Are We Ready to Recommend Capsaicin for Disorders Other Than Neuropathic Pain?
Abstract
:1. Introduction
2. Metabolism and Bioactivity
3. TRPV1-Dependent Mechanisms of Action
4. TRPV1-Independent Mechanisms of Action
5. Anti-Inflammatory and Gastrointestinal Effects of Capsaicin
6. Capsaicin and Non-Communicable Chronic Diseases
7. Effect of Capsaicin on Metabolic Syndrome and Obesity
7.1. Effects of Capsaicin on Food Intake and Satiety
7.2. Effect of Capsaicin on Obesogenic Dysbiosis
7.3. Effect of Non-Pungent Capsinoids and Spice Foods
8. Adverse Effects
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rogers, J.; Urbina, S.L.; Taylor, L.W.; Wilborn, C.D.; Purpura, M.; Jäger, R.; Juturu, V. Capsaicinoids supplementation decreases percent body fat and fat mass: Adjustment using covariates in a post hoc analysis. BMC Obes. 2018, 5, 22. [Google Scholar] [CrossRef]
- Chanda, S.; Bashir, M.; Babbar, S.; Koganti, A.; Bley, K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab. Dispos. 2008, 36, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Saudale, F.; Duval, C.; Keshtkar, S.; Groener, J.E.M.; Van Rooijen, N.; Staels, B.; Kersten, S.; Müller, M. Kupffer cells promote hepatic steatosis via interleukin-1β-dependent suppression of peroxisome proliferator-activated receptor α activity. Hepatology 2010, 51, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Kida, R.; Yoshida, H.; Murakami, M.; Shirai, M.; Hashimoto, O.; Kawada, T.; Matsui, T.; Funaba, M. Direct action of capsaicin in brown adipogenesis and activation of brown adipocytes. Cell Biochem. Funct. 2016, 34, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yang, G.; Sun, T.; Jie, T.; Zhu, C.; Yu, H.; Cheng, Y.; Yang, Z.; Xu, M.; Jiang, Y.; et al. Capsaicin receptor TRPV1 maintains quiescence of hepatic stellate cells in the liver via recruitment of SARM1. J. Hepatol. 2023, 78, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Aoki-Nonaka, Y.; De Jong, P.R.; Nohara, L.L.; Xu, H.; Stanwood, S.R.; Srikanth, S.; Lee, J.; To, K.; Abramson, L.; et al. The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4+ T cells. Nat. Immunol. 2014, 15, 1055–1063. [Google Scholar] [CrossRef]
- Negri, S.; Faris, P.; Rosti, V.; Antognazza, M.R.; Lodola, F.; Moccia, F. Endothelial TRPV1 as an Emerging Molecular Target to Promote Therapeutic Angiogenesis. Cells 2020, 9, 1341. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, X.; Cheng, W.; Yang, W.; Yu, P.; Song, Z.; Yarov-Yarovoy, V.; Zheng, J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015, 11, 518–524. [Google Scholar] [CrossRef]
- Forstenpointner, J.; Naleschinski, D.; Wasner, G.; Hüllemann, P.; Binder, A.; Baron, R. Sensitized vasoactive C-nociceptors: Key fibers in peripheral neuropathic pain. Pain Rep. 2019, 4, e709. [Google Scholar] [CrossRef]
- Irving, G.A.; Backonja, M.; Rauck, R.; Webster, L.R.; Tobias, J.K.; Vanhove, G.F. NGX-4010, a capsaicin 8% dermal patch, administered alone or in combination with systemic neuropathic pain medications, reduces pain in patients with postherpetic neuralgia. Clin. J. Pain 2012, 28, 101–107. [Google Scholar] [CrossRef]
