Association of Chrononutrition Indices with Anthropometric Parameters, Academic Performance, and Psychoemotional State of Adolescents: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Subject and Study Design
2.2. Instruments
2.2.1. Personal Information
2.2.2. Academic Performance
2.2.3. Anthropometric Characteristics
2.2.4. Meal Timing
2.2.5. ZSDS
2.2.6. YFAS-C
2.2.7. The Validity of the Instruments Used
2.3. Data and Statistical Analyses
2.3.1. Multiple Regression
2.3.2. Logistic Regression
3. Results
4. Discussion
5. Conclusions
6. Future Studies and Practical Recommendations
6.1. Future Studies
6.2. Practical Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aschoff, J.; Wever, R. The circadian system of man. In Biological Rhythms; Springer: Boston, MA, USA, 1981; pp. 311–331. [Google Scholar]
- Smolensky, M.H.; Hermida, R.C.; Reinberg, A.; Sackett-Lundeen, L.; Portaluppi, F. Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol. Int. 2016, 33, 1101–1119. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Touitou, D.; Reinberg, A. Disruption of adolescents’ circadian clock: The vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J. Physiol. 2016, 110 Pt B, 467–479. [Google Scholar] [CrossRef]
- Anisimov, V.N. Carcinogenesis and Aging; CRC Press: Boca Raton, FL, USA, 1987; Volumes 1–2. [Google Scholar]
- Schernhammer, E.S.; Laden, F.; Spezer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Fuchs, C.S.; Colditz, G.A. Night-shift work and risk of colorectal cancer in the nurses’ health study. J. Natl. Cancer Inst. 2003, 95, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Haus, E.; Reinberg, A.; Mauvieux, B.; Le Floc’h, N.; Sackett-Lundeen, L.; Touitou, Y. Risk of obesity in male shift workers: A chronophysiological approach. Chronobiol. Int. 2016, 33, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Kantermann, T.; Eastman, C.I. Circadian phase, circadian period and chronotype are reproducible over months. Chronobiol. Int. 2018, 35, 280–288. [Google Scholar] [CrossRef]
- Duffy, J.F.; Wright, K.P., Jr. Entrainment of the human circadian system by light. J. Biol. Rhythm. 2005, 20, 326–338. [Google Scholar] [CrossRef]
- Hankins, M.W.; Peirson, S.N.; Foster, R.G. Melanopsin: An exciting photopigment. Trends Neurosci. 2008, 31, 27–36. [Google Scholar] [CrossRef]
- Stephan, F.K. The “other” circadian system: Food as a Zeitgeber. J. Biol. Rhythm. 2002, 17, 284–292. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Antle, M.C. Entrainment of circadian clocks in mammals by arousal and food. Essays Biochem. 2011, 49, 119–136. [Google Scholar]
- Smit, A.N.; Patton, D.F.; Michalik, M.; Opiol, H.; Mistlberger, R.E. Dopaminergic regulation of circadian food anticipatory activity rhythms in the rat. PLoS ONE 2013, 8, e82381. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal timing regulates the human circadian system. Curr. Biol. 2017, 27, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Stutz, B.; Buyken, A.E.; Schadow, A.M.; Jankovic, N.; Alexy, U.; Krueger, B. Associations of chronotype and social jetlag with eating jetlag and their changes among German students during the first COVID-19 lockdown. The Chronotype and Nutrition study. Appetite 2023, 180, 106333. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Ng, C.M.; Tang, S.Y.; Kok, E.Y. Weight status of working adults: The effects of eating misalignment, chronotype, and eating jetlag during mandatory confinement. Chronobiol. Int. 2023, 40, 406–415. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.G.; Reedy, J.; Graubard, B.I.; Kant, A.K.; Czajkowski, S.M.; Berrigan, D. Circadian timing of eating and BMI among adults in the American Time Use Survey. Int. J. Obes. 2022, 46, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Popp, C.J.; Curran, M.; Wang, C.; Prasad, M.; Fine, K.; Gee, A.; Nair, N.; Perdomo, K.; Chen, S.; Hu, L.; et al. Temporal eating patterns and eating windows among adults with overweight or obesity. Nutrients 2021, 13, 4485. [Google Scholar] [CrossRef]
- Lucassen, E.A.; Zhao, X.; Rother, K.I.; Mattingly, M.S.; Courville, A.B.; de Jonge, L.; Csako, G.; Cizza, G.; Sleep Extension Study Group. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS ONE 2013, 8, e56519. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef]
