Bacillus Subtilis (BG01-4TM) Improves Self-Reported Symptoms for Constipation, Indigestion, and Dyspepsia: A Phase 1/2A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Intervention
2.4. Outcome Measures
2.5. Statistical Analysis
2.5.1. Primary Outcomes
2.5.2. Exploratory Outcomes
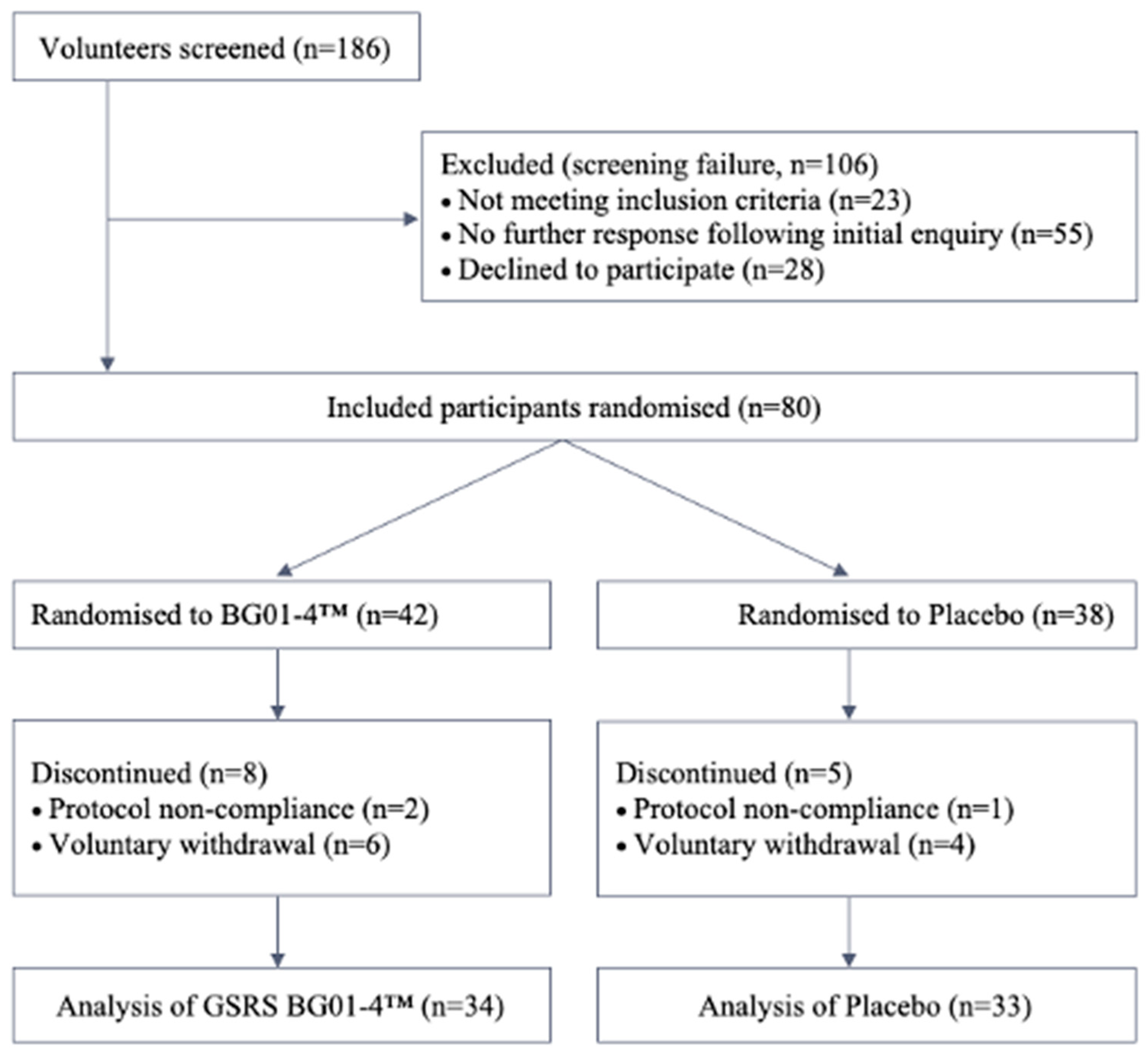
3. Results
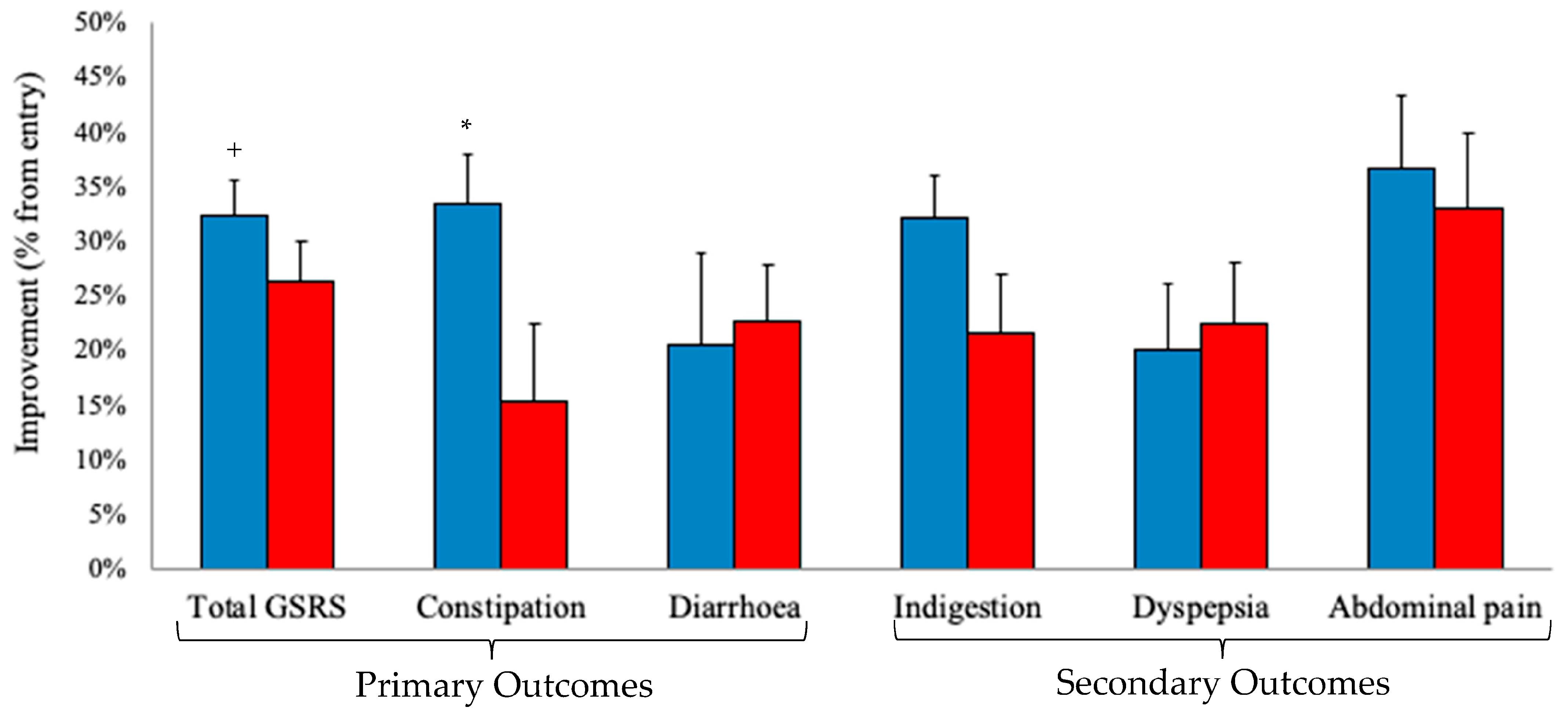
3.1. Primary and Secondary Outcomes
3.2. Exploratory Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talley, N. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol. Motil. 2008, 20, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D. Introduction. The Rome Foundation and Rome III. Neurogastroenterol. Motil. 2007, 19, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.; Spiller, R. Irritable bowel syndrome: A little understood organic bowel disease? Lancet 2002, 360, 555–564. [Google Scholar] [CrossRef]
- Cuentas, A.M.; Deaton, J.; Khan, S.; Davidson, J.; Ardita, C. The effect of Bacillus subtilis DE111 on the daily bowel movement profile for people with occasional gastrointestinal irregularity. J. Probiotics Health 2017, 5, 4. [Google Scholar] [CrossRef]
- Penet, C.; Kramer, R.; Little, R.; Spears, J.L.; Parker, J.; Iyer, J.K.; Guthrie, N.; Evans, M. A randomized, double-blind, placebo-controlled, parallel study evaluating the efficacy of Bacillus subtilis MB40 to reduce abdominal discomfort, gas, and bloating. Altern. Ther. Health Med. 2021, 27, 146–157. [Google Scholar] [PubMed]
- Seo, A.Y.; Kim, N.; Oh, D.H. Abdominal bloating: Pathophysiology and treatment. J. Neurogastroenterol. Motil. 2013, 19, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Palsson, O.S.; Törnblom, H.; Sperber, A.D.; Whitehead, W.E.; Simrén, M. The prevalence and impact of overlapping Rome IV-diagnosed functional gastrointestinal disorders on somatization, quality of life, and healthcare utilization: A cross-sectional general population study in three countries. Am. J. Gastroenterol. 2018, 113, 86–96. [Google Scholar] [CrossRef]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef]
- Iovino, P.; Bucci, C.; Tremolaterra, F.; Santonicola, A.; Chiarioni, G. Bloating and functional gastro-intestinal disorders: Where are we and where are we going? World J. Gastroenterol. 2014, 20, 14407. [Google Scholar] [CrossRef]