- Huang, C.-J.; Pu, C.-M.; Su, S.-Y.; Lo, S.-L.; Lee, C.H.; Yen, Y.-H. Improvement of wound healing by capsaicin through suppression of the inflammatory response and amelioration of the repair process. Mol. Med. Rep. 2023, 28, 1–13. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Thongin, S.; Den-udom, T.; Uppakara, K.; Sriwantana, T.; Sibmooh, N.; Laolob, T.; Boonthip, C.; Wichai, U.; Muta, K.; Ketsawatsomkron, P. Beneficial effects of capsaicin and dihydrocapsaicin on endothelial inflammation, nitric oxide production and antioxidant activity. Biomed. Pharmacother. 2022, 154, 113521. [Google Scholar] [CrossRef]
- Nawaka, N.; Wanmasae, S.; Makarasen, A.; Dechtrirat, D.; Techasakul, S.; Jeenduang, N. Allicin and Capsaicin Ameliorated Hypercholesterolemia by Upregulating LDLR and Downregulating PCSK9 Expression in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 14299. [Google Scholar] [CrossRef]
- Ursu, D.; Knopp, K.; Beattie, R.E.; Liu, B.; Sher, E. Pungency of TRPV1 agonists is directly correlated with kinetics of receptor activation and lipophilicity. Eur. J. Pharmacol. 2010, 641, 114–122. [Google Scholar] [CrossRef]
- Lim, S.G.; Seo, S.E.; Jo, S.; Kim, K.H.; Kim, L.; Kwon, O.S. Highly Efficient Real-Time TRPV1 Screening Methodology for Effective Drug Candidates. ACS Omega 2022, 7, 36441–36447. [Google Scholar] [CrossRef]
- Marzęda, P.; Wróblewska-Łuczka, P.; Florek-Łuszczki, M.; Drozd, M.; Góralczyk, A.; Łuszczki, J.J. Comparison of the Anticancer Effects of Arvanil and Olvanil When Combined with Cisplatin and Mitoxantrone in Various Melanoma Cell Lines-An Isobolographic Analysis. Int. J. Mol. Sci. 2022, 23, 14192. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar] [PubMed]
- Christensen, J.D.; Lo Vecchio, S.; Andersen, H.H.; Elberling, J.; Arendt-Nielsen, L. Effect of Topical Analgesia on Desensitization Following 8% Topical Capsaicin Application. J. Pain 2021, 22, 778–788. [Google Scholar] [CrossRef] [PubMed]
- van Neerven, S.G.A.; Mouraux, A. Capsaicin-Induced Skin Desensitization Differentially Affects A-Delta and C-Fiber-Mediated Heat Sensitivity. Front. Pharmacol. 2020, 11, 615. [Google Scholar] [CrossRef]
- Balleza-Tapia, H.; Dolz-Gaiton, P.; Andrade-Talavera, Y.; Fisahn, A. Capsaicin-Induced Impairment of Functional Network Dynamics in Mouse Hippocampus via a TrpV1 Receptor-Independent Pathway: Putative Involvement of Na+/K+-ATPase. Mol. Neurobiol. 2020, 57, 1170–1185. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.W.; Zurawski, T.H.; Dong, X.; Oliver Dolly, J. Population Coding of Capsaicin Concentration by Sensory Neurons Revealed Using Ca2+ Imaging of Dorsal Root Ganglia Explants from Adult pirt-GCaMP3 Mouse. Cell. Physiol. Biochem. 2021, 55, 428–448. [Google Scholar]
- Arora, V.; Campbell, J.N.; Chung, M.K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef] [PubMed]
- Irandoost, P.; Lotfi Yagin, N.; Namazi, N.; Keshtkar, A.; Farsi, F.; Mesri Alamdari, N.; Vafa, M. The effect of Capsaicinoids or Capsinoids in red pepper on thermogenesis in healthy adults: A systematic review and meta-analysis. Phyther. Res. 2021, 35, 1358–1377. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salari, S.; Baluchnejadmojarad, T.; Roghani, M. Capsaicin protects against septic acute liver injury by attenuation of apoptosis and mitochondrial dysfunction. Heliyon 2023, 9, e14205. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, X.; Zhang, T.; Wu, X.; Fan, D.; Hu, Y.; Ding, J.; Yang, X.; Lou, J.; Du, Q.; et al. Beneficial effects of dietary capsaicin in gastrointestinal health and disease. Exp. Cell Res. 2022, 417, 113227. [Google Scholar] [CrossRef]
