The Association among Urinary Lead and Cadmium, Serum Adiponectin, and Serum Apoptotic Microparticles in a Young Taiwanese Population
Abstract
1. Introduction
2. Materials and Methods
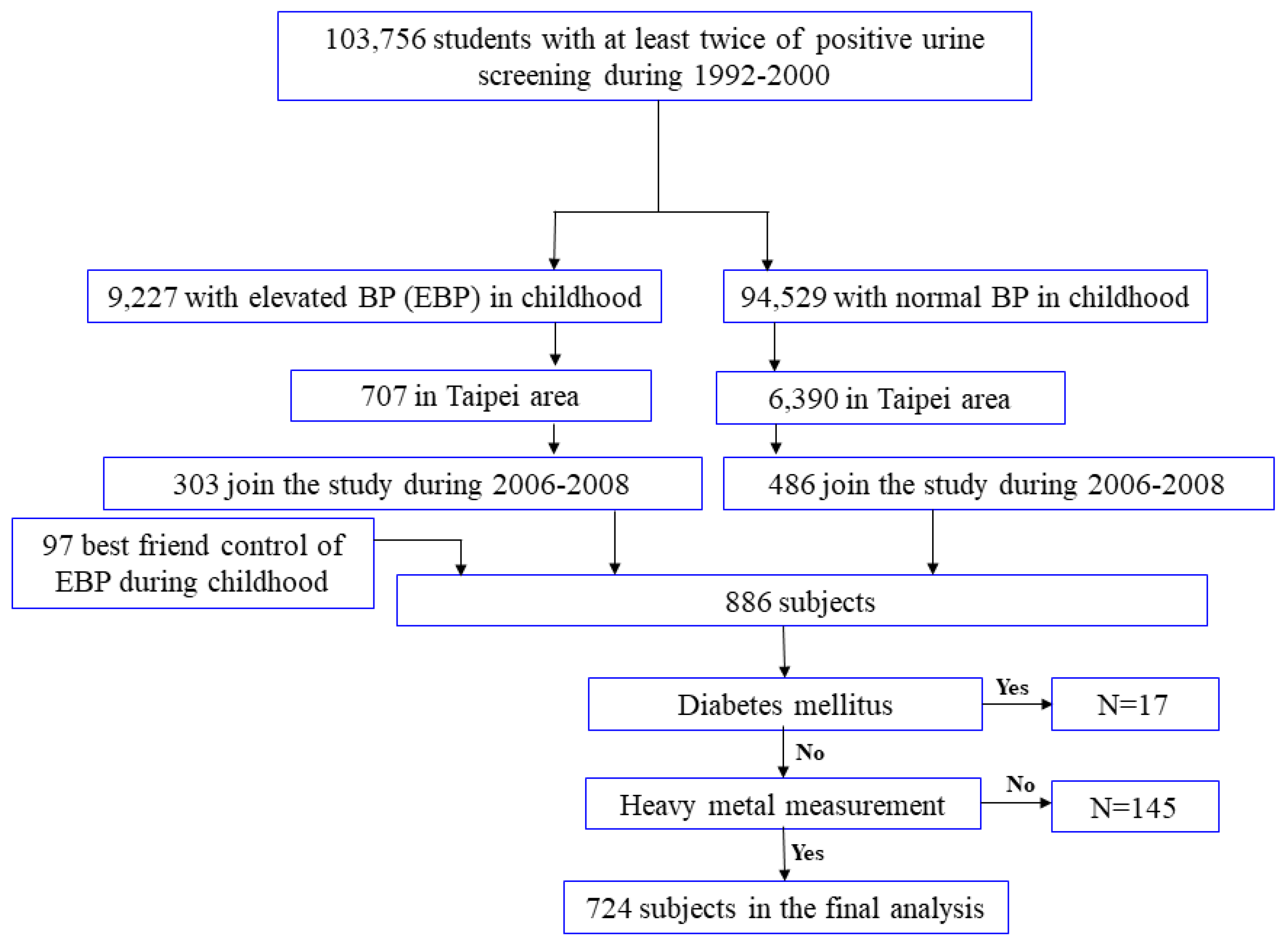
2.1. Study Population
2.2. Measurement of Serum Adiponectin, Urinary Pb and Cd Levels
2.3. Measurement of the Concentration of Apoptotic Microparticles in Serum
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 10 Chemicals of Public Health Concern. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 15 August 2023).
