Eight Weeks of Bifidobacterium lactis BL-99 Supplementation Improves Lipid Metabolism and Sports Performance through Short-Chain Fatty Acids in Cross-Country Skiers: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Group
2.2. Probiotic Supplementation Program
2.3. Energy Intake and Expenditure
2.3.1. Dietary Records
2.3.2. Training Load and Energy Expenditure during Training
2.4. Body Composition
2.5. Maximal Oxygen Consumption (VO2max)
2.6. Isokinetic Muscle Strength Test
2.7. Hematological–Biochemical Analysis
2.8. Fecal Metagenomic Analysis
- (1)
- Extraction of microbiome DNA and metagenome library preparation
- (2)
- Bioinformatic Analysis
2.9. Targeted Metabolomic Analysis of Blood
2.10. Data Analysis
3. Results
3.1. Diary Nutrition
3.2. Training Load and Energy Expenditure during Training
3.3. Body Composition
3.4. Maximal Oxygen Consumption (VO2max)
3.5. Isokinetic Muscle Strength Test
3.6. Hematological–Biochemical Profiling
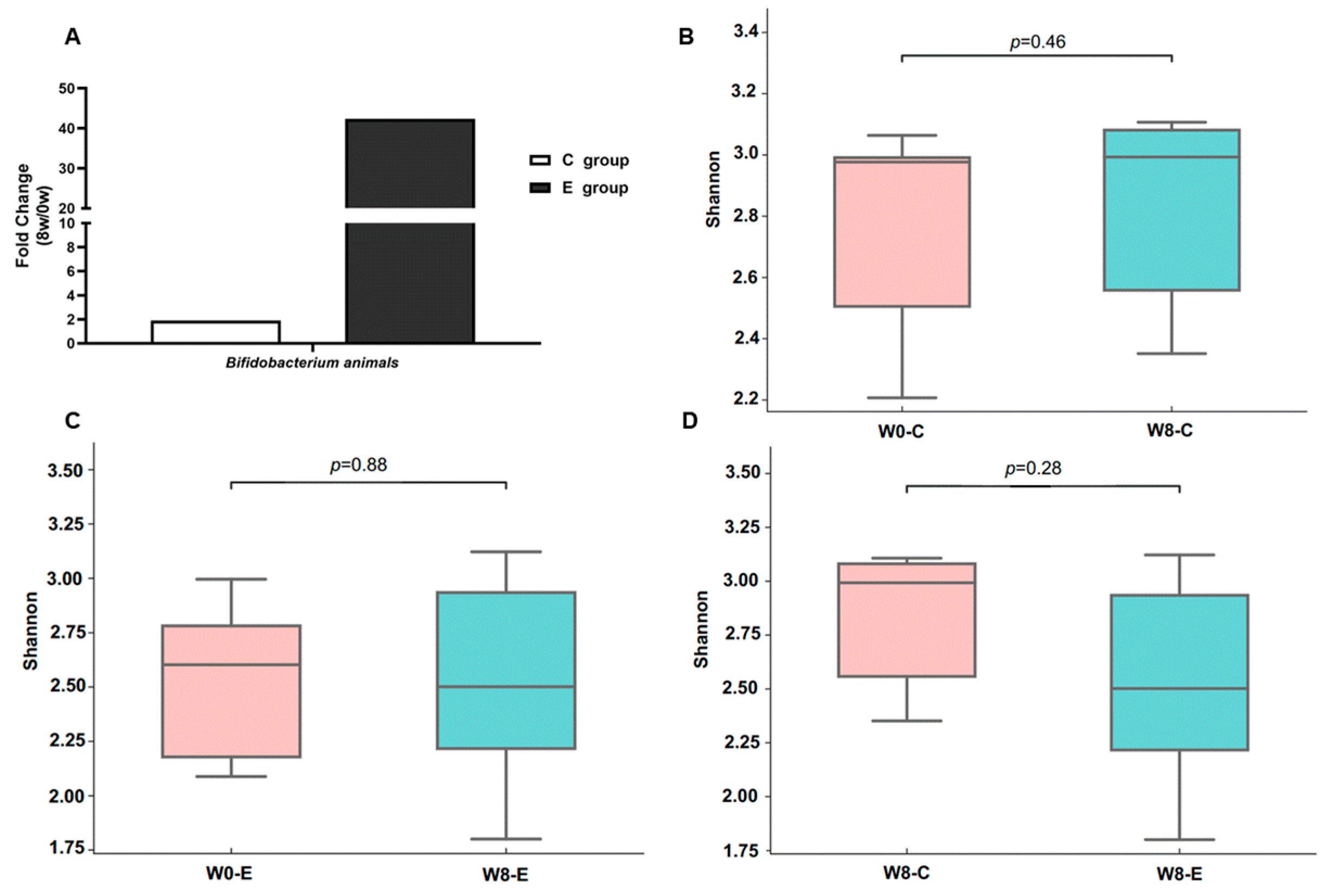
3.7. Fecal Metagenomics
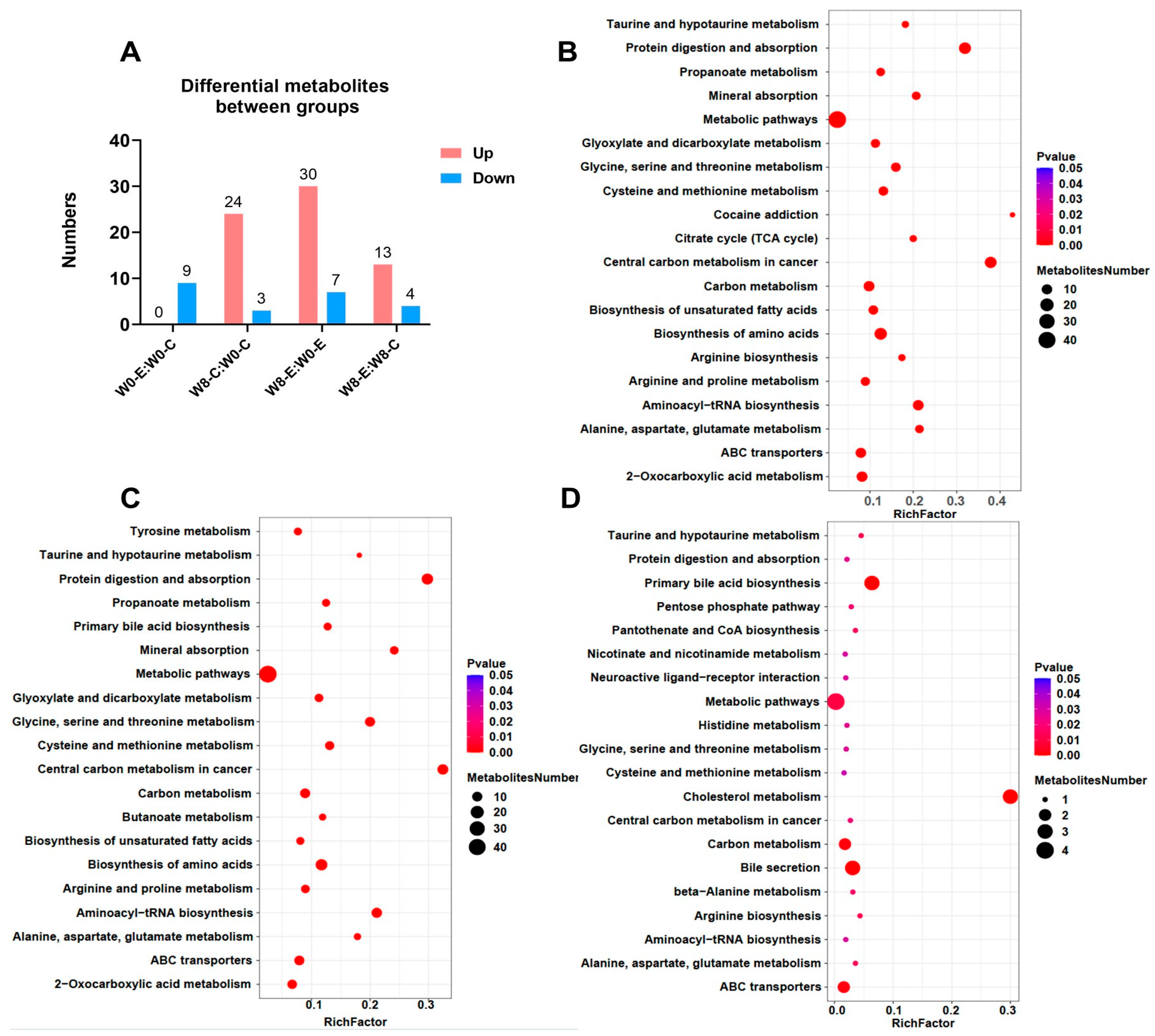
3.8. Plasma-Targeted Metabolomics
3.8.1. Overview of Plasma-Targeted Metabolomic Analysis
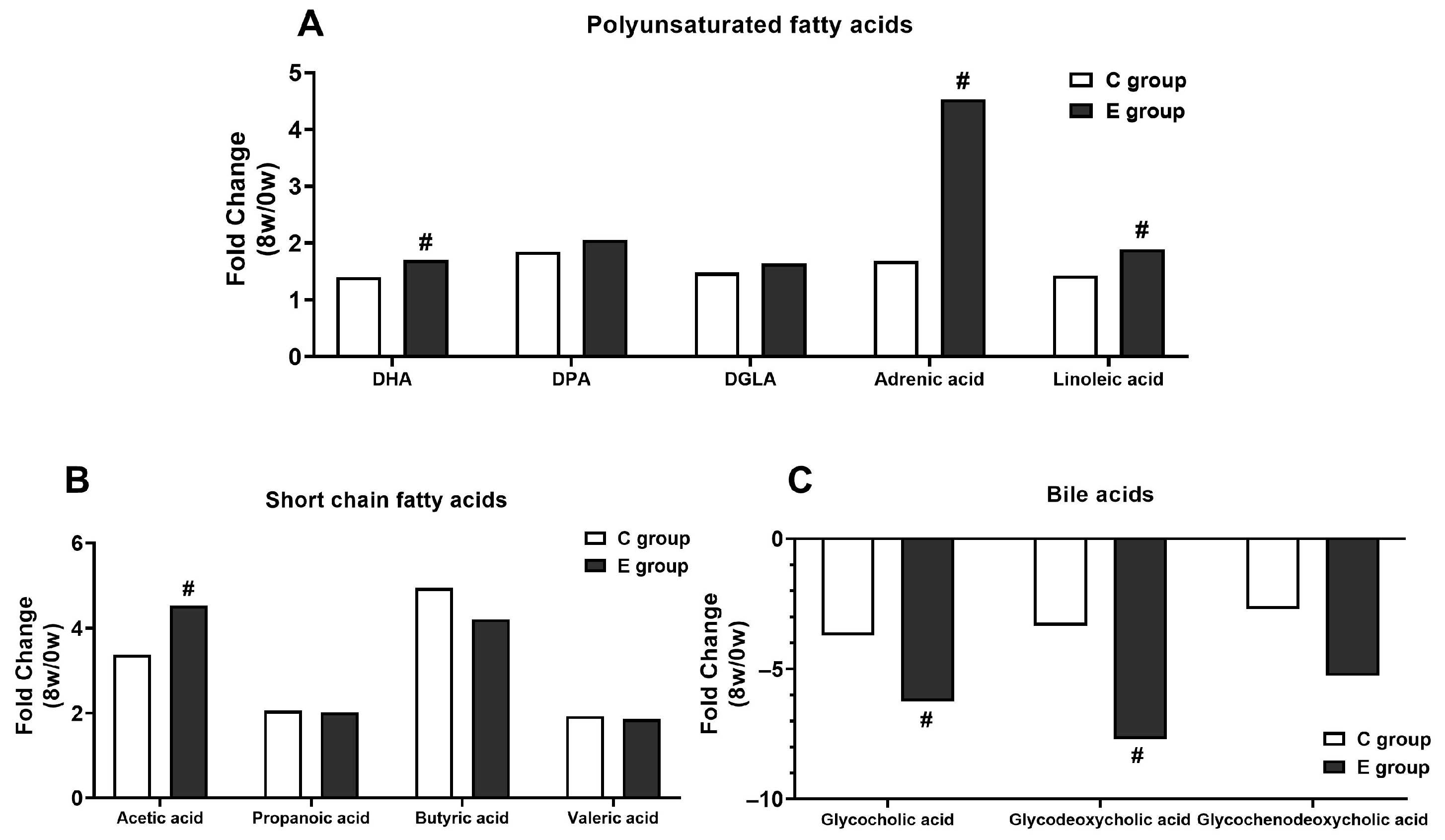
3.8.2. The Most Regulated Lipid-Metabolism-Related Metabolites
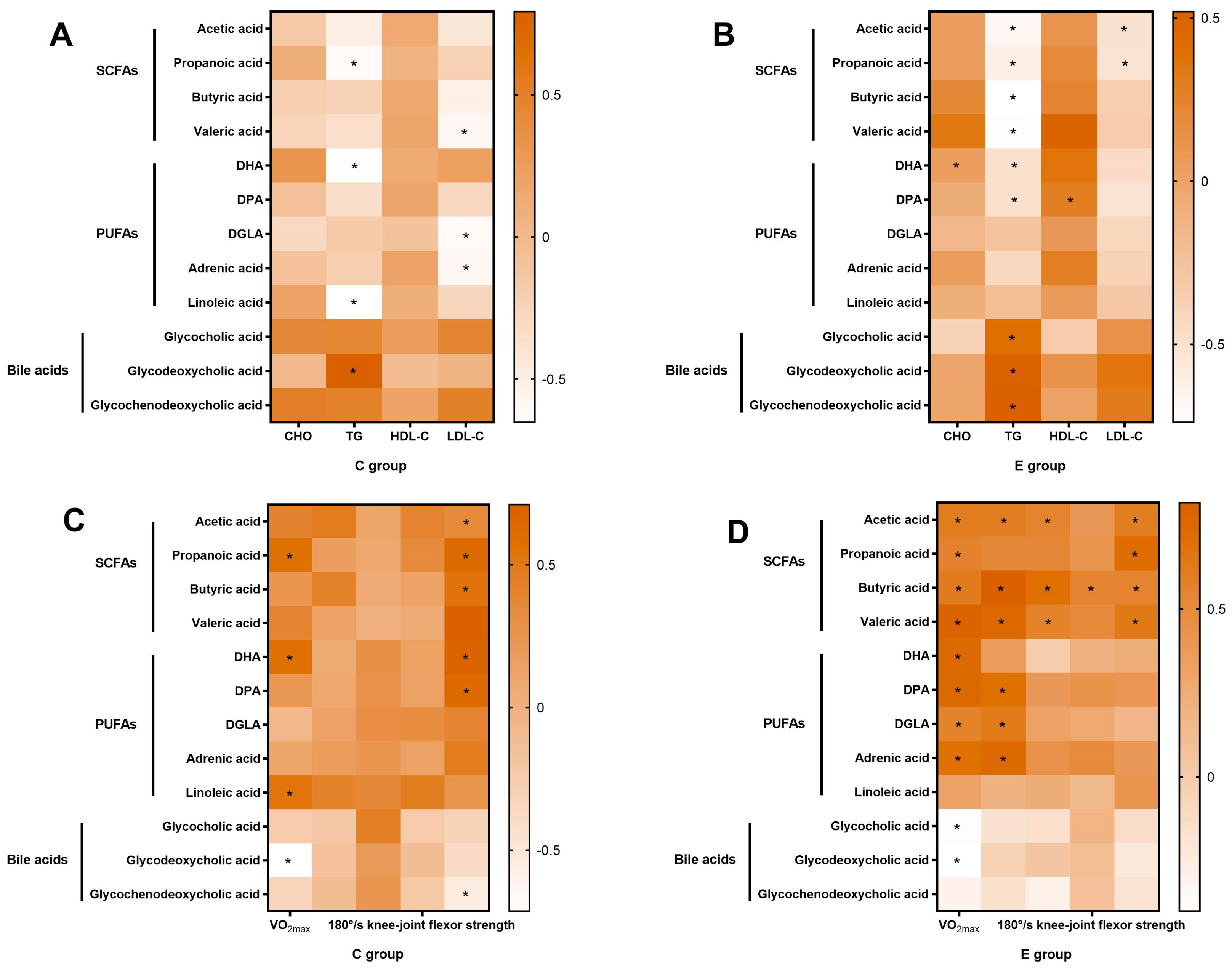
3.9. Correlation Analysis
3.9.1. Correlation Analysis of Bifidobacterium animalis and the Most Regulated Metabolites
3.9.2. Analysis of the Correlation of the Most Regulated Metabolites with Lipid-Metabolism-Related Indicators and Sports Performance
4. Discussion
4.1. BL-99 Supplementation Increases Bifidobacterium Abundance
4.2. BL-99 Supplementation Combined with Training Ameliorated Lipid Metabolism through Short-Chain Fatty Acids
4.3. BL-99 Supplementation Combined with Training Increased Muscle Strength and VO2max through Short-Chain Fatty Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A.; et al. Probiotic Administration Increases Amino Acid Absorption from Plant Protein: A Placebo-Controlled, Randomized, Double-Blind, Multicenter, Crossover Study. Probiotics Antimicrob. Proteins 2020, 12, 1330–1339. [Google Scholar] [CrossRef]
- Keller, D.; Van Dinter, R.; Cash, H.; Farmer, S.; Venema, K. Bacillus coagulans GBI-30, 6086 increases plant protein digestion in a dynamic, computer-controlled in vitro model of the small intestine (TIM-1). Benef. Microbes 2017, 8, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Hazards, E.P.o.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA J. 2020, 18, e05966. [Google Scholar] [CrossRef]