- Stocks, N.P.; Gonzalez-Chica, D.; Hay, P. Impact of gastrointestinal conditions, restrictive diets and mental health on health-related quality of life: Cross-sectional population-based study in Australia. BMJ Open 2019, 9, e026035. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Devapatla, S.; Lawrence, P.; Brenna, J.T. Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr. Res. 2008, 64, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Park, H.G.; Xu, C.; Lawrence, P.; Su, X.; Wijendran, V.; Walker, W.A.; Kothapalli, K.S.; Brenna, J.T. Human fetal intestinal epithelial cells metabolize and incorporate branched chain fatty acids in a structure specific manner. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Wang, D.; Lawrence, P.; Wang, X.; Kothapalli, K.S.; Greenwald, J.; Liu, R.; Park, H.G.; Brenna, J.T. BCFA-enriched vernix-monoacylglycerol reduces LPS-induced inflammatory markers in human enterocytes in vitro. Pediatr. Res. 2018, 83, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Greenwald, J.; Kothapalli, K.; Park, H.; Liu, R.; Mendralla, E.; Lawrence, P.; Wang, X.; Brenna, J. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, T. Iso-and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, T. Fatty acids of the genus Bacillus: An example of branched-chain preference. Bacteriol. Rev. 1977, 41, 391–418. [Google Scholar] [CrossRef] [PubMed]
- Errington, J.; van der Aart, L.T. Microbe Profile: Bacillus subtilis: Model organism for cellular development, and industrial workhorse. Microbiology 2020, 166, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; Palsson, O.S.; Maier, D.; Carroll, I.; Galanko, J.A.; Leyer, G.; Ringel, Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: A double-blind study. J. Clin. Gastroenterol. 2011, 45, 518–525. [Google Scholar] [CrossRef]
- Dai, C.; Zheng, C.-Q.; Jiang, M.; Ma, X.-Y.; Jiang, L.-J. Probiotics and irritable bowel syndrome. World J. Gastroenterol. 2013, 19, 5973. [Google Scholar] [CrossRef]
- Hanifi, A.; Culpepper, T.; Mai, V.; Anand, A.; Ford, A.; Ukhanova, M.; Christman, M.; Tompkins, T.; Dahl, W. Evaluation of Bacillus subtilis R0179 on gastrointestinal viability and general wellness: A randomised, double-blind, placebo-controlled trial in healthy adults. Benef. Microbes 2015, 6, 19–27. [Google Scholar] [CrossRef]
- Rogers, R. Bacillus isolates from refrigerated doughs, wheat flour, and wheat. Cereal Chem. 1978, 55, 671–674. [Google Scholar]
- Cruz Ramos, H.; Hoffmann, T.; Marino, M.; Nedjari, H.; Presecan-Siedel, E.; Dreesen, O.; Glaser, P.; Jahn, D. Fermentative metabolism of Bacillus subtilis: Physiology and regulation of gene expression. J. Bacteriol. 2000, 182, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Desfougères, T.; Lobach, A.R.; Simon, R.; Pélerin, F.; Jüsten, P.; Urdaci, M.C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2017, 83, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.; Sim, D.; O’Donnell-Megaro, A.; Bauman, D.; Barbano, D.; Brenna, J. Branched chain fatty acid content of United States retail cow’s milk and implications for dietary intake. Lipids 2011, 46, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-Chain Fatty Acids-An Underexplored Class of Dairy-Derived Fatty Acids. Nutrients 2020, 12, 2875. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.E.; Brenna, J.T.; Dvorak, B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.; Benati, G.; Strocchi, A.; Sorge, M.; Gasbarrini, G. Treatment with Bacillus subtilis reduces intestinal hydrogen production in patients with gaseous symptoms. Curr. Ther. Res. 1992, 52, 144–151. [Google Scholar] [CrossRef]
- Kim, Y.G.; Moon, J.T.; Lee, K.M.; Chon, N.R.; Park, H. The effects of probiotics on symptoms of irritable bowel syndrome. Korean J. Gastroenterol. 2006, 47, 413–419. [Google Scholar]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.E5. [Google Scholar] [CrossRef]
- Bulbuloglu, S.; Gunes, H.; Saritas, S. The effect of long-term immunosuppressive therapy on gastrointestinal symptoms after kidney transplantation. Transpl. Immunol. 2022, 70, 101515. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.S.; Sardá, F.A.H.; Giuntini, E.B.; Gumbrevicius, I.; Morais, M.B.d.; Menezes, E.W.d. Translation and validation of the brazilian portuguese version of the Gastrointestinal Symptom Rating Scale (GSRS) Questionnaire. Arq. Gastroenterol. 2016, 53, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.; Asti, T.A.; Kaya, N. Reliability and validity of the Turkish version of the Gastrointestinal Symptom Rating Scale. Gastroenterol. Nurs. 2017, 40, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Revicki, D.A.; Wood, M.; Wiklund, I.; Crawley, J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual. Life Res. 1997, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, I.; Fullerton, S.; Hawkey, C.; Jones, R.; Longstreth, G.; Mayer, E.; Peacock, R.; Wilson, I.; Naesdal, J. An irritable bowel syndrome-specific symptom questionnaire: Development and validation. Scand. J. Gastroenterol. 2003, 38, 947–954. [Google Scholar] [PubMed]
- Dimenäs, E.; Glise, H.; Hallerbäck, B.; Hernqvist, H.; Svedlund, J.; Wiklund, I. Quality of life in patients with upper gastrointestinal symptoms: An improved evaluation of treatment regimens? Scand. J. Gastroenterol. 1993, 28, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Dimenäs, E.; Glise, H.; Hallerbäck, B.; Hernqvist, H.; Svedlund, J.; Wiklund, I. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand. J. Gastroenterol. 1995, 30, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Dimenäs, E.; Carlsson, G.; Glise, H.; Israelsson, B.; Wiklund, I. Relevance of norm values as part of the documentation of quality of life instruments for use in upper gastrointestinal disease. Scand. J. Gastroenterol. 1996, 31, 8–13. [Google Scholar] [CrossRef]