- Makarem, N.; Sears, D.D.; St-Onge, M.P.; Zuraikat, F.M.; Gallo, L.C.; Talavera, G.A.; Castaneda, S.F.; Lai, Y.; Aggarwal, B. Variability in daily eating patterns and eating jetlag are associated with worsened cardiometabolic risk profiles in the American Heart Association Go Red for Women Strategically Focused Research Network. J. Am. Heart Assoc. 2021, 10, e022024. [Google Scholar] [CrossRef]
- Tahara, Y.; Makino, S.; Suiko, T.; Nagamori, Y.; Iwai, T.; Aono, M.; Shibata, S. Association between irregular meal timing and the mental health of Japanese workers. Nutrients 2021, 13, 2775. [Google Scholar] [CrossRef]
- Alsayid, M.; Khan, M.O.; Adnan, D.; Rasmussen, H.E.; Keshavarzian, A.; Bishehsari, F. Behavioral circadian phenotypes are associated with the risk of elevated body mass index. Eat. Weight Disord. 2022, 27, 1395–1403. [Google Scholar] [CrossRef]
- Zerón-Rugerio, M.F.; Hernáez, Á.; Porras-Loaiza, A.P.; Cambras, T.; Izquierdo-Pulido, M. Eating jet lag: A marker of the variability in meal timing and its association with body mass index. Nutrients 2019, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Wirz-Justice, A. Diurnal variation of depressive symptoms. Dialogues Clin. Neurosci. 2008, 10, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Chellappa, S.L. Endogenous circadian rhythms in mood and well-being. Sleep Health, 2023; in press. [Google Scholar]
- Lewy, A.J. Circadian misalignment in mood disturbances. Curr. Psychiatry Rep. 2009, 11, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Wyse, C.A.; Celis Morales, C.A.; Graham, N.; Fan, Y.; Ward, J.; Curtis, A.M.; Mackay, D.; Smith, D.J.; Bailey, M.E.S.; Biello, S.; et al. Adverse metabolic and mental health outcomes associated with shiftwork in a population-based study of 277,168 workers in UK biobank. Ann. Med. 2017, 49, 411–420. [Google Scholar] [CrossRef]
- Qian, J.; Vujovic, N.; Nguyen, H.; Rahman, N.; Heng, S.W.; Amira, S.; Scheer, F.A.J.L.; Chellappa, S.L. Daytime eating prevents mood vulnerability in night work. Proc. Natl. Acad. Sci. USA 2022, 119, e2206348119. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-P.; Fang, H.-J.; Jia, C.-X. The Serial mediation of the association between breakfast skipping and suicidality by weight status and depressive symptoms: Findings from the National Youth Risk Behavior Surveys of the United States. Nutrients 2022, 14, 956. [Google Scholar] [CrossRef]
- Adolphus, K.; Lawton, C.L.; Dye, L. The effects of breakfast on behavior and academic performance in children and adolescents. Front. Hum. Neurosci. 2013, 7, 425. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Komada, Y.; Okajima, I.; Kitamura, S.; Inoue, Y. A survey on social jetlag in Japan: A nationwide, cross-sectional internet survey. Sleep Biol. Rhythm. 2019, 17, 417–422. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Tserne, T.A.; Panev, A.S.; Kuznetsova, E.S.; Petrova, N.B.; Timonin, V.D.; Kolomeichuk, S.N.; Vinogradova, I.A.; Kovyazina, M.S.; Khokhlov, N.A.; et al. Seven-year survey of sleep timing in Russian children and adolescents: Chronic 1-h forward transition of social clock is associated with increased social jetlag and winter pattern of mood seasonality. Biol. Rhythm Res. 2017, 48, 3–12. [Google Scholar] [CrossRef]
- Haraszti, R.Á.; Ella, K.; Gyöngyösi, N.; Roenneberg, T.; Káldi, K. Social jetlag negatively correlates with academic performance in undergraduates. Chronobiol. Int. 2014, 31, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Levandovski, R.; Dantas, G.; Fernandes, L.C.; Caumo, W.; Torres, I.; Roenneberg, T.; Hidalgo, M.P.; Allebrandt, K.V. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 2011, 28, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef]
- Stice, E.; Whitenton, K. Risk factors for body dissatisfaction in adolescent girls: A longitudinal investigation. Dev. Psychol. 2002, 38, 669. [Google Scholar] [CrossRef]
- Sample Size Calculation and Sample Size Justification. Available online: https://www.statisticssolutions.com/dissertation-resources/sample-size-calculation-and-sample-size-justification/sample-size-formula (accessed on 18 October 2023).