- Bouyer, P.G.; Tang, X.; Weber, C.R.; Shen, L.; Turner, J.R.; Matthews, J.B. Capsaicin induces NKCC1 internalization and inhibits chloride secretion in colonic epithelial cells independently of TRPV1. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G142–G156. [Google Scholar] [CrossRef]
- Ching, L.C.; Kou, Y.R.; Shyue, S.K.; Su, K.H.; Wei, J.; Cheng, L.C.; Yu, Y.B.; Pan, C.C.; Lee, T.S. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc. Res. 2011, 91, 492–501. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef]
- Joung, E.J.; Li, M.H.; Lee, H.G.; Somparn, N.; Jung, Y.S.; Na, H.K.; Kim, S.H.; Cha, Y.N.; Surh, Y.J. Capsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K-Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential target. Antioxid. Redox Signal. 2007, 9, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Boreddy, S.R.; Srivastava, S.K. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE 2011, 6, e20151. [Google Scholar] [CrossRef]
- Sharma, N.; Phan, H.T.T.; Yoda, T.; Shimokawa, N.; Vestergaard, M.C.; Takagi, M. Effects of Capsaicin on Biomimetic Membranes. Biomimetics 2019, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Isaev, D.; Yang, K.H.S.; Shabbir, W.; Howarth, F.C.; Oz, M. Capsaicin Inhibits Multiple Voltage-Gated Ion Channels in Rabbit Ventricular Cardiomyocytes in TRPV1-Independent Manner. Pharmaceuticals 2022, 15, 1187. [Google Scholar] [CrossRef] [PubMed]
- Szoka, L.; Palka, J. Capsaicin up-regulates pro-apoptotic activity of thiazolidinediones in glioblastoma cell line. Biomed. Pharmacother. 2020, 132, 110741. [Google Scholar] [CrossRef]
- Bort, A.; Sánchez, B.G.; Mateos-Gómez, P.A.; Díaz-Laviada, I.; Rodríguez-Henche, N. Capsaicin targets lipogenesis in hepG2 cells through AMPK activation, AKT inhibition and ppars regulation. Int. J. Mol. Sci. 2019, 20, 1660. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef]
- Zeng, H.; Shi, N.; Peng, W.; Yang, Q.; Ren, J.; Yang, H.; Chen, L.; Chen, Y.; Guo, J. Effects of Capsaicin on Glucose Uptake and Consumption in Hepatocytes. Molecules 2023, 28, 5258. [Google Scholar] [CrossRef]
- Wan, H.; Chen, X.Y.; Zhang, F.; Chen, J.; Chu, F.; Sellers, Z.M.; Xu, F.; Dong, H. Capsaicin inhibits intestinal Cl− secretion and promotes Na+ absorption by blocking TRPV4 channels in healthy and colitic mice. J. Biol. Chem. 2022, 298, 101847. [Google Scholar] [CrossRef]
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances Against Human Cancer. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef]
- Babbar, S.; Chanda, S.; Bley, K. Inhibition and induction of human cytochrome P450 enzymes in vitro by capsaicin. Xenobiotica 2010, 40, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Mahalak, K.K.; Bobokalonov, J.; Firrman, J.; Williams, R.; Evans, B.; Fanelli, B.; Soares, J.W.; Kobori, M.; Liu, L. Analysis of the Ability of Capsaicin to Modulate the Human Gut Microbiota In Vitro. Nutrients 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cai, Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones 2017, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, X.; Chen, Y.; Zhang, D.; Chen, D.; Chen, L.; Lin, J. Study on the Effect of Capsaicin on the Intestinal Flora through High-Throughput Sequencing. ACS Omega 2020, 5, 1246–1253. [Google Scholar] [CrossRef]