- The Jamovi Project (2021), Version 2.2, Computer Software; Sydney, Australia, 2021. Available online: https://www.jamovi.org (accessed on 21 August 2022).
- Al-Fataftah, A.-R.; Abdelqader, A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim. Feed Sci. Technol. 2014, 198, 279–285. [Google Scholar] [CrossRef]
- Park, I.; Lee, Y.; Goo, D.; Zimmerman, N.; Smith, A.; Rehberger, T.; Lillehoj, H.S. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult. Sci. 2020, 99, 725–733. [Google Scholar] [CrossRef]
- Qiu, K.; Li, C.L.; Wang, J.; Qi, G.H.; Gao, J.; Zhang, H.J.; Wu, S.G. Effects of Dietary Supplementation with Bacillus subtilis, as an Alternative to Antibiotics, on Growth Performance, Serum Immunity, and Intestinal Health in Broiler Chickens. Front. Nutr. 2021, 8, 786878. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Wakana, N.; Suzuki, Y.; Nukui, M.; Daimatsu, T.; Tanaka, E.; Tanaka, K.; Koga, Y.; Nakajima, Y.; Nakazawa, H. Treatment of natto, a fermented soybean preparation, to prevent excessive plasma vitamin K concentrations in patients taking warfarin. J. Nutr. Sci. Vitaminol. 2006, 52, 297–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhatt, V.D.; Vaidya, Y.H.; Kunjadia, P.D.; Kunjadia, A.P.; Patel, R. Isolation and characterization of probiotic bacteria from human milk. Int. J. Pharm. Sci. Health Care 2012, 3, 62–70. [Google Scholar]
- Freedman, K.E.; Hill, J.L.; Wei, Y.; Vazquez, A.R.; Grubb, D.S.; Trotter, R.E.; Wrigley, S.D.; Johnson, S.A.; Foster, M.T.; Weir, T.L. Examining the gastrointestinal and immunomodulatory effects of the novel probiotic Bacillus subtilis DE111. Int. J. Mol. Sci. 2021, 22, 2453. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, F.; Zhang, J. Effect of Combined Live Probiotics Alleviating the Gastrointestinal Symptoms of Functional Bowel Disorders. Gastroenterol. Res. Pract. 2020, 2020, 4181748. [Google Scholar] [CrossRef] [PubMed]
- Östlund-Lagerström, L.; Kihlgren, A.; Repsilber, D.; Björkstén, B.; Brummer, R.J.; Schoultz, I. Probiotic administration among free-living older adults: A double blinded, randomized, placebo-controlled clinical trial. Nutr. J. 2015, 15, 80. [Google Scholar] [CrossRef]
- Fikree, A.; Byrne, P. Management of functional gastrointestinal disorders. Clin. Med. 2021, 21, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Slaets, H.; De Paepe, K.; Ceulemans, M.; Wetzels, S.; Geboers, K.; Toth, J.; Thys, W.; Dybajlo, R.; Walgraeve, D. Efficacy and safety of spore-forming probiotics in the treatment of functional dyspepsia: A pilot randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2021, 6, 784–792. [Google Scholar] [CrossRef]