- Bujang, M.A.; Sa’at, N.; Sidik, T.M.I.T.A.B.; Joo, L.C. Sample s guidelines for logistic regression from observational studies with large population: Emphasis on the accuracy between statistics and parameters based on real life clinical data. Malays. J. Med. Sci. 2018, 25, 122–130. [Google Scholar] [CrossRef]
- Zung, W.W. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Roberto, C.; Seamans, M.; Corbin, W.; Brownell, K. Preliminary validation of the Yale Food Addiction Scale for children. Eat. Behav. 2013, 14, 508–512. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Popov, S.V.; Smirnov, V.V.; Dorogina, O.I.; Pecherkina, A.A.; Symaniuk, E.E. Later school start time is associated with better academic performance, sleep-wake rhythm characteristics, and eating behavior. Chronobiol. Int. 2022, 39, 1444–1453. [Google Scholar] [CrossRef]
- de Oris, M.; Onyango, A.W.; Borghi, E.; Siyama, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Swainson, M.G.; Batterham, A.M.; Tsakirides, C.; Rutherford, Z.H.; Hind, K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 2017, 12, e0177175. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W. The depression status inventory: An adjunct to the self-rating depression scale. J. Clin. Psychol. 1972, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Passik, S.D.; Lundberg, J.C.; Rosenfeld, B.; Kirsh, K.L.; Donaghy, K.; Theobald, D.; Lundberg, E.; Dugan, W. Factor analysis of the Zung Self-Rating Depression Scale in a large ambulatory oncology sample. Psychosomatics 2000, 41, 121–127. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Tserne, T.A.; Bakutova, L.A. Food addiction in Russian adolescents: Associations with age, sex, weight, and depression. Eur. Eat. Disord. Rev. 2018, 26, 671–678. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Popov, S.V.; Pecherkina, A.A.; Dorogina, O.I.; Martinson, E.A.; Vetosheva, V.I.; Gubin, D.G.; Solovieva, S.V.; Turovinina, E.F.; Symaniuk, E.E. Food addiction in young adult residents of Russia: Associations with emotional and anthropometric characteristics. Eur. Eat. Disord. Rev. 2020, 28, 465–472. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Tserne, T.A.; Popov, S.V.; Bakutova, L.A.; Pecherkina, A.A.; Dorogina, O.I.; Martinson, E.A.; Vetosheva, V.I.; Gubin, D.G.; Solovieva, S.V.; et al. Food preferences and YFAS/YFAS-C scores in schoolchildren and university students. Eat. Weight Disord. 2021, 26, 2333–2343. [Google Scholar] [CrossRef]
- Tserne, T.A.; Borisenkov, M.F.; Popov, S.V.; Bakutova, L.A.; Jongte, L.; Trivedi, A.K.; Pecherkina, A.A.; Dorogina, O.I.; Martinson, E.A.; Vetosheva, V.I.; et al. Food addiction and weight in students with high academic performance. Public Health Nutr. 2021, 24, 6027–6033. [Google Scholar] [CrossRef] [PubMed]