- Dai, Z.; Li, S.; Meng, Y.; Zhao, Q.; Zhang, Y.; Suonan, Z.; Sun, Y.; Shen, Q.; Liao, X.; Xue, Y. Capsaicin Ameliorates High-Fat Diet-Induced Atherosclerosis in ApoE−/− Mice via Remodeling Gut Microbiota. Nutrients 2022, 14, 4334. [Google Scholar] [CrossRef]
- Garufi, A.; Pistritto, G.; Cirone, M.; D’Orazi, G. Reactivation of mutant p53 by capsaicin, the major constituent of peppers. J. Exp. Clin. Cancer Res. 2016, 35, 1–9. [Google Scholar] [CrossRef]
- Cheng, P.; Wu, J.; Zong, G.; Wang, F.; Deng, R.; Tao, R.; Qian, C.; Shan, Y.; Wang, A.; Zhao, Y.; et al. Capsaicin shapes gut microbiota and pre-metastatic niche to facilitate cancer metastasis to liver. Pharmacol. Res. 2023, 188, 106643. [Google Scholar] [CrossRef]
- Rosca, A.E.; Iesanu, M.I.; Zahiu, C.D.M.; Voiculescu, S.E.; Paslaru, A.C.; Zagrean, A.M. Capsaicin and gut microbiota in health and disease. Molecules 2020, 25, 5681. [Google Scholar] [CrossRef]
- Braga Ferreira, L.G.; Faria, J.V.; dos Santos, J.P.S.; Faria, R.X. Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur. J. Pharmacol. 2020, 887, 173356. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, W.; Lu, C.; Guo, Y.; Chen, X.; Chen, J.; Xu, F.; Wan, H.; Dong, H. Beneficial effect of capsaicin via TRPV4/EDH signals on mesenteric arterioles of normal and colitis mice. J. Adv. Res. 2022, 39, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Vanilloid receptor TRPV1: Hot on the tongue and inflaming the colon. Neurogastroenterol. Motil. 2004, 16, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Sarkar, D.; Khan, U.; Karmakar, B.C.; Paul, S.; Mukhopadhyay, A.K.; Dutta, S.; Bhattacharya, S. Capsaicin Inhibits Inflammation and Gastric Damage during H. pylori Infection by Targeting NF-kB–miRNA Axis. Pathogens 2022, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Tang, X.; Cui, S.; Zhang, Q.; Liu, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. Capsaicin, the Spicy Ingredient of Chili Peppers: Effects on Gastrointestinal Tract and Composition of Gut Microbiota at Various Dosages. Foods 2022, 11, 686. [Google Scholar] [CrossRef]
- Liu, Y.P.; Dong, F.X.; Chai, X.; Zhu, S.; Zhang, B.L.; Gao, D.S. Role of Autophagy in Capsaicin-Induced Apoptosis in U251 Glioma Cells. Cell. Mol. Neurobiol. 2016, 36, 737–743. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, J.; Ma, Z.; Liu, W.; Yang, F.; Yang, Z.; Wang, K.; Wang, X.; He, D.; Li, L.; et al. Capsaicin enhances anti-proliferation efficacy of pirarubicin via activating TRPV1 and inhibiting PCNA nuclear translocation in 5637 cells. Mol. Med. Rep. 2016, 13, 881–887. [Google Scholar] [CrossRef]
- Liang, W.; Lan, Y.; Chen, C.; Song, M.; Xiao, J.; Huang, Q.; Cao, Y.; Ho, C.-T.; Lu, M. Modulating effects of capsaicin on glucose homeostasis and the underlying mechanism. Crit. Rev. Food Sci. Nutr. 2023, 63, 3634–3652. [Google Scholar] [CrossRef]
- Ferdowsi, P.V.; Ahuja, K.D.K.; Beckett, J.M.; Myers, S. TRPV1 Activation by Capsaicin Mediates Glucose Oxidation and ATP Production Independent of Insulin Signalling in Mouse Skeletal Muscle Cells. Cells 2021, 10, 1560. [Google Scholar] [CrossRef]