- He, B.L.; Xiong, Y.; Hu, T.G.; Zong, M.H.; Wu, H. Bifidobacterium spp. as functional foods: A review of current status, challenges, and strategies. Crit. Rev. Food Sci. Nutr. 2022, 63, 8048–8065. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sanchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Bernini, L.J.; Simao, A.N.; Alfieri, D.F.; Lozovoy, M.A.; Mari, N.L.; de Souza, C.H.; Dichi, I.; Costa, G.N. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: A randomized trial. Effects of probiotics on metabolic syndrome. Nutrition 2016, 32, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]
- Leite, G.S.F.; Resende Master Student, A.S.; West, N.P.; Lancha, A.H., Jr. Probiotics and sports: A new magic bullet? Nutrition 2019, 60, 152–160. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Huang, C.C.; Liu, H.C.; Lee, M.C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients 2020, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of Probiotic (Bifidobacterium longum 35624) Supplementation on Exercise Performance, Immune Modulation, and Cognitive Outlook in Division I Female Swimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Perez de Heredia, F.; Gomez-Martinez, S.; Marcos, A. The Role of Probiotics on the Microbiota: Effect on Obesity. Nutr. Clin. Pract. 2016, 31, 387–400. [Google Scholar] [CrossRef]
- Jong-Yeon, K.; Hickner, R.C.; Dohm, G.L.; Houmard, J.A. Long- and medium-chain fatty acid oxidation is increased in exercise-trained human skeletal muscle. Metabolism 2002, 51, 460–464. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport. Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Gasparini Neto, V.H.; Santos Neves, L.N.; Kalva-Filho, C.A.; Schwingel, P.A.; Leite, R.D.; Carletti, L. Cardiopulmonary Exercise Testing with Elastic Resistance: A New Reproducible Proposal for Determination of Ventilatory Thresholds and Maximum Oxygen Consumption. J. Sports Sci. Med. 2022, 21, 426–434. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinformatics 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Prasad, J.; Gill, H.; Stevenson, L.; Gopal, P. Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J. Nutr. Health Aging 2007, 11, 26–31. [Google Scholar]