- Borisenkov, M.F.; Popov, S.V.; Smirnov, V.V.; Gubin, D.G.; Petrov, I.M.; Vasilkova, T.N.; Solovieva, S.V.; Martinson, E.A.; Pecherkina, A.A.; Dorogina, O.I.; et al. Association between food addiction and time perspective during COVID-19 isolation. Eat. Weight Disord. 2022, 27, 1585–1591. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Tserne, T.A.; Bakutova, L.A.; Gubin, D.G. Food addiction and emotional eating are associated with intradaily rest–activity rhythm variability. Eat. Weight Disord. 2022, 27, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Borisenkov, M.F.; Popov, S.V.; Tserne, T.A.; Bakutova, L.A.; Pecherkina, A.A.; Dorogina, O.I.; Martinson, E.A.; Vetosheva, V.I.; Gubin, D.G.; Solovieva, S.V.; et al. Food addiction and symptoms of depression among inhabitants of the European North of Russia: Associations with sleep characteristics and photoperiod. Eur. Eat. Disord. Rev. 2020, 28, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K. Alternatives to P value: Confidence interval and effect size. Korean J. Anesthesiol. 2016, 69, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics notes: Quartiles, quintiles, centiles, and other quantiles. Brit. Med. J. 1994, 309, 996. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Matsui, K.; Komada, Y.; Okajima, I.; Takaesu, Y.; Kuriyama, K.; Inoue, Y. A cross-sectional study of evening hyperphagia and nocturnal ingestion: Core constituents of night eating syndrome with different background factors. Nutrients 2021, 13, 4179. [Google Scholar] [CrossRef]
- Muntaner-Mas, A.; Martínez-Gómez, D.; Castro-Piñero, J.; Fernandez-Santos, J.R.; Salmon, J.; Veiga, Ó.L.; Esteban-Cornejo, I. Objectively measured physical activity and academic performance in school-aged youth: The UP&DOWN longitudinal study. Scand. J. Med. Sci. Sports 2021, 31, 2230–2240. [Google Scholar]
- Sun, Y.; Shi, L.; Bao, Y.; Sun, Y.; Shi, J.; Lu, L. The bidirectional relationship between sleep duration and depression in community-dwelling middle-aged and elderly individuals: Evidence from a longitudinal study. Sleep Med. 2018, 52, 221–229. [Google Scholar] [CrossRef]
- Wu, J.; Wu, H.; Wang, J.; Guo, L.; Deng, X.; Lu, C. Associations between sleep duration and overweight/obesity: Results from 66,817 Chinese adolescents. Sci. Rep. 2015, 5, 16686. [Google Scholar] [CrossRef]