- Xia, Y.; Lee, G.; Yamamoto, M.; Takahashi, H.; Kuda, T. Detection of indigenous gut bacteria related to red chilli pepper (Capsicum annuum) in murine caecum and human faecal cultures. Mol. Biol. Rep. 2022, 49, 10239–10250. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wang, M.L.; He, Y.T.; Guo, S.W.; Li, T.T.; Peng, W.J.; Luo, D. Capsaicin ameliorates intermittent high glucose-mediated endothelial senescence via the TRPV1/SIRT1 pathway. Phytomedicine 2022, 100, 154081. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Guo, L.; Chen, L.; Zhang, M.; Chen, X.; Li, J.; Zhang, L. TRPV1 activation inhibits phenotypic switching and oxidative stress in vascular smooth muscle cells by upregulating PPARα. Biochem. Biophys. Res. Commun. 2021, 545, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, D.; Huang, J.; Hu, Y.; Xu, Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J. Clin. Pharm. Ther. 2020, 45, 16–28. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health: Table 1. Open Hear. 2015, 2, e000262. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-X.X.; Ren, H.; Gao, Y.-F.F.; Lee, C.-Y.Y.; Li, S.-F.F.; Zhang, F.; Li, L.; Chen, H. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Front. Physiol. 2017, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Ji, X.; Xue, C.; Liang, H.; Ge, Y.; He, X.; Zhang, L.; Bian, K.; Zhang, L. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro. Biomed. Pharmacother. 2017, 94, 898–908. [Google Scholar] [CrossRef]
- Szallasi, A. Capsaicin for Weight Control: “Exercise in a Pill” (or Just Another Fad)? Pharmaceuticals 2022, 15, 851. [Google Scholar] [CrossRef]
- Wang, M.; Huang, W.; Xu, Y. Effects of spicy food consumption on overweight/obesity, hypertension and blood lipids in China: A meta-analysis of cross-sectional studies. Nutr. J. 2023, 22, 29. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Fu, L.; Wang, Y.; He, M.; Zhao, L.; Liao, X. Extraction, purification, bioactivity and pharmacological effects of capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5322–5348. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Maraschin, M.; de Bairros, Â.d.F.M.; Pedreschi, R. Factors affecting the capsaicinoid profile of hot peppers and biological activity of their non-pungent analogs (Capsinoids) present in sweet peppers. Crit. Rev. Food Sci. Nutr. 2021, 61, 649–665. [Google Scholar] [CrossRef]
- Gupta, R.; Kapoor, B.; Gulati, M.; Kumar, B.; Gupta, M.; Singh, S.K.; Awasthi, A. Sweet pepper and its principle constituent capsiate: Functional properties and health benefits. Crit. Rev. Food Sci. Nutr. 2022, 62, 7370–7394. [Google Scholar] [CrossRef] [PubMed]
- Sanati, S.; Razavi, B.M.; Hosseinzadeh, H. A review of the effects of Capsicum annuum L. and its constituent, capsaicin, in metabolic syndrome. Iran. J. Basic Med. Sci. 2018, 21, 439–448. [Google Scholar] [PubMed]
- Ávila, D.L.; Nunes, N.A.M.; Almeida, P.H.R.F.; Gomes, J.A.S.; Rosa, C.O.B.; Alvarez-Leite, J.I. Signaling Targets Related to Antiobesity Effects of Capsaicin: A Scoping Review. Adv. Nutr. 2021, 12, 2232–2243. [Google Scholar] [CrossRef]