- Tremblay, A.; Fatani, A.; Ford, A.L.; Piano, A.; Nagulesapillai, V.; Auger, J.; MacPherson, C.W.; Christman, M.C.; Tompkins, T.A.; Dahl, W.J. Safety and Effect of a Low- and High-Dose Multi-Strain Probiotic Supplement on Microbiota in a General Adult Population: A Randomized, Double-Blind, Placebo-Controlled Study. J. Diet. Suppl. 2021, 18, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Fang, T.J.; Ho, H.H.; Chen, J.F.; Kuo, Y.W.; Huang, Y.Y.; Tsai, S.Y.; Wu, S.F.; Lin, H.C.; Yeh, Y.T. A multi-strain probiotic blend reshaped obesity-related gut dysbiosis and improved lipid metabolism in obese children. Front. Nutr. 2022, 9, 922993. [Google Scholar] [CrossRef]
- Chen, M.; Liu, C.; Dai, M.; Wang, Q.; Li, C.; Hung, W. Bifidobacterium lactis BL-99 modulates intestinal inflammation and functions in zebrafish models. PLoS ONE 2022, 17, e0262942. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Liu, W.H.; Zheng, H.; Feng, H.; Zhao, W.; Hung, W.L.; Li, H. Bifidobacterium lactis BL-99 protects mice with osteoporosis caused by colitis via gut inflammation and gut microbiota regulation. Food Funct. 2022, 13, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Zhao, W.; Liu, W.H.; Li, Y.; Li, N.; Hong, Y.; Cui, J.; Shang, X.; Feng, H.; Hung, W.L.; et al. Bifidobacterium animalis subsp. lactis BL-99 ameliorates colitis-related lung injury in mice by modulating short-chain fatty acid production and inflammatory monocytes/macrophages. Food Funct. 2023, 14, 1099–1112. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef]
- Latino, F.; Cataldi, S.; Carvutto, R.; De Candia, M.; D’Elia, F.; Patti, A.; Bonavolonta, V.; Fischetti, F. The Importance of Lipidomic Approach for Mapping and Exploring the Molecular Networks Underlying Physical Exercise: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8734. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Liu, J.; Johnson, R.; Dillon, S.; Jankowski, C.M.; Kroehl, M.; Robertson, C.E.; Frank, D.N.; Tuncil, Y.; Higgins, J.; et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther. Adv. Infect. Dis. 2021, 8, 20499361211027067. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, Z.; Xing, X.; Fan, Y.; Zhang, Y.; Nan, B.; Li, X.; Wang, Y.; Liu, J. The ameliorative effect of probiotics on diet-induced lipid metabolism disorders: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Guo, S.; Chen, M.; Wu, T.; Liu, K.; Zhang, H.; Wang, J. Probiotic Bifidobacterium animalis ssp. lactis Probio-M8 improves the properties and organic acid metabolism of fermented goat milk. J. Dairy. Sci. 2022, 105, 9426–9438. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honore, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, W.H.; Li, J.; Chow, S.K.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the gut microbiota and sarcopenia: A systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Lugli, G.A.; Duranti, S.; Turroni, F.; Mancabelli, L.; Ferrario, C.; Mangifesta, M.; Hevia, A.; Viappiani, A.; Scholz, M.; et al. Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Sci. Rep. 2015, 5, 15782. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Chambers, E.; Ni Lochlainn, M.; Witard, O.C. Mechanisms Linking the Gut-Muscle Axis With Muscle Protein Metabolism and Anabolic Resistance: Implications for Older Adults at Risk of Sarcopenia. Front. Physiol. 2021, 12, 770455. [Google Scholar] [CrossRef]
- Buigues, C.; Fernandez-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martinez, R.; Martinez-Martinez, M.; Verdejo, Y.; Mascaros, M.C.; Peris, C.; Cauli, O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [PubMed]
- Smarkusz-Zarzecka, J.; Ostrowska, L.; Leszczynska, J.; Orywal, K.; Cwalina, U.; Pogodzinski, D. Analysis of the Impact of a Multi-Strain Probiotic on Body Composition and Cardiorespiratory Fitness in Long-Distance Runners. Nutrients 2020, 12, 3758. [Google Scholar] [CrossRef]
- Soares, A.D.N.; Wanner, S.P.; Morais, E.S.S.; Hudson, A.S.R.; Martins, F.S.; Cardoso, V.N. Supplementation with Saccharomyces boulardii Increases the Maximal Oxygen Consumption and Maximal Aerobic Speed Attained by Rats Subjected to an Incremental-Speed Exercise. Nutrients 2019, 11, 2352. [Google Scholar] [CrossRef]
- Salarkia, N.; Ghadamli, L.; Zaeri, F.; Sabaghian Rad, L. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: A randomized controlled trial. Med. J. Islam. Repub. Iran. 2013, 27, 141–146. [Google Scholar]
- Takahashi, E.; Yamada, M.; Saito, M.; Kuboyama, M.; Ogasa, K. Differentiation of cultured Friend leukemia cells induced by short-chain fatty acids. Gan 1975, 66, 577–580. [Google Scholar]
- Perrine, S.P.; Wargin, W.A.; Boosalis, M.S.; Wallis, W.J.; Case, S.; Keefer, J.R.; Faller, D.V.; Welch, W.C.; Berenson, R.J. Evaluation of safety and pharmacokinetics of sodium 2,2 dimethylbutyrate, a novel short chain fatty acid derivative, in a phase 1, double-blind, placebo-controlled, single-dose, and repeat-dose studies in healthy volunteers. J. Clin. Pharmacol. 2011, 51, 1186–1194. [Google Scholar] [CrossRef][Green Version]