- Pursey, K.M.; Stanwell, P.; Gearhardt, A.N.; Collins, C.E.; Burrows, T.L. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: A systematic review. Nutrients 2014, 6, 4552–4590. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Yokum, S.; Orr, P.T.; Stice, E.; Corbin, W.R.; Brownell, K.D. Neural correlates of food addiction. Arch. Gen. Psychiatry 2011, 68, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.; Weiß, A.; Schulte, E.M.; Meule, A.; Ellrott, T. Prevalence of ‘food addiction’ as measured with the Yale Food Addiction Scale 2.0 in a representative German sample and its association with sex, age and weight categories. Obes. Facts 2017, 10, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Wiss, D. Clinical considerations of ultra-processed food addiction across weight classes: An eating disorder treatment and care perspective. Curr. Addict. Rep. 2022, 9, 255–267. [Google Scholar] [CrossRef]
- Agüera, Z.; Vintró-Alcaraz, C.; Baenas, I.; Granero, R.; Sánchez, I.; Sánchez-González, J.; Menchón, J.M.; Jiménez-Murcia, S.; Treasure, J.; Fernández-Aranda, F. Lifetime weight course as a phenotypic marker of severity and therapeutic response in patients with eating disorders. Nutrients 2021, 13, 2034. [Google Scholar] [CrossRef] [PubMed]
- Borisenkov, M.F.; Popov, S.V.; Smirnov, V.V.; Gubin, D.G.; Petrov, I.M.; Vasilkova, T.N.; Solovieva, S.V.; Martinson, E.A.; Pecherkina, A.A.; Dorogina, O.I.; et al. Associations among sleep-wake rhythm characteristics, time perspective and psycho-emotional state during COVID-19 isolation. Biol. Rhythm Res. 2022, 53, 1770–1781. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Apostolidou, M.K.; Atsiova, M.B.; Filippidou, A.K.; Florou, A.K.; Gousiou, D.S.; Katsara, A.R.; Mantzari, S.N.; Padouva-Markoulaki, M.; Papatriantafyllou, E.I.; et al. Self-reported changes in anxiety, depression and suicidality during the COVID-19 lockdown in Greece. J. Affect. Disord. 2021, 279, 624–629. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Tserne, T.; Bakutova, L.; Smirnov, V.; Popov, S. Afternoon school shift is associated with increased risk of overweight/obesity in 11-14-year-old females with early and intermediate chronotype. Pediatr. Obes. 2023, 18, e13039. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Gallop, M.R.; Flores Ramos, S.; Zarrinpar, A.; Broussard, J.L.; Chondronikola, M.; Chaix, A.; Klein, S. Complex physiology and clinical implications of time-restricted eating. Physiol. Rev. 2022, 102, 1991–2034. [Google Scholar] [CrossRef]
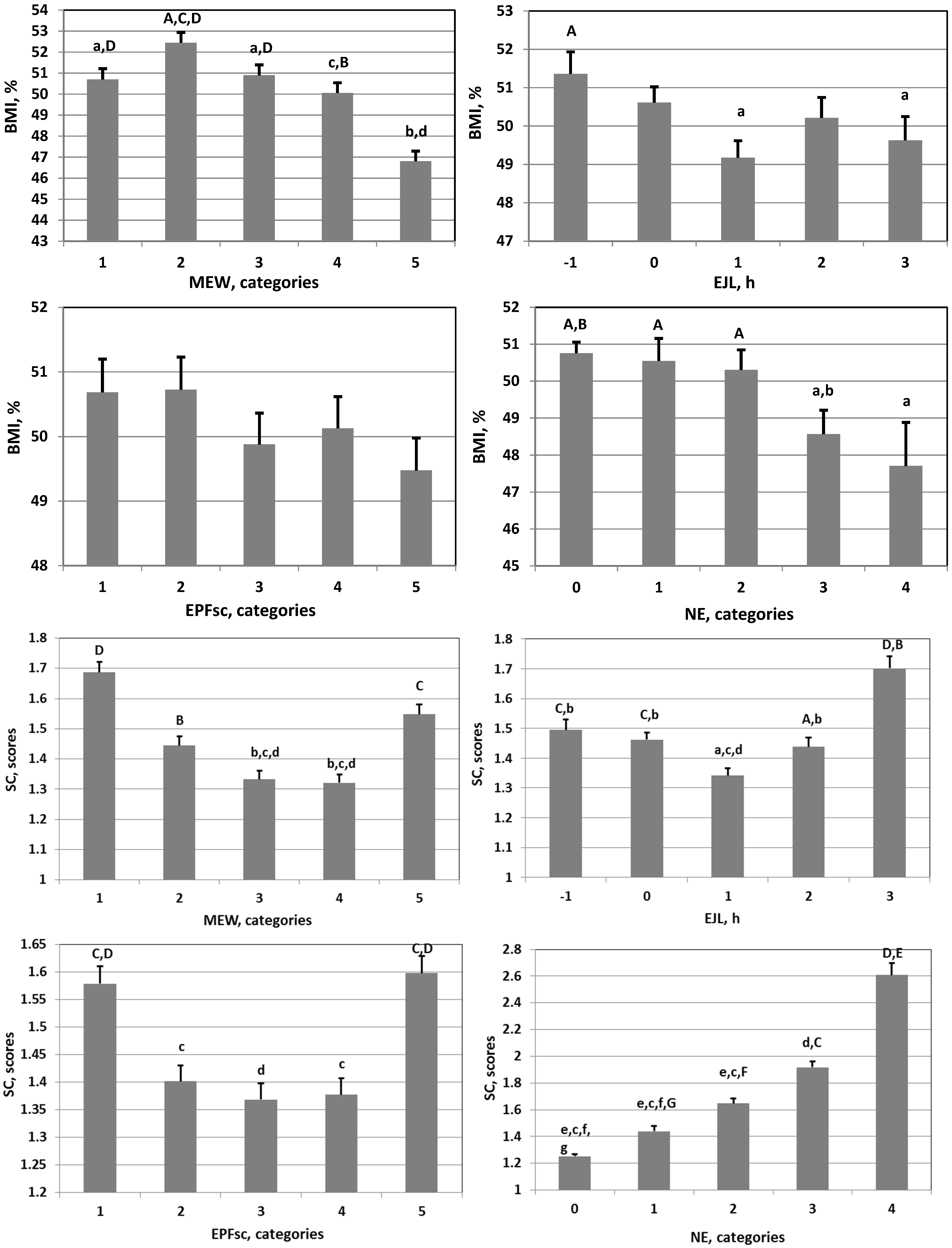

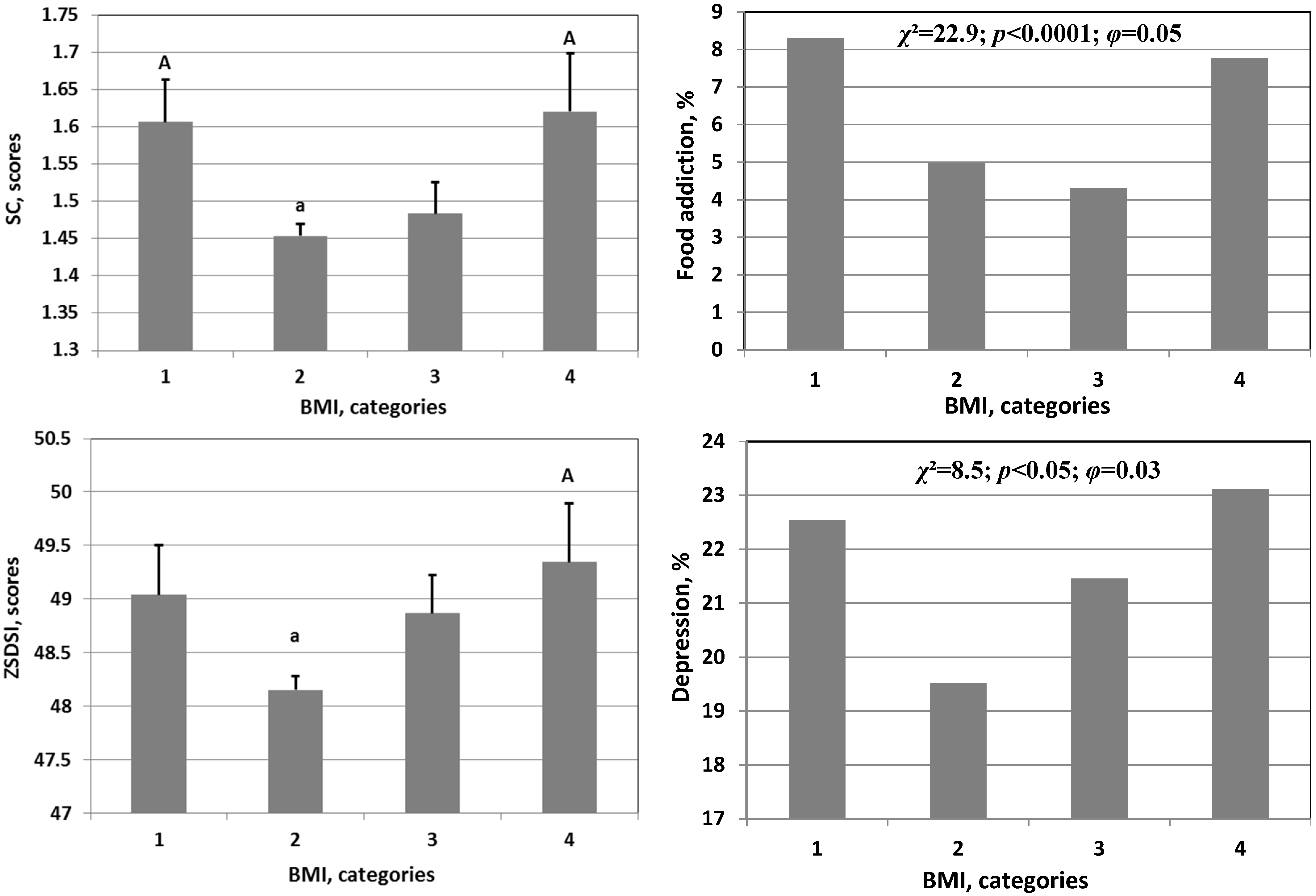
| Parameter | Abbreviation | M | SD | S | K |
|---|---|---|---|---|---|
| Age | 14.20 | 1.70 | 0.10 | 0.20 | |
| Body mass index | BMI | 19.96 | 4.14 | 8.06 | 170.72 |
| BMI% | 50.25 | 24.09 | −0.02 | −0.44 | |
| Waist-to-height ratio | WHtR | 0.39 | 0.05 | 0.44 | 2.07 |
| WHtRc | 0.37 | 0.05 | 0.12 | −0.11 | |
| Academic performance | GPA | 4.09 | 0.53 | −0.08 | −0.83 |
| Depression | ZSDSI | 48.42 | 12.20 | 0.41 | −0.19 |
| Symptom counts of food addiction | SC | 1.48 | 1.49 | 1.33 | 1.68 |