- Sanjay, M.; Sharma, A.; Lee, H.J. Role of Phytoconstituents as PPAR Agonists: Implications for Neurodegenerative Disorders. Biomedicines 2021, 9, 1914. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Baskaran, P.; Thyagarajan, B. Troglitazone activates TRPV1 and causes deacetylation of PPARγ in 3T3-L1 cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 445–453. [Google Scholar] [CrossRef]
- Baboota, R.K.; Singh, D.P.; Sarma, S.M.; Kaur, J.; Sandhir, R.; Boparai, R.K.; Kondepudi, K.K.; Bishnoi, M. Capsaicin induces “Brite” phenotype in differentiating 3T3-L1 preadipocytes. PLoS ONE 2014, 9, e103093. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liu, D.Y.; Ma, L.Q.; Luo, Z.D.; Cao, T.B.; Zhong, J.; Yan, Z.C.; Wang, L.J.; Zhao, Z.G.; Zhu, S.J.; et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007, 100, 1063–1070. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Li, Y.; Liang, X.; Sun, Q.; Yu, H.; Zhong, J.; Ni, Y.; Chen, J.; Zhao, Z.; et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ Influx. Cardiovasc. Diabetol. 2015, 14, 1–14. [Google Scholar] [CrossRef]
- Kida, R.; Noguchi, T.; Murakami, M.; Hashimoto, O.; Kawada, T.; Matsui, T.; Funaba, M. Supra-pharmacological concentration of capsaicin stimulates brown adipogenesis through induction of endoplasmic reticulum stress. Sci. Rep. 2018, 8, 845. [Google Scholar] [CrossRef]
- Fan, L.; Xu, H.; Yang, R.; Zang, Y.; Chen, J.; Qin, H. Combination of Capsaicin and Capsiate Induces Browning in 3T3-L1 White Adipocytes via Activation of the Peroxisome Proliferator-Activated Receptor γ/β3-Adrenergic Receptor Signaling Pathways. J. Agric. Food Chem. 2019, 67, 6232–6240. [Google Scholar] [CrossRef]
- Thornton, T.; Mills, D.; Bliss, E. Capsaicin: A Potential Treatment to Improve Cerebrovascular Function and Cognition in Obesity and Ageing. Nutrients 2023, 15, 1537. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Howarth, F.C. Transient receptor potential vanilloid 1 (TRPV1)-independent actions of capsaicin on cellular excitability and ion transport. Med. Res. Rev. 2023, 43, 1038–1067. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The Effects of Capsaicin and Capsiate on Energy Balance: Critical Review and Meta-analyses of Studies in Humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-J.; Lee, H.-B.; Yoo, G.; Park, M.; Lee, C.-H.; Choi, I.; Park, H.-Y. Anti-obesity effects of red pepper (Capsicum annuum L.) leaf extract on 3T3-L1 preadipocytes and high fat diet-fed mice. Food Funct. 2023, 14, 292–304. [Google Scholar] [CrossRef]
- Lu, M.; Cao, Y.; Xiao, J.; Song, M.; Ho, C.T. Molecular mechanisms of the anti-obesity effect of bioactive ingredients in common spices: A review. Food Funct. 2018, 9, 4569–4581. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda-Solís, A.; Sánchez, J.; Portillo, M.P.; Palou, A.; Picó, C. Combination of capsaicin and hesperidin reduces the effectiveness of each compound to decrease the adipocyte size and to induce browning features in adipose tissue of western diet fed rats. J. Agric. Food Chem. 2018, 66, 9679–9689. [Google Scholar] [CrossRef]
- Silvester, A.J.; Aseer, K.R.; Yun, J.W. Dietary polyphenols and their roles in fat browning. J. Nutr. Biochem. 2019, 64, 1–12. [Google Scholar] [CrossRef]