- Zeng, H.; Huang, C.; Lin, S.; Zheng, M.; Chen, C.; Zheng, B.; Zhang, Y. Lotus Seed Resistant Starch Regulates Gut Microbiota and Increases Short-Chain Fatty Acids Production and Mineral Absorption in Mice. J. Agric. Food Chem. 2017, 65, 9217–9225. [Google Scholar] [CrossRef]
- Michalickova, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Nikolic, I.; Djordjevic, B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016, 41, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Wu, M.F.; Lee, M.C.; Huang, C.C. Exercise training combined with Bifidobacterium longum OLP-01 treatment regulates insulin resistance and physical performance in db/db mice. Food Funct. 2021, 12, 7728–7740. [Google Scholar] [CrossRef]
- Batatinha, H.; Tavares-Silva, E.; Leite, G.S.F.; Resende, A.S.; Albuquerque, J.A.T.; Arslanian, C.; Fock, R.A.; Lancha, A.H., Jr.; Lira, F.S.; Kruger, K.; et al. Probiotic supplementation in marathonists and its impact on lymphocyte population and function after a marathon: A randomized placebo-controlled double-blind study. Sci. Rep. 2020, 10, 18777. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Hsu, Y.J.; Ho, H.H.; Chang, Y.C.; Kuo, Y.W.; Yeh, Y.T.; Tsai, S.Y.; Chen, C.W.; Chen, J.F.; Huang, C.C.; et al. Bifidobacterium longum subsp. longum OLP-01 Supplementation during Endurance Running Training Improves Exercise Performance in Middle- and Long-Distance Runners: A Double-Blind Controlled Trial. Nutrients 2020, 12, 1972. [Google Scholar] [CrossRef] [PubMed]

| Index | C Group (n = 8) | E Group (n = 7) |
|---|---|---|
| Age (years) | 19.3 ± 0.7 | 19.6 ± 1.1 |
| Training years | 7.3 ± 0.3 | 7.5 ± 0.3 |
| Height (cm) | 178.6 ± 5.5 | 176.1 ± 3.7 |
| Body mass (kg) | 59.5 ± 8.4 | 59.9 ± 5.2 |
| Fat mass (kg) | 3.4 ± 1.3 | 3.5 ± 1.1 |
| Muscle mass (kg) | 31.8 ± 4.5 | 32.0 ± 1.2 |
| Body Mass Index (BMI) (kg/m2) | 18.6 ± 2.1 | 19.2 ± 1.1 |
| Time | Index | C Group (n = 8) | E Group (n = 7) | p Value | Cohen’s d Effect Size |
|---|---|---|---|---|---|
| Week-0 | Total energy (kJ) | 3138.5 ± 304.6 | 3159.9 ± 196.3 | 0.63 | −0.08 |
| Carbohydrate (g) | 404.1 ± 56.1 | 402.9 ± 25.7 | 0.91 | 0.03 | |
| Protein (g) | 145.1 ± 8.8 | 148.4 ± 9.5 | 0.76 | −0.36 | |