- Ang, D.; Talley, N.J.; Simrén, M.; Janssen, P.; Boeckxstaens, G.; Tack, J. Endpoints used in functional dyspepsia drug therapy trials. Aliment. Pharmacol. Ther. 2011, 33, 634–649. [Google Scholar] [CrossRef]
- Labellarte, G.; Cooper, S.; Maher, M. Tolerance and efficacy of a probiotic supplement delivered in capsule form. FASEB J. 2015, 29, 924.33. [Google Scholar] [CrossRef]
- Riezzo, G.; Orlando, A.; D’Attoma, B.; Linsalata, M.; Martulli, M.; Russo, F. Randomised double blind placebo controlled trial on Lactobacillus reuteri DSM 17938: Improvement in symptoms and bowel habit in functional constipation. Benef. Microbes 2018, 9, 51–60. [Google Scholar] [CrossRef]
| Group | Abdominal Pain | Dyspepsia | Indigestion | Constipation | Diarrhoea | Total Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 2-wk | 4-wk | Pre | 2-wk | 4-wk | Pre | 2-wk | 4-wk | Pre | 2-wk | 4-wk | Pre | 2-wk | 4-wk | Pre | 2-wk | 4-wk | |
| BG01-4™ | 7.22 (±2.01) | 5.15 (±2.25) | 4.41 (±2.15) | 6.26 (±2.65) | 5.41 (±2.66) | 4.81 * (±2.57) | 15.00 (±3.55) | 11.30 (±3.61) | 10.00 * (±3.41) | 11.67 (±4.88) | 9.93 (±4.58) | 7.85 * (±4.46) | 8.41 (±4.28) | 7.44 (±4.26) | 5.81 (±2.87) | 48.56 (±9.45) | 39.22 (±10.56) | 32.89 * (±10.60) |
| Placebo | 7.43 (±1.89) | 5.07 (±2.68) | 4.86 (±2.52) | 8.36 (±4.00) | 6.11 (±3.76) | 6.29 (±3.46) | 15.29 (±4.38) | 10.71 (±5.16) | 11.14 (±4.58) | 10.43 (±4.52) | 7.55 (±4.13) | 8.32 (±4.08) | 10.79 (±5.45) | 7.39 (±4.23) | 8.18 (±5.13) | 52.64 (±10.89) | 36.96 (±14.67) | 39.07 (±14.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patch, C.; Pearce, A.J.; Cheng, M.; Boyapati, R.; Brenna, J.T. Bacillus Subtilis (BG01-4TM) Improves Self-Reported Symptoms for Constipation, Indigestion, and Dyspepsia: A Phase 1/2A Randomized Controlled Trial. Nutrients 2023, 15, 4490. https://doi.org/10.3390/nu15214490
Patch C, Pearce AJ, Cheng M, Boyapati R, Brenna JT. Bacillus Subtilis (BG01-4TM) Improves Self-Reported Symptoms for Constipation, Indigestion, and Dyspepsia: A Phase 1/2A Randomized Controlled Trial. Nutrients. 2023; 15(21):4490. https://doi.org/10.3390/nu15214490
Chicago/Turabian StylePatch, Craig, Alan J. Pearce, Mek Cheng, Ray Boyapati, and J. Thomas Brenna. 2023. "Bacillus Subtilis (BG01-4TM) Improves Self-Reported Symptoms for Constipation, Indigestion, and Dyspepsia: A Phase 1/2A Randomized Controlled Trial" Nutrients 15, no. 21: 4490. https://doi.org/10.3390/nu15214490
APA StylePatch, C., Pearce, A. J., Cheng, M., Boyapati, R., & Brenna, J. T. (2023). Bacillus Subtilis (BG01-4TM) Improves Self-Reported Symptoms for Constipation, Indigestion, and Dyspepsia: A Phase 1/2A Randomized Controlled Trial. Nutrients, 15(21), 4490. https://doi.org/10.3390/nu15214490