- Takeda, Y.; Dai, P. Capsaicin directly promotes adipocyte browning in the chemical compound-induced brown adipocytes converted from human dermal fibroblasts. Sci. Rep. 2022, 12, 6612. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Tang, Y.; Yin, H.; Liu, X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Siebert, E.; Lee, S.Y.; Prescott, M.P. Chili pepper preference development and its impact on dietary intake: A narrative review. Front. Nutr. 2022, 9, 1039207. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Peppers and Their Constituents against Obesity. Biol. Futur. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef] [PubMed]
- Smeets, A.J.; Westerterp-Plantenga, M.S. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. Eur. J. Nutr. 2009, 48, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Van Avesaat, M.; Troost, F.J.; Westerterp-Plantenga, M.S.; Helyes, Z.; Le Roux, C.W.; Dekker, J.; Masclee, A.A.M.; Keszthelyi, D. Capsaicin-induced satiety is associated with gastrointestinal distress but not with the release of satiety hormones. Am. J. Clin. Nutr. 2016, 103, 305–313. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Casnici, C.; Marelli, O.; De Col, A.; Tamini, S.; Lucchetti, E.; Tringali, G.; De Micheli, R.; Abbruzzese, L.; Bortolotti, M.; et al. Acute administration of capsaicin increases resting energy expenditure in young obese subjects without affecting energy intake, appetite, and circulating levels of orexigenic/anorexigenic peptides. Nutr. Res. 2018, 52, 71–79. [Google Scholar] [CrossRef]
- de Moura e Silva, V.E.L.; Cholewa, J.M.; Jäger, R.; Zanchi, N.E.; de Freitas, M.C.; de Moura, R.C.; Barros, E.M.L.; Antunes, B.M.; Caperuto, E.C.; Ribeiro, S.L.G.; et al. Chronic capsiate supplementation increases fat-free mass and upper body strength but not the inflammatory response to resistance exercise in young untrained men: A randomized, placebo-controlled and double-blind study. J. Int. Soc. Sports Nutr. 2021, 18, 50. [Google Scholar] [CrossRef]
- Zsiborás, C.; Mátics, R.; Hegyi, P.; Balaskó, M.; Pétervári, E.; Szabó, I.; Sarlós, P.; Mikó, A.; Tenk, J.; Rostás, I.; et al. Capsaicin and capsiate could be appropriate agents for treatment of obesity: A meta-analysis of human studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1419–1427. [Google Scholar] [CrossRef]
- Wang, D.; Guo, S.; He, H.; Gong, L.; Cui, H. Gut Microbiome and Serum Metabolome Analyses Identify Unsaturated Fatty Acids and Butanoate Metabolism Induced by Gut Microbiota in Patients with Chronic Spontaneous Urticaria. Front. Cell. Infect. Microbiol. 2020, 10, 24. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Mahajan, N.; Kaur, J.; Devi, K.; Dharavath, R.N.; Singh, R.P.; Kondepudi, K.K.; Bishnoi, M. Mucin secretory action of capsaicin prevents high fat diet-induced gut barrier dysfunction in C57BL/6 mice colon. Biomed. Pharmacother. 2022, 145, 112452. [Google Scholar] [CrossRef]
- Xia, J.; Gu, L.; Guo, Y.; Feng, H.; Chen, S.; Jurat, J.; Fu, W.; Zhang, D. Gut Microbiota Mediates the Preventive Effects of Dietary Capsaicin Against Depression-like Behavior Induced by Lipopolysaccharide in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 627608. [Google Scholar] [CrossRef]