| Fat (g) | 106.8 ± 8.4 | 109.0 ± 8.4 | 0.38 | −0.26 | |
| Week-8 | Total energy (kJ) | 3239.5 ± 915.2 | 3006.1 ± 622.8 | 0.36 | 0.30 |
| Carbohydrate (g) | 373.5 ± 113.9 | 383.6 ± 76.9 | 0.16 | −0.10 | |
| Protein (g) | 166.4 ± 38.4 | 148.6 ± 29.2 | 0.26 | 0.52 | |
| Fat (g) | 127.9 ± 44.1 | 106.6 ± 29.2 | 0.71 | 0.57 |
| Time | Index | C Group (n = 8) | E Group (n = 7) | p Value | Cohen’s d Effect Size |
|---|---|---|---|---|---|
| Week-1 | TRIMP | 126.6 ± 30.9 | 124.9 ± 21.3 | 0.71 | 0.06 |
| EE (Kcal) | 2055.6 ± 306.5 | 2156.4 ± 389.8 | 0.73 | −0.28 | |
| Week-2 | TRIMP | 207.3 ± 59.8 | 209.2 ± 55.8 | 0.35 | −0.03 |
| EE (Kcal) | 2697.9 ± 423.8 | 2778.6 ± 498.3 | 0.14 | −0.17 | |
| Week-3 | TRIMP | 150.3 ± 46.2 | 175.4 ± 57.2 | 0.50 | −0.48 |
| EE (Kcal) | 2041.4 ± 299.1 | 2207.2 ± 352.7 | 0.07 | −0.51 | |
| Week-4 | TRIMP | 168.5 ± 45.1 | 165.1 ± 46.3 | 0.61 | 0.07 |
| EE (Kcal) | 2221.6 ± 392.7 | 2207.5 ± 406.4 | 0.15 | 0.04 | |
| Week-5 | TRIMP | 166.9 ± 38.0 | 161.0 ± 49.3 | 0.57 | 0.13 |
| EE (Kcal) | 2062.5 ± 234.9 | 2135.8 ± 410.4 | 0.17 | −0.22 | |
| Week-6 | TRIMP | 232.6 ± 73.1 | 232.1 ± 40.1 | 0.67 | 0.01 |
| EE (Kcal) | 2399.4 ± 457.4 | 2458.4 ± 341.7 | 0.30 | −0.15 | |
| Week-7 | TRIMP | 217.7 ± 31.3 | 214.0 ± 48.5 | 0.25 | 0.09 |
| EE (Kcal) | 2455.0 ± 299.8 | 2212.4 ± 330.1 | 0.47 | 0.77 | |
| Week-8 | TRIMP | 134.5 ± 32.2 | 135.9 ± 43.4 | 0.20 | −0.04 |
| EE (Kcal) | 1872.4 ± 239.4 | 1562.2 ± 424.1 | 0.44 | 0.90 |
| Time | Index | C Group (n = 8) | E Group (n = 7) | p Value | Cohen’s d Effect Size |
|---|---|---|---|---|---|
| Week-0 | Body mass (kg) | 59.5 ± 8.4 | 59.9 ± 5.2 | 0.93 | −0.06 |
| Fat-free mass (kg) | 56.2 ± 7.4 | 56.4 ± 5.0 | 0.94 | −0.03 | |
| Body fat (kg) | 3.4 ± 1.3 | 3.5 ± 1.2 | 0.90 | −0.08 | |
| Body fat percentage (%) | 5.6 ± 1.6 | 5.8 ± 2.0 | 0.82 | −0.11 | |
| Week-8 | Body mass (kg) | 62.0 ± 8.0 | 62.1 ± 5.3 | 0.98 | −0.01 |
| Fat-free mass (kg) | 57.5 ± 7.0 | 57.6 ± 5.0 | 0.98 | −0.02 | |
| Body fat (kg) | 4.4 ± 0.5 | 4.5 ± 0.4 | 0.98 | −0.22 | |
| Body fat percentage (%) | 7.1 ± 1.9 | 7.2 ± 1.6 | 0.90 | −0.06 |
| Time | Index (mL/Kg) | C Group (n = 8) | E Group (n = 7) | p Value | Cohen’s d Effect Size |
| Week-0 | VO2max | 55.9 ± 4.4 | 55.8 ± 5.4 | 0.56 | 0.02 |
| Week-8 | VO2max | 61.8 ± 3.2 * | 64.5 ± 2.6 *# | 0.02 | −0.93 |
| Group | Index (mL/Kg) | Week-0 | Week-8 | p Value | Cohen’s d Effect Size |