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, G.; Wang, Z.; Zhou, H.; He, P.; Liu, Y.; Jin, T. The association between lead and cadmium co-exposure and renal dysfunction. Ecotoxicol. Environ. Saf. 2019, 173, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, J.; Niu, J.; Wang, H.; Li, X. Association between cadmium and lead co-exposure, blood pressure, and hypertension: A cross-sectional study from northwest China. Hum. Ecol. Risk Assess. Int. J. 2022, 28, 471–489. [Google Scholar] [CrossRef]
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef]
- Lamas, G.A.; Ujueta, F.; Navas-Acien, A. Lead and Cadmium as Cardiovascular Risk Factors: The Burden of Proof Has Been Met. J. Am. Heart Assoc. 2021, 10, e018692. [Google Scholar] [CrossRef]
- Sevim, Ç.; Doğan, E.; Comakli, S. Cardiovascular disease and toxic metals. Curr. Opin. Toxicol. 2020, 19, 88–92. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, H.L.; Hwang, Y.T.; Huang, P.C.; Wang, C.; Sung, F.C.; Wu, C.; Su, T.C. Urinary heavy metals, DNA methylation, and subclinical atherosclerosis. Ecotoxicol. Environ. Saf. 2020, 204, 111039. [Google Scholar] [CrossRef]
- Hong, D.; Bai, Y.P.; Gao, H.C.; Wang, X.; Li, L.F.; Zhang, G.G.; Hu, C.P. Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis 2014, 235, 310–317. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Q.; Liu, J.; Li, R.; Wang, D.; Peng, W.; Wu, C. Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol. Res. 2021, 168, 105599. [Google Scholar] [CrossRef]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef]
- Lee, C.K.; Wu, C.; Lin, C.Y.; Huang, P.C.; Sung, F.C.; Su, T.C. Positive Association between Endothelium-Platelet Microparticles and Urinary Concentration of Lead and Cadmium in Adolescents and Young Adults. Nutrients 2021, 13, 2913. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Szydełko, J.; Trojanowska, P.; Dąbrowska, I.; Szydełko-Gorzkowicz, M.; Litwińczuk, M. Adiponectin as novel biomarker of endothelial dysfunction in insulin resistance and obesity–a narrative review. J. Educ. Health Sport 2020, 10, 591–606. [Google Scholar] [CrossRef]
- Ehsan, M.; Singh, K.K.; Lovren, F.; Pan, Y.; Quan, A.; Mantella, L.-E.; Sandhu, P.; Teoh, H.; Al-Omran, M.; Verma, S. Adiponectin limits monocytic microparticle-induced endothelial activation by modulation of the AMPK, Akt and NFκB signaling pathways. Atherosclerosis 2016, 245, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Inami, N.; Shouzu, A.; Omoto, S.; Kimura, Y.; Takahashi, N.; Tanaka, A.; Urase, F.; Maeda, Y.; Ohtani, H.; et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets 2009, 20, 16–22. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Di Palo, C.; Gicchino, M.; Petrizzo, M.; Bellastella, G.; Saccomanno, F.; Giugliano, D. Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes--a randomized controlled trial. Diabetes Obes. Metab. 2011, 13, 439–445. [Google Scholar] [CrossRef]
- Meyer, D.N.; Crofts, E.J.; Akemann, C.; Gurdziel, K.; Farr, R.; Baker, B.B.; Weber, D.; Baker, T.R. Developmental exposure to Pb(2+) induces transgenerational changes to zebrafish brain transcriptome. Chemosphere 2020, 244, 125527. [Google Scholar] [CrossRef]