| Night eating | NE | 0.98 | 1.26 | 0.91 | −0.50 |
| Eating window | MEW | 9.68 | 3.90 | −1.11 | 0.38 |
| MEW1 | 1.90 | 0.59 | 0.71 | −0.32 | |
| Eating jetlag | EJL | 1.21 | 1.83 | 0.25 | 4.13 |
| EJL1 | 1.60 | 1.51 | 0.56 | −0.98 | |
| Eating phase | EPFc | 15.52 | 2.40 | −0.31 | 1.15 |
| EPFc1 | 1.61 | 0.44 | 0.85 | 0.32 |
| Parameter | Gradation | Abbreviation | N | % |
|---|---|---|---|---|
| All | 12,759 | |||
| Sex | Females | F | 7292 | 57.2 |
| Males | M | 5467 | 42.8 | |
| BMIc | Underweight | U | 818 | 6.4 |
| Normal weight | N | 10,126 | 79.4 | |
| Overweight | Ov | 1281 | 10.0 | |
| Obese | Ob | 534 | 4.2 | |
| Ov/Ob | 1815 | 14.2 | ||
| WHtRc | ≤0.29 | 411 | 3.2 | |
| 0.3–0.39 | 6904 | 54.1 | ||
| 0.4–0.49 | 5169 | 40.5 | ||
| ≥0.5 | 275 | 2.2 | ||
| WHtRc1 | No central adiposity | WHtR < 0.5 | 12,484 | 97.8 |
| Central adiposity | WHtR ≥ 0.5 | 275 | 2.2 | |
| Academic performance | Low | GPAL | 1775 | 13.9 |
| Mean | GPAM | 7634 | 59.8 | |
| High | GPAH | 3350 | 26.3 | |
| Depression | No | ZSDSIc1 | 7200 | 56.4 |
| Minimal/Mild | ZSDSIc2 | 2987 | 23.4 | |
| Moderate/Significant | ZSDSIc3 | 1977 | 15.5 | |
| Severe/Extreme | ZSDSIc4 | 595 | 4.7 | |
| Depression | 2573 | 20.2 | ||
| Food addiction | Yes | FA0 | 12,082 | 94.7 |
| No | FA1 | 677 | 5.3 |
| Parameter | Extremely Low | Optimal | Extremely High |
|---|---|---|---|
| MEW, h | ≤7.5 | 7.51–12.5 | >12.5 |
| EJL, h | ≤−1 | −0.99–3 | >3 |
| EPFc, h | ≤13.5 | 13.51–17.33 | >17.33 |
| NE | – | ≤1–3 per year | ≥1–3 per month |
| # | Dependent Variable | Predictor | B | β | P | R2 | ∆R2 | VIF |
|---|---|---|---|---|---|---|---|---|
| 1 | BMI% | MEW | −0.191 | −0.031 | 0.001 | 0.001 | 0.001 | 1.005 |
| Sex | 5.217 | 0.107 | 0.000 | 0.012 | 0.011 | 1.003 | ||
| Age | −1.062 | −0.072 | 0.000 | 0.017 | 0.005 | 1.056 | ||
| 2 | BMI% | NE | −0.691 | −0.036 | 0.000 | 0.001 | 0.001 | 1.005 |
| Age | −0.968 | −0.065 | 0.000 | 0.005 | 0.004 | 1.008 | ||
| 3 | WHtR | NE | −0.002 | −0.038 | 0.000 | 0.001 | 0.001 | 1.006 |
| Age | −0.002 | −0.052 | 0.000 | 0.003 | 0.002 | 1.025 | ||
| Lat | 0.005 | 0.035 | 0.001 | 0.004 | 0.001 | 1.047 | ||
| 4 | GPA | EJL1 | −0.130 | −0.088 | 0.000 | 0.008 | 0.008 | 1.001 |
| Season | −0.005 | −0.031 | 0.001 | 0.009 | 0.001 | 1.125 | ||
| 5 | GPA | MEW1 | −0.086 | −0.095 | 0.000 | 0.009 | 0.009 | 1.005 |
| Season | −0.006 | −0.034 | 0.000 | 0.011 | 0.002 | 1.126 | ||
| 6 | GPA | EPFc1 | −0.110 | −0.091 | 0.000 | 0.008 | 0.008 | 1.007 |
| Season | −0.007 | −0.038 | 0.000 | 0.011 | 0.003 | 1.086 | ||
| 7 | GPA | NE | −0.086 | −0.205 | 0.000 | 0.044 | 0.044 | 1.004 |
| Season | −0.005 | −0.030 | 0.001 | 0.046 | 0.002 | 1.129 | ||
| 8 | ZSDSI | EJL1 | 0.840 | 0.103 | 0.000 | 0.011 | 0.011 | 1.009 |
| Season | 0.274 | 0.068 | 0.000 | 0.015 | 0.004 | 1.017 | ||