| C group (n = 8) | VO2max | 55.9 ± 4.4 | 61.8 ± 3.2 * | 0.01 | −1.53 |
| E group (n = 7) | VO2max | 55.8 ± 5.4 | 64.5 ± 2.6 *# | 0.01 | −2.05 |
| Time | Index (Right + Left, Nm/Kg) | C Group (n = 8) | E Group (n = 7) | p Value | Cohen’s d Effect Size |
| Week-0 | 60°/s knee joint flexor strength | 2.3 ± 0.4 | 2.0 ± 0.4 | 0.11 | 0.75 |
| 60°/s knee joint extensor strength | 5.2 ± 0.6 | 5.0 ± 0.4 | 0.55 | 0.39 | |
| 180°/s knee joint flexor strength | 2.1 ± 0.2 | 2.2 ± 0.3 | 0.56 | −0.39 | |
| 180°/s knee joint extensor strength | 3.9 ± 0.4 | 4.0 ± 0.5 | 0.61 | −0.22 | |
| Week-8 | 60°/s knee joint flexor strength | 2.8 ± 0.5 | 2.9 ± 0.6 * | 0.70 | −0.18 |
| 60°/s knee joint extensor strength | 5.1 ± 0.2 | 5.7 ± 0.4 *# | 0.00 | −1.90 | |
| 180°/s knee joint flexor strength | 2.3 ± 0.5 | 2.5 ± 0.6 | 0.59 | −0.36 | |
| 180°/s knee joint extensor strength | 4.5 ± 0.5 * | 4.8 ± 0.6 * | 0.24 | −0.54 | |
| Group | Index (Right + Left, Nm/Kg) | Week-0 | Week-8 | p Value | Cohen’s d Effect Size |
| C group (n = 8) | 60°/s knee joint flexor strength | 2.3 ± 0.4 | 2.8 ± 0.5 | 0.06 | −1.10 |
| 60°/s knee joint extensor strength | 5.2 ± 0.6 | 5.1 ± 0.2 | 0.77 | 0.22 | |
| 180°/s knee joint flexor strength | 2.1 ± 0.2 | 2.3 ± 0.5 | 0.24 | −0.53 | |
| 180°/s knee joint extensor strength | 3.9 ± 0.4 | 4.5 ± 0.5 * | 0.02 | −1.33 | |
| E group (n = 7) | 60°/s knee joint flexor strength | 2.0 ± 0.4 | 2.9 ± 0.6 * | 0.00 | −1.77 |
| 60°/s knee joint extensor strength | 5.0 ± 0.4 | 5.7 ± 0.4 *# | 0.01 | −1.75 | |
| 180°/s knee joint flexor strength | 2.2 ± 0.3 | 2.5 ± 0.6 | 0.25 | −0.63 | |
| 180°/s knee joint extensor strength | 4.0 ± 0.5 | 4.8 ± 0.6 * | 0.02 | −1.45 |
| Time | Index | C Group (n = 8) | E Group (n = 7) | p Value | Cohen’s d Effect Size |
| Week-0 | GLOB (g/L) | 28.9 ± 3.0 | 26.3 ± 1.4 | 0.77 | 1.11 |
| ALB (g/L) | 47.2 ± 1.7 | 46.6 ± 1.4 | 0.48 | 0.38 | |
| TG (mmol/L) | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.19 | 0.39 | |
| T-CHO (mmol/L) | 3.2 ± 0.4 | 3.4 ± 0.7 | 0.67 | −0.35 | |
| HDL-C (mmol/L) | 1.4 ± 0.2 | 1.5 ± 0.2 | 0.55 | −0.50 | |
| LDL-C (mmol/L) | 2.0 ± 0.5 | 2.1 ± 0.6 | 0.87 | −0.18 | |
| Week-8 | GLOB (g/L) | 28.5 ± 1.9 | 25.4 ± 2.0 | 0.05 | 1.59 |
| ALB (g/L) | 45.6 ± 1.5 | 44.8 ± 0.8 * | 0.22 | 0.67 | |
| TG (mmol/L) | 0.3 ± 0.1 * | 0.3 ± 0.1 * | 0.52 | 0.00 | |
| T-CHO (mmol/L) | 3.3 ± 0.5 | 3.2 ± 0.5 | 0.67 | 0.20 | |