- Kawakami, T.; Nishiyama, K.; Kadota, Y.; Sato, M.; Inoue, M.; Suzuki, S. Cadmium modulates adipocyte functions in metallothionein-null mice. Toxicol. Appl. Pharm. 2013, 272, 625–636. [Google Scholar] [CrossRef]
- Wu, C.-J.; Ho, A.-C.; Chen, S.-Y.; Pan, C.-H.; Chuang, H.-C.; Lai, C.-H. Exposure to Heavy Metals and Serum Adiponectin Levels among Workers: A 2-Year Follow-Up Study. Metabolites 2023, 13, 158. [Google Scholar] [CrossRef]
- Ashley-Martin, J.; Dodds, L.; Arbuckle, T.E.; Ettinger, A.S.; Shapiro, G.D.; Fisher, M.; Taback, S.; Bouchard, M.F.; Monnier, P.; Dallaire, R.; et al. Maternal blood metal levels and fetal markers of metabolic function. Environ. Res. 2015, 136, 27–34. [Google Scholar] [CrossRef]
- Kupsco, A.; Kioumourtzoglou, M.A.; Just, A.C.; Amarasiriwardena, C.; Estrada-Gutierrez, G.; Cantoral, A.; Sanders, A.P.; Braun, J.M.; Svensson, K.; Brennan, K.J.M.; et al. Prenatal Metal Concentrations and Childhood Cardiometabolic Risk Using Bayesian Kernel Machine Regression to Assess Mixture and Interaction Effects. Epidemiology 2019, 30, 263–273. [Google Scholar] [CrossRef]
- Valcke, M.; Ouellet, N.; Dubé, M.; Laouan Sidi, E.A.; LeBlanc, A.; Normandin, L.; Balion, C.; Ayotte, P. Biomarkers of cadmium, lead and mercury exposure in relation with early biomarkers of renal dysfunction and diabetes: Results from a pilot study among aging Canadians. Toxicol. Lett. 2019, 312, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Karvonen-Gutierrez, C.A.; Mukherjee, B.; Herman, W.H.; Park, S.K. Urinary metals and adipokines in midlife women: The Study of Women’s Health Across the nation (SWAN). Environ. Res. 2021, 196, 110426. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lee, H.L.; Hwang, Y.T.; Wang, C.; Hsieh, C.J.; Wu, C.; Sung, F.C.; Su, T.C. The association between urine di-(2-ethylhexyl) phthalate metabolites, global DNA methylation, and subclinical atherosclerosis in a young Taiwanese population. Environ. Pollut. 2020, 265 Pt B, 114912. [Google Scholar] [CrossRef]
- Nie, J.M.; Li, H.F. Metformin in combination with rosiglitazone contribute to the increased serum adiponectin levels in people with type 2 diabetes mellitus. Exp. Ther. Med. 2017, 14, 2521–2526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.W.; Chen, H.Y.; Li, W.F.; Liou, S.H.; Chen, C.J.; Wu, J.H.; Wang, S.L. The association between total urinary arsenic concentration and renal dysfunction in a community-based population from central Taiwan. Chemosphere 2011, 84, 17–24. [Google Scholar] [CrossRef]
- Tsai, T.L.; Kuo, C.C.; Pan, W.H.; Chung, Y.T.; Chen, C.Y.; Wu, T.N.; Wang, S.L. The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int. 2017, 92, 710–720. [Google Scholar] [CrossRef]
- Landers-Ramos, R.Q.; Addison, O.A.; Beamer, B.; Katzel, L.I.; Blumenthal, J.B.; Robinson, S.; Hagberg, J.M.; Prior, S.J. Circulating microparticle concentrations across acute and chronic cardiovascular disease conditions. Physiol. Rep. 2020, 8, e14534. [Google Scholar] [CrossRef]