| Lat | −1.269 | −0.042 | 0.000 | 0.017 | 0.002 | 1.029 | ||
| 9 | ZSDSI | MEW1 | 2.734 | 0.131 | 0.000 | 0.018 | 0.018 | 1.007 |
| Season | 0.269 | 0.067 | 0.000 | 0.022 | 0.004 | 1.017 | ||
| Lat | −1.340 | −0.044 | 0.000 | 0.023 | 0.001 | 1.030 | ||
| 10 | ZSDSI | EPFc1 | 3.449 | 0.124 | 0.000 | 0.016 | 0.016 | 1.010 |
| Season | 0.267 | 0.066 | 0.000 | 0.020 | 0.004 | 1.018 | ||
| Lat | −1.405 | −0.046 | 0.000 | 0.021 | 0.001 | 1.032 | ||
| 11 | ZSDSI | NE | 2.319 | 0.237 | 0.000 | 0.053 | 0.053 | 1.005 |
| Sex | −0.270 | −0.068 | 0.000 | 0.095 | 0.042 | 1.018 | ||
| Lat | −1.616 | −0.053 | 0.000 | 0.099 | 0.004 | 1.032 | ||
| 12 | SC | EJL1 | 0.358 | 0.087 | 0.000 | 0.008 | 0.008 | 1.003 |
| Sex | −0.224 | −0.075 | 0.000 | 0.013 | 0.006 | 1.000 | ||
| 13 | SC | MEW1 | 0.253 | 0.100 | 0.000 | 0.010 | 0.010 | 1.006 |
| Sex | −0.229 | −0.076 | 0.000 | 0.016 | 0.006 | 1.001 | ||
| 14 | SC | EPFc1 | 0.299 | 0.089 | 0.000 | 0.007 | 0.007 | 1.009 |
| Sex | −0.230 | −0.077 | 0.000 | 0.013 | 0.006 | 1.001 | ||
| 15 | SC | NE | 0.031 | 0.037 | 0.000 | 0.046 | 0.046 | 1.023 |
| Season | 0.015 | 0.030 | 0.001 | 0.047 | 0.001 | 1.018 |
| # | Dependent Variable | Predictor | B | ExpB | [95% CI] | &P | Omnibus Test | Hosmer-Lemeshov Test | ||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | P | χ2 | P | |||||||
| 1 | Ov/Ob | MEW | −0.030 | 0.971 | [0.958–0.984] | 0.000 | 326.838 | 0.000 | 10.482 | 0.233 |
| Age | −0.130 | 0.878 | [0.849–0.908] | 0.000 | ||||||
| Sex | 0.783 | 2.188 | [1.966–2.435] | 0.000 | ||||||
| 2 | GPAL | MEW1 | 0.148 | 0.878 | [1.117–1.204] | 0.000 | 407.995 | 0.000 | 15.954 | 0.430 |
| Sex | −0.878 | 2.188 | [0.373–0.462] | 0.000 | ||||||
| 3 | Depression | EJL1 | 0.725 | 2.065 | [1.830–2.330] | 0.000 | 404.923 | 0.000 | 4.184 | 0.840 |
| Age | 0.042 | 1.043 | [1.013–1.074] | 0.005 | ||||||
| Sex | −0.746 | 0.474 | [0.429–0.524] | 0.000 | ||||||
| Season | 0.027 | 0.974 | [0.958–0.990] | 0.002 | ||||||
| Lat | −0.208 | 0.813 | [0.715–0.924] | 0.002 | ||||||
| 4 | Depression | MEW1 | 0.441 | 1.554 | [1.440–1.678] | 0.000 | 396.172 | 0.000 | 9.137 | 0.331 |
| Age | 0.043 | 1.044 | [1.014–1.075] | 0.004 | ||||||
| Sex | −0.755 | 0.470 | [0.425–0.520] | 0.000 | ||||||
| Season | 0.026 | 0.975 | [0.959–0.991] | 0.002 | ||||||
| Lat | −0.227 | 0.797 | [0.701–0.906] | 0.001 | ||||||
| 5 | Depression | EPFc1 | 0.528 | 1.696 | [1.531–1.879] | 0.000 | 370.568 | 0.000 | 6.905 | 0.547 |
| Age | 0.044 | 1.045 | [1.015–1.076] | 0.003 | ||||||
| Sex | −0.758 | 0.469 | [0.424–0.518] | 0.000 | ||||||
| Season | 0.025 | 0.976 | [0.960–0.992] | 0.004 | ||||||
| Lat | −0.235 | 0.790 | [0.695–0.898] | 0.000 | ||||||
| 6 | Depression | NE | 0.316 | 1.371 | [1.324–1.420] | 0.000 | 581.241 | 0.000 | 4.047 | 0.853 |
