aulino, P.; Thomas-Ahner, J.; Wang, J.; Bloomston, M.; Muscarella, P.; Nau, P.; et al. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Investig. 2013, 123, 4821–4835. [Google Scholar] [CrossRef] [PubMed]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; Mcanally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and Class II HDACs Control Neurogenic Muscle Atrophy by Inducing E3 Ubiquitin Ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [PubMed]
| Males | WTNL | WTL | APCNL | APCL |
| $ Body Mass (g) | 29.5 ± 0.8 a | 29.7 ± 0.8 a | 25.0 ± 0.8 b | 21.9 ± 0.5 c |
| * Soleus (mg/mm) | 0.59 ± 0.04 | 0.56 ± 0.03 | 0.47 ± 0.02 | 0.49 ± 0.03 |
| * Plantaris (mg/mm) | 1.08 ± 0.04 | 1.11 ± 0.02 | 0.84 ± 0.04 | 0.74 ± 0.05 |
| *# Gastrocnemius (mg/mm) | 8.54 ± 0.20 | 7.99 ± 0.30 | 5.62 ± 0.30 | 4.92 ± 0.30 |
| * Tibialis Anterior (mg/mm) | 3.00 ± 0.07 | 3.25 ± 0.06 | 1.98 ± 0.20 | 1.94 ± 0.08 |
| * Heart (mg) | 131.6 ± 4.50 | 130.9 ± 3.10 | 128.2 ± 5.61 | 116.1 ± 3.62 |
| * Spleen (mg) | 90.1 ± 2.32 | 97.1 ± 2.15 | 515.7 ± 39.30 | 492.5 ± 39.40 |
| Liver (mg) | 1364.30 ± 46.50 | 1318.1 ± 34.30 | 1306.3 ± 142.50 | 1185.4 ± 77.90 |
| * Gonadal Fat (mg) | 601.25 ± 85.8 | 596.13 ± 84.30 | 25.47 ± 6.80 | 49.57 ± 12.30 |
| Total Polyp count | ND | ND | 46 ± 4 | 34 ± 5 |
| Females | WTNL | WTL | APCNL | APCL |
| * Body Mass (g) | 23.2 ± 0.7 | 22.9 ± 0.7 | 19.3 ± 0.6 | 19.3 ± 0.9 |
| * Soleus (mg/mm) | 0.57 ± 0.04 | 0.39 ± 0.04 | 0.39 ± 0.02 | 0.44 ± 0.04 |
| * Plantaris (mg/mm) | 0.87 ± 0.05 | 0.86 ± 0.04 | 0.58 ± 0.04 | 0.59 ± 0.04 |
| * Gastrocnemius (mg/mm) | 6.44 ± 0.10 | 6.39 ± 0.10 | 4.24 ± 0.30 | 4.20 ± 0.30 |
| * Tibialis Anterior (mg/mm) | 2.49 ± 0.10 | 2.28 ± 0.09 | 1.59 ± 0.09 | 1.48 ± 0.10 |
| * Heart (mg) | 104.1 ± 4.30 | 109.60 ± 50 | 114.40 ± 5.50 | 123.90 ± 5.70 |
| * Spleen (mg) | 89.1 ± 4.50 | 85.70 ± 4.20 | 378.7 ± 39.30 | 346.00 ± 48.10 |
| # Liver (mg) | 953.9 ± 18.10 | 880.10 ± 39.10 | 1011.00 ± 43.90 | 851.20 ± 97.00 |
| * Gonadal Fat (mg) | 516.8 ± 68.20 | 409.63 ± 53.10 | 97.83 ± 32.10 | ND |
| Total Polyp count | ND | ND | 29.8 ± 4 | 38.5 ± 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schrems, E.R.; Haynie, W.S.; Perry, R.A., Jr.; Morena, F.; Cabrera, A.R.; Rosa-Caldwell, M.E.; Greene, N.P.; Washington, T.A. Leucine Supplementation Exacerbates Morbidity in Male but Not Female Mice with Colorectal Cancer-Induced Cachexia. Nutrients 2023, 15, 4570. https://doi.org/10.3390/nu15214570
Schrems ER, Haynie WS, Perry RA Jr., Morena F, Cabrera AR, Rosa-Caldwell ME, Greene NP, Washington TA. Leucine Supplementation Exacerbates Morbidity in Male but Not Female Mice with Colorectal Cancer-Induced Cachexia. Nutrients. 2023; 15(21):4570. https://doi.org/10.3390/nu15214570
Chicago/Turabian StyleSchrems, Eleanor R., Wesley S. Haynie, Richard A. Perry, Jr., Francielly Morena, Ana Regina Cabrera, Megan E. Rosa-Caldwell, Nicholas P. Greene, and Tyrone A. Washington. 2023. "Leucine Supplementation Exacerbates Morbidity in Male but Not Female Mice with Colorectal Cancer-Induced Cachexia" Nutrients 15, no. 21: 4570. https://doi.org/10.3390/nu15214570
APA StyleSchrems, E. R., Haynie, W. S., Perry, R. A., Jr., Morena, F., Cabrera, A. R., Rosa-Caldwell, M. E., Greene, N. P., & Washington, T. A. (2023). Leucine Supplementation Exacerbates Morbidity in Male but Not Female Mice with Colorectal Cancer-Induced Cachexia. Nutrients, 15(21), 4570. https://doi.org/10.3390/nu15214570