- Pirro, M.; Schillaci, G.; Paltriccia, R.; Bagaglia, F.; Menecali, C.; Mannarino, M.R.; Capanni, M.; Velardi, A.; Mannarino, E. Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2530–2535. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Bratseth, V.; Ritschel, V.; Andersen, G.; Halvorsen, S.; Eritsland, J.; Arnesen, H.; Badimon, L.; Seljeflot, I. Monocyte-derived circulating microparticles (CD14(+), CD14(+)/CD11b(+) and CD14(+)/CD142(+)) are related to long-term prognosis for cardiovascular mortality in STEMI patients. Int. J. Cardiol. 2017, 227, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Zambrano, J.P.; Virani, S.S.; Jimenez, J.J.; Jy, W.; Ahn, E.; Horstman, L.L.; Castellanos, A.; Myerburg, R.J.; Ahn, Y.S. Correlation between apoptotic endothelial microparticles and serum interleukin-6 and C-reactive protein in healthy men. Am. J. Cardiol. 2005, 95, 1258–1260. [Google Scholar] [CrossRef]
- Sommar, J.N.; Hedmer, M.; Lundh, T.; Nilsson, L.; Skerfving, S.; Bergdahl, I.A. Investigation of lead concentrations in whole blood, plasma and urine as biomarkers for biological monitoring of lead exposure. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-H.; Cheng, Z.-P.; Lu, M.; Lin, W.-Y.; Luo, L.-L.; Ming, Z.-Y.; Hu, Y. Adiponectin receptor agonist AdipoRon modulates human and mouse platelet function. Acta Pharmacol. Sin. 2023, 44, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Long, Y.; Yu, Y.R.; Li, M.R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK–eNOS Pathway. Int. J. Obes. 2010, 34, 165–171. [Google Scholar] [CrossRef]
- Cui, X.-J.; Lin, X.; Zhong, J.-Y.; Li, S.; He, J.-Y.; Ni, Y.-Q.; Zhan, J.-K.; Liu, Y.-S. Adiponectin attenuates the premature senescence of vascular smooth muscle cells induced by high glucose through mTOR signaling pathway. Aging Med. 2020, 3, 178–187. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 916. [Google Scholar] [CrossRef]
- Patwa, J.; Flora, S.J.S. Heavy Metal-Induced Cerebral Small Vessel Disease: Insights into Molecular Mechanisms and Possible Reversal Strategies. Int. J. Mol. Sci. 2020, 21, 3862. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Smolik, R. The effect of combined exposure to lead and cadmium on serum lipids and lipid peroxides level in rats. Int. J. Occup. Med. Environ. Health 1994, 7, 263–271. [Google Scholar]
- Ahn, J.; Kim, N.S.; Lee, B.K.; Park, J.; Kim, Y. Association of Blood Pressure with Blood Lead and Cadmium Levels in Korean Adolescents: Analysis of Data from the 2010–2016 Korean National Health and Nutrition Examination Survey. J. Korean Med. Sci. 2018, 33, e278. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Hsu, S.H.-J.; Chen, C.-W.; Wang, C.; Sung, F.-C.; Su, T.-C. Association of Urinary Lead and Cadmium Levels, and Serum Lipids with Subclinical Arteriosclerosis: Evidence from Taiwan. Nutrients 2023, 15, 571. [Google Scholar] [CrossRef] [PubMed]

| Numbers (%) | |
|---|---|
| Age (years): 12–19 | 231 (31.9) |
| 20–30 | 493 (68.1) |
| Sex: Male | 290 (40.1) |
| Female | 434 (59.9) |
| BMI: <24 | 570 (78.7) |
| ≥24 | 154 (21.3) |
| Smoking: Active | 122 (16.9) |
| Not active | 602 (83.1) |
| Drinking: Current | 69 (9.5) |
| Not current | 654 (90.5) |
| Household income: <TWD 50,000 | 282 (39.1) |
| ≥TWD 50,000 | 440 (60.9) |
| Hypertension: Yes | 54 (7.5) |
| No | 670 (92.5) |