| Age | 0.052 | 1.053 | [1.023–1.085] | 0.000 | ||||||
| Sex | −0.775 | 0.461 | [0.417–0.509] | 0.000 | ||||||
| Season | 0.030 | 0.970 | [0.954–0.986] | 0.000 | ||||||
| Lat | −0.277 | 0.758 | [0.666–0.862] | 0.000 | ||||||
| 7 | FA | EJL1 | 0.677 | 1.968 | [1.612–2.402] | 0.000 | 122.042 | 0.000 | 5.705 | 0.680 |
| Age | 0.067 | 1.070 | [1.016–1.125] | 0.010 | ||||||
| Sex | −0.661 | 0.516 | [0.430–0.621] | 0.000 | ||||||
| Season | 0.051 | 0.950 | [0.922–0.979] | 0.001 | ||||||
| 8 | FA | MEW1 | 0.380 | 1.463 | [1.279–1.673] | 0.000 | 111.488 | 0.000 | 13.058 | 0.110 |
| Age | 0.069 | 1.071 | [1.019–1.127] | 0.008 | ||||||
| Sex | −0.671 | 0.511 | [0.425–0.615] | 0.000 | ||||||
| Season | 0.050 | 0.951 | [0.923–0.980] | 0.001 | ||||||
| 9 | FA | EPFc1 | 0.520 | 1.682 | [1.407–2.012] | 0.000 | 112.776 | 0.000 | 6.930 | 0.544 |
| Age | 0.069 | 1.071 | [1.018–1.127] | 0.008 | ||||||
| Sex | −0.676 | 0.508 | [0.423–0.611] | 0.000 | ||||||
| Season | 0.049 | 0.952 | [0.924–0.981] | 0.001 | ||||||
| 10 | FA | NE | 0.307 | 1.360 | [1.282–1.443] | 0.000 | 178.961 | 0.000 | 11.599 | 0.170 |
| Age | 0.080 | 1.083 | [1.031–1.139] | 0.002 | ||||||
| Sex | −0.646 | 0.524 | [0.438–0.627] | 0.000 | ||||||
| Season | 0.055 | 0.947 | [0.919–0.975] | 0.000 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisenkov, M.F.; Tserne, T.A.; Popov, S.V.; Smirnov, V.V.; Dorogina, O.I.; Pecherkina, A.A.; Symaniuk, E.E. Association of Chrononutrition Indices with Anthropometric Parameters, Academic Performance, and Psychoemotional State of Adolescents: A Cross-Sectional Study. Nutrients 2023, 15, 4521. https://doi.org/10.3390/nu15214521
Borisenkov MF, Tserne TA, Popov SV, Smirnov VV, Dorogina OI, Pecherkina AA, Symaniuk EE. Association of Chrononutrition Indices with Anthropometric Parameters, Academic Performance, and Psychoemotional State of Adolescents: A Cross-Sectional Study. Nutrients. 2023; 15(21):4521. https://doi.org/10.3390/nu15214521
Chicago/Turabian StyleBorisenkov, Mikhail F., Tatyana A. Tserne, Sergey V. Popov, Vasily V. Smirnov, Olga I. Dorogina, Anna A. Pecherkina, and Elvira E. Symaniuk. 2023. "Association of Chrononutrition Indices with Anthropometric Parameters, Academic Performance, and Psychoemotional State of Adolescents: A Cross-Sectional Study" Nutrients 15, no. 21: 4521. https://doi.org/10.3390/nu15214521
APA StyleBorisenkov, M. F., Tserne, T. A., Popov, S. V., Smirnov, V. V., Dorogina, O. I., Pecherkina, A. A., & Symaniuk, E. E. (2023). Association of Chrononutrition Indices with Anthropometric Parameters, Academic Performance, and Psychoemotional State of Adolescents: A Cross-Sectional Study. Nutrients, 15(21), 4521. https://doi.org/10.3390/nu15214521