- Xia, Y.; Kuda, T.; Yamamoto, M.; Yano, T.; Nakamura, A.; Takahashi, H. The effect of Sichuan pepper on gut microbiota in mice fed a high-sucrose and low-dietary fibre diet. Appl. Microbiol. Biotechnol. 2023, 107, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, S.; Li, F.; Xu, X.; Xing, H. Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity. Biomolecules 2022, 12, 1871. [Google Scholar] [CrossRef]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. MBio 2017, 8, 10–1128. [Google Scholar] [CrossRef]
- Manca, C.; Lacroix, S.; Pérusse, F.; Flamand, N.; Chagnon, Y.; Drapeau, V.; Tremblay, A.; Di Marzo, V.; Silvestri, C. Oral Capsaicinoid Administration Alters the Plasma Endocannabinoidome and Fecal Microbiota of Reproductive-Aged Women Living with Overweight and Obesity. Biomedicines 2021, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral administration of Flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 2020, 47, 6717–6725. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Huang, Z.; Liu, H. Spicy Food and Chili Peppers and Multiple Health Outcomes: Umbrella Review. Mol. Nutr. Food Res. 2022, 66, 2200167. [Google Scholar] [CrossRef]
- Zanzer, Y.C.; Plaza, M.; Dougkas, A.; Turner, C.; Östman, E. Black pepper-based beverage induced appetite-suppressing effects without altering postprandial glycaemia, gut and thyroid hormones or gastrointestinal well-being: A randomized crossover study in healthy subjects. Food Funct. 2018, 9, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Pandian, A.; Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin. Phytoscience 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Tang, W.; Mao, D.; Liu, X.; Qian, W.; Dai, Y.; Chen, L.; Ding, X. Spicy food consumption is associated with abdominal obesity among Chinese Han population aged 30–79 years in the Sichuan Basin: A population-based cross-sectional study. BMC Public Health 2022, 22, 1881. [Google Scholar] [CrossRef]
- Wen, Q.; Wei, Y.; Du, H.; Lv, J.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.Y.; Chen, Y.Y.; Shi, L.; et al. Characteristics of spicy food consumption and its relation to lifestyle behaviours: Results from 0.5 million adults. Int. J. Food Sci. Nutr. 2021, 72, 569–576. [Google Scholar] [CrossRef]
- Duranova, H.; Valkova, V.; Gabriny, L. Chili peppers (Capsicum spp.): The spice not only for cuisine purposes: An update on current knowledge. Phytochem. Rev. 2022, 21, 1379–1413. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The Two Faces of Capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef] [PubMed]

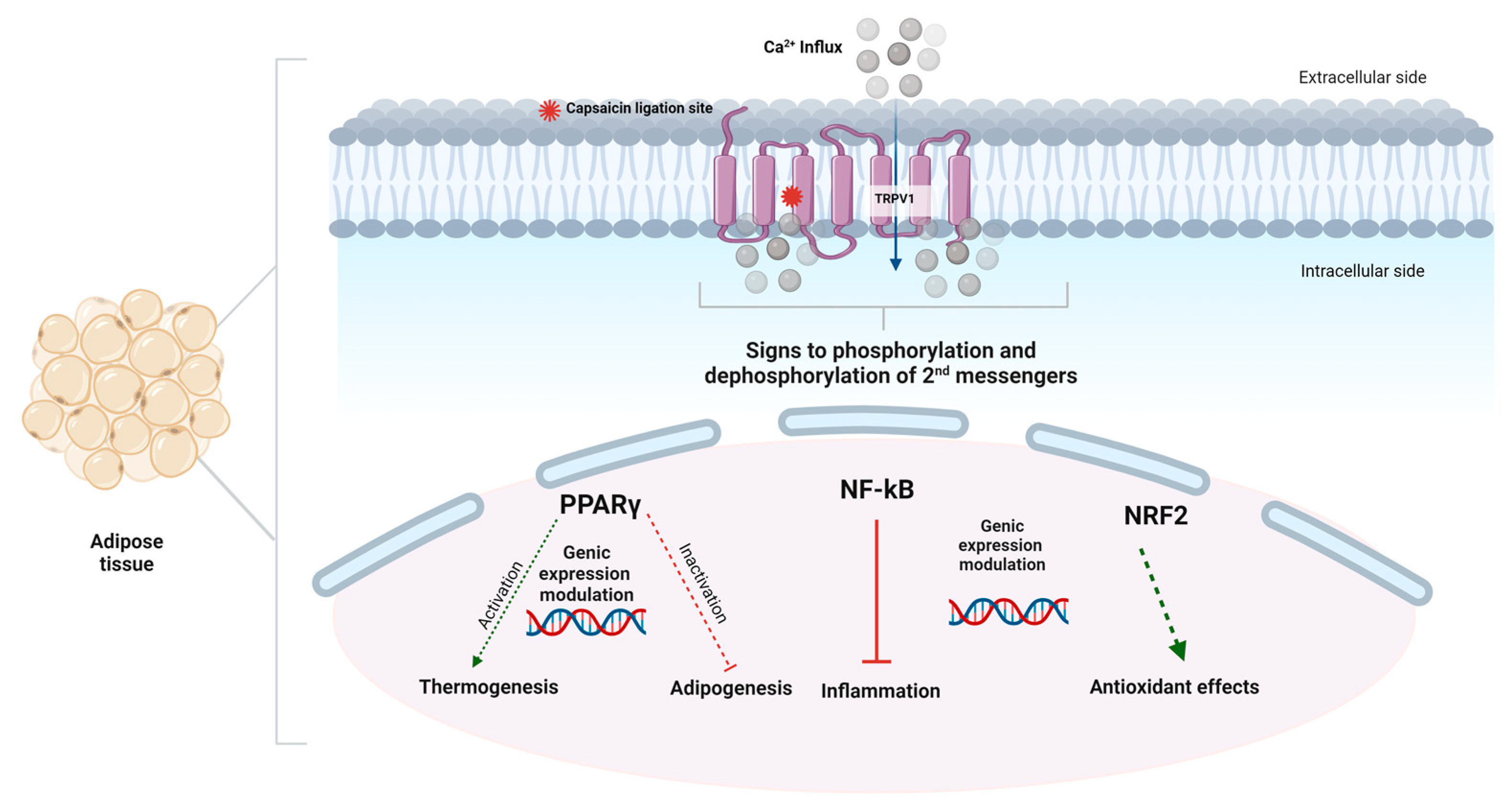
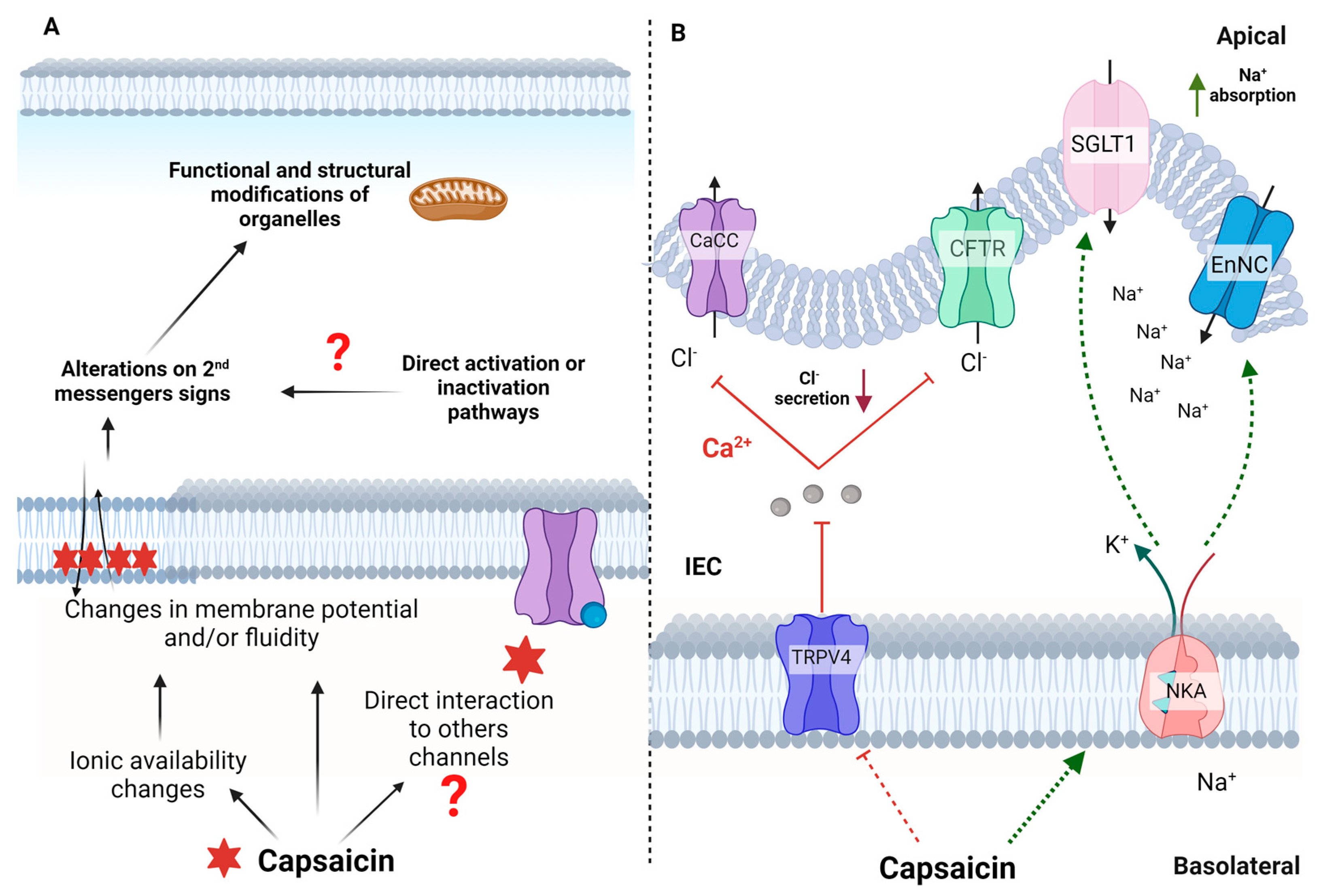

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.L.; Santos, E.A.; Alvarez-Leite, J.I. Are We Ready to Recommend Capsaicin for Disorders Other Than Neuropathic Pain? Nutrients 2023, 15, 4469. https://doi.org/10.3390/nu15204469
Silva JL, Santos EA, Alvarez-Leite JI. Are We Ready to Recommend Capsaicin for Disorders Other Than Neuropathic Pain? Nutrients. 2023; 15(20):4469. https://doi.org/10.3390/nu15204469
Chicago/Turabian StyleSilva, Janayne L., Elandia A. Santos, and Jacqueline I. Alvarez-Leite. 2023. "Are We Ready to Recommend Capsaicin for Disorders Other Than Neuropathic Pain?" Nutrients 15, no. 20: 4469. https://doi.org/10.3390/nu15204469
APA StyleSilva, J. L., Santos, E. A., & Alvarez-Leite, J. I. (2023). Are We Ready to Recommend Capsaicin for Disorders Other Than Neuropathic Pain? Nutrients, 15(20), 4469. https://doi.org/10.3390/nu15204469