| Mean (SD) | Geometric Mean (Geometric SD) | |
|---|---|---|
| Adiponectin (ng/mL) a | 14,401.22 (40,206.03) | 6404.14 (2.99) |
| Urinary heavy metals | ||
| Pb (μg/g creatinine) | 6.98 (16.57) | 1.47 (5.27) |
| Pb (μg/L) | 9.22 (17.53) | 2.18 (5.17) |
| Cd (μg/g creatinine) | 1.43 (2.64) | 0.62 (3.51) |
| Cd (μg/L) | 2.00 (2.73) | 0.92 (3.63) |
| Apoptotic microparticles (counts/µL) | ||
| CD31+/CD42a− | 243.73 (367.80) | 166.47 (3.46) |
| CD31+/CD42a+ | 11,432.68 (18,428.00) | 3725.29 (5.46) |
| CD14 | 123.46 (74.35) | 108.03 (1.67) |
| Ln Adiponectin | Ln CD31+/CD42a− | Ln CD31+/CD42a+ | Ln CD14 | |
|---|---|---|---|---|
| (ng/mL) | (counts/µL) | (counts/µL) | (counts/µL) | |
| n = 362 | n = 722 | n = 722 | n = 722 | |
| Ln adiponectin (ng/mL) | −0.186 (0.064) | −0.277 (0.090) | −0.020 (0.027) | |
| p value | 0.004 * | 0.002 * | 0.465 * | |
| Ln Pb (μg/g creatinine) | −0.169 (0.032) | 0.349 (0.025) | 0.320 (0.038) | 0.067 (0.012) |
| p value | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| Ln Cd (μg/g creatinine) | −0.192 (0.045) | 0.433 (0.033) | 0.417 (0.050) | 0.071 (0.015) |
| p value | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| Adiponectin (ng/mL) | ||||
|---|---|---|---|---|
| Heavy Metals (μg/g Creatinine) | Geometric Mean (SE) | p | p for Trend | |
| Pb | ≤0.36 (≤25%ile) | 7065.65 (1.15) | Reference | <0.001 |
| ≤0.61 (≤50%ile) | 8014.44 (1.14) | 1.000 | ||
| ≤2.00 (≤75%ile) | 8160.00 (1.14) | 1.000 | ||
| >2.00 (>75%ile) | 3725.66 (1.14) | <0.001 | ||
| Cd | ≤0.20 (≤25%ile) | 8184.52 (1.14) | Reference | <0.001 |
| ≤0.32 (≤50%ile) | 6967.42 (1.14) | 1.000 | ||
| ≤0.75 (≤75%ile) | 7608.34 (1.14) | 1.000 | ||
| >0.75 (>75%ile) | 4101.06 (1,14) | <0.001 | ||
| Pb ≤ 50%ile and Cd ≤ 50%ile | Pb > 50%ile and Cd ≤ 50%ile | Pb ≤ 50%ile and Cd > 50%ile | Pb > 50%ile and Cd > 50%ile | ||
|---|---|---|---|---|---|
| Number | 191 | 34 | 34 | 103 | |
| Adiponectin (ng/mL) | Geometric mean (S.E.) | 7427.91 (1.11) | 8586.96 (1.21) | 10,107.17 (1.21) | 3987.82 (1.13) |
| p value | Reference | 0.442 | 0.101 | <0.001 | |
| p for trend | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Wang, C.-K.; Sung, F.-C.; Su, T.-C. The Association among Urinary Lead and Cadmium, Serum Adiponectin, and Serum Apoptotic Microparticles in a Young Taiwanese Population. Nutrients 2023, 15, 4528. https://doi.org/10.3390/nu15214528
Lin C-Y, Wang C-K, Sung F-C, Su T-C. The Association among Urinary Lead and Cadmium, Serum Adiponectin, and Serum Apoptotic Microparticles in a Young Taiwanese Population. Nutrients. 2023; 15(21):4528. https://doi.org/10.3390/nu15214528
Chicago/Turabian StyleLin, Chien-Yu, Chi-Kang Wang, Fung-Chang Sung, and Ta-Chen Su. 2023. "The Association among Urinary Lead and Cadmium, Serum Adiponectin, and Serum Apoptotic Microparticles in a Young Taiwanese Population" Nutrients 15, no. 21: 4528. https://doi.org/10.3390/nu15214528
APA StyleLin, C.-Y., Wang, C.-K., Sung, F.-C., & Su, T.-C. (2023). The Association among Urinary Lead and Cadmium, Serum Adiponectin, and Serum Apoptotic Microparticles in a Young Taiwanese Population. Nutrients, 15(21), 4528. https://doi.org/10.3390/nu15214528