| HDL-C (mmol/L) | 1.5 ± 0.2 | 1.5 ± 0.2 | 0.77 | 0.00 | |
| LDL-C (mmol/L) | 1.6 ± 0.5 | 1.6 ± 0.4 * | 0.91 | 0.00 | |
| Group | Index | Week-0 | Week-8 | p Value | Cohen’s d Effect Size |
| C group (n = 8) | GLOB (g/L) | 28.9 ± 3.0 | 28.5 ± 1.9 | 0.77 | 0.16 |
| ALB (g/L) | 47.2 ± 1.7 | 45.6 ± 1.5 | 0.08 | 1.00 | |
| TG (mmol/L) | 0.7 ± 0.3 | 0.3 ± 0.1 * | 0.00 | 1.79 | |
| T-CHO (mmol/L) | 3.2 ± 0.4 | 3.3 ± 0.5 | 0.75 | −0.22 | |
| HDL-C (mmol/L) | 1.4 ± 0.2 | 1.5 ± 0.2 | 0.30 | −0.50 | |
| LDL-C (mmol/L) | 2.0 ± 0.5 | 1.6 ± 0.5 | 0.11 | −0.80 | |
| E group (n = 7) | GLOB (g/L) | 26.3 ± 1.4 | 25.4 ± 2.0 | 0.44 | 0.52 |
| ALB (g/L) | 46.6 ± 1.4 | 44.8 ± 0.8 * | 0.01 | 1.58 | |
| TG (mmol/L) | 0.6 ± 0.2 | 0.3 ± 0.1 * | 0.01 | 1.90 | |
| T-CHO (mmol/L) | 3.4 ± 0.7 | 3.2 ± 0.5 | 0.62 | 0.33 | |
| HDL-C (mmol/L) | 1.5 ± 0.2 | 1.5 ± 0.2 | 0.95 | 0.00 | |
| LDL-C (mmol/L) | 2.1 ± 0.6 | 1.6 ± 0.4 * | 0.04 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Rui, Z.; Mao, L.; Chang, Y.; Shao, J.; Chen, Y.; Han, Q.; Sui, X.; An, N.; Li, H.; et al. Eight Weeks of Bifidobacterium lactis BL-99 Supplementation Improves Lipid Metabolism and Sports Performance through Short-Chain Fatty Acids in Cross-Country Skiers: A Preliminary Study. Nutrients 2023, 15, 4554. https://doi.org/10.3390/nu15214554
Li T, Rui Z, Mao L, Chang Y, Shao J, Chen Y, Han Q, Sui X, An N, Li H, et al. Eight Weeks of Bifidobacterium lactis BL-99 Supplementation Improves Lipid Metabolism and Sports Performance through Short-Chain Fatty Acids in Cross-Country Skiers: A Preliminary Study. Nutrients. 2023; 15(21):4554. https://doi.org/10.3390/nu15214554
Chicago/Turabian StyleLi, Tieying, Zihan Rui, Letian Mao, Yashan Chang, Jing Shao, Yue Chen, Qi Han, Xuemei Sui, Nan An, Haoqiu Li, and et al. 2023. "Eight Weeks of Bifidobacterium lactis BL-99 Supplementation Improves Lipid Metabolism and Sports Performance through Short-Chain Fatty Acids in Cross-Country Skiers: A Preliminary Study" Nutrients 15, no. 21: 4554. https://doi.org/10.3390/nu15214554
APA StyleLi, T., Rui, Z., Mao, L., Chang, Y., Shao, J., Chen, Y., Han, Q., Sui, X., An, N., Li, H., Feng, H., Jiang, T., & Wang, Q. (2023). Eight Weeks of Bifidobacterium lactis BL-99 Supplementation Improves Lipid Metabolism and Sports Performance through Short-Chain Fatty Acids in Cross-Country Skiers: A Preliminary Study. Nutrients, 15(21), 4554. https://doi.org/10.3390/nu15214554







