Lactobacillus paragasseri SBT2055 Activates Plasmacytoid Dendritic Cells and Improves Subjective Symptoms of Common Cold in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Parallel-Group Comparative Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Preparation of LG2055
2.1.2. Flow Cytometry Analysis
2.1.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.1.4. Statistical Analysis
2.2. Clinical Study
2.2.1. Participants
2.2.2. Test Samples
2.2.3. Study Design
2.2.4. Outcomes
2.2.5. Physical Health Questionnaire
2.2.6. Salivary sIgA, Serum IgA, Serum IgG, and NK Cell Activity
2.2.7. pDC Activity
2.2.8. Analysis of Fecal Microbiota
2.2.9. Safety Assessments
2.2.10. Sample Size
2.2.11. Randomization
2.2.12. Statistical Analysis
3. Results
3.1. In Vitro Experiments
3.2. Clinical Study
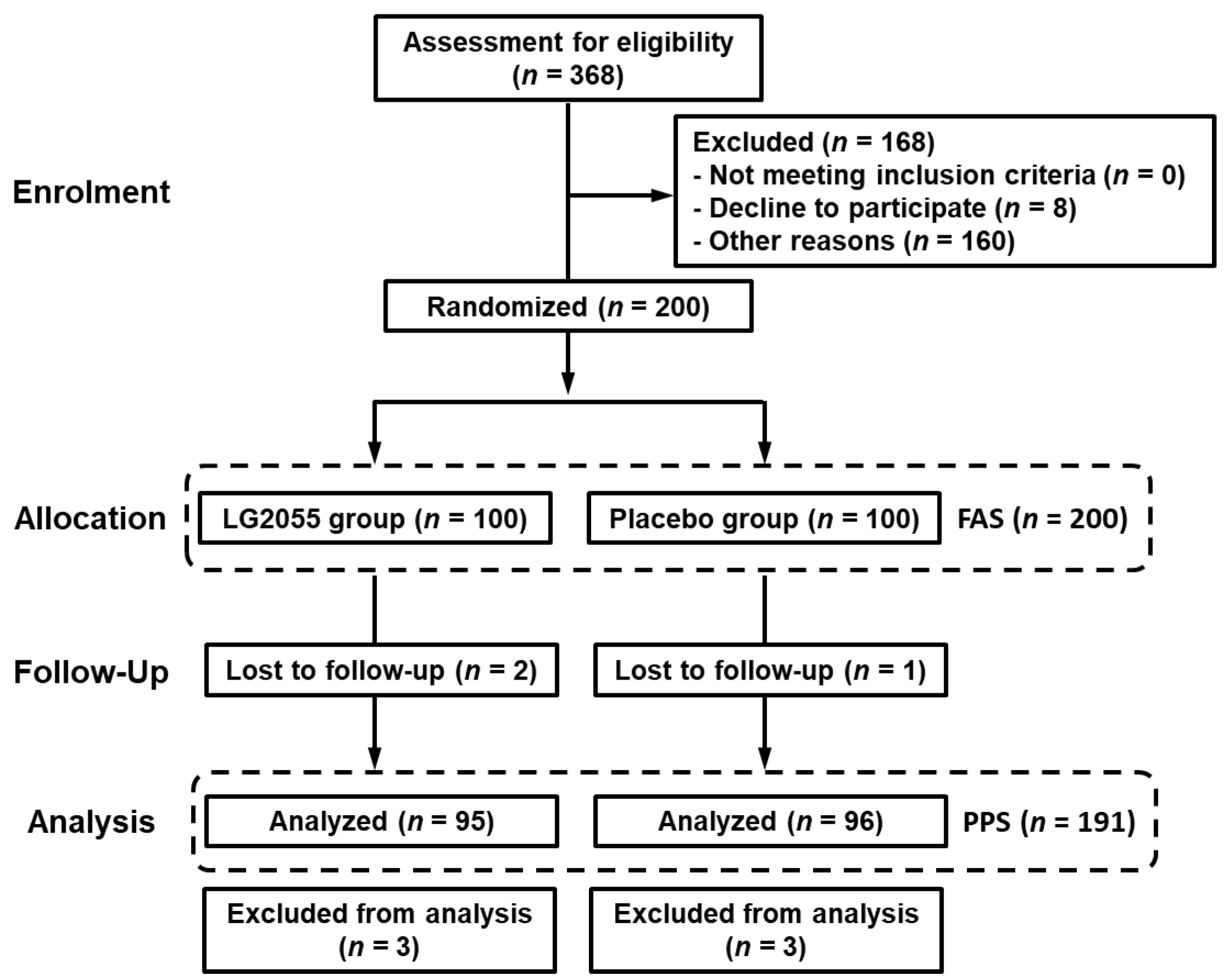
3.2.1. Participants
3.2.2. Background of Participants
3.2.3. Intake Ratio
3.2.4. Primary Outcome
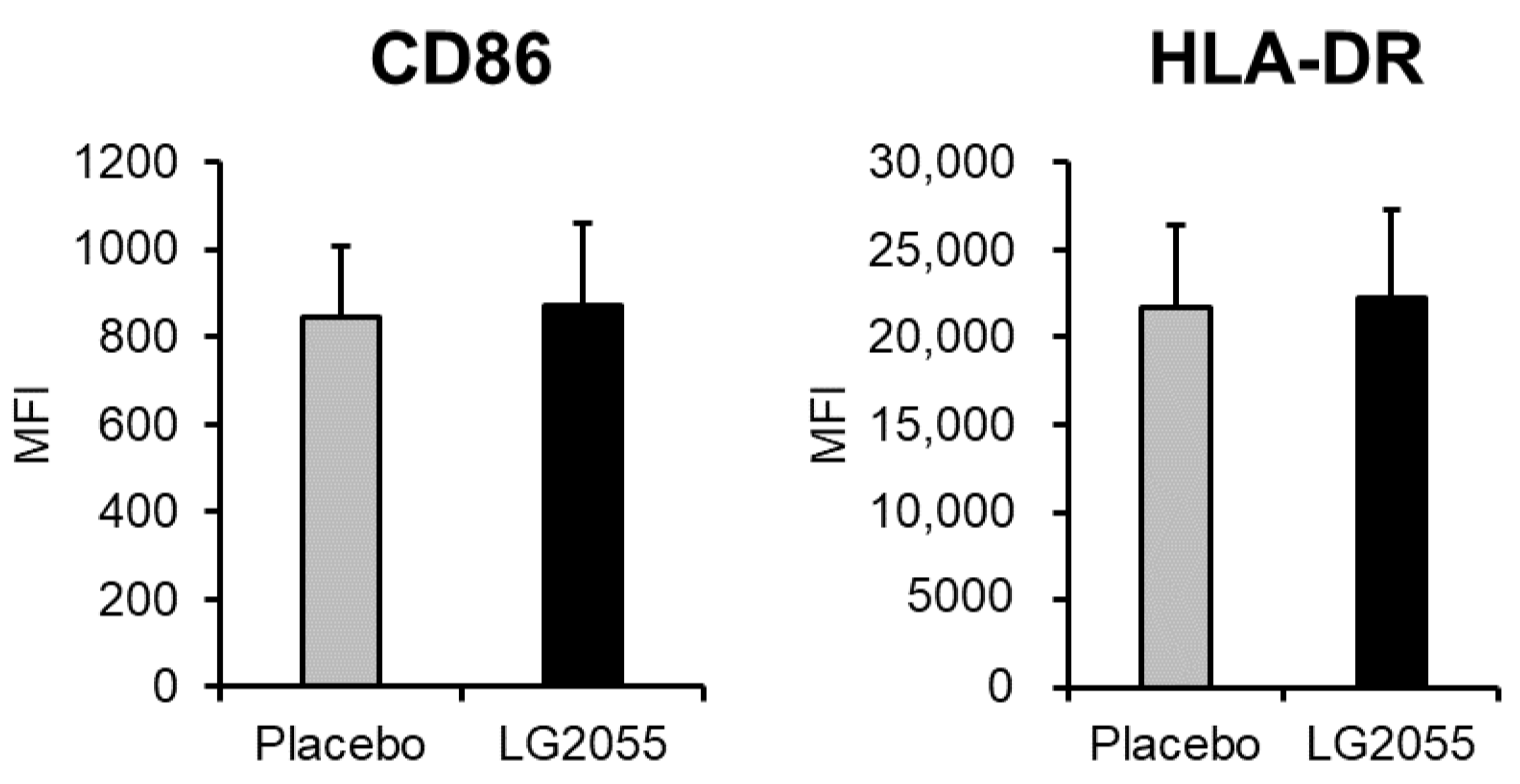
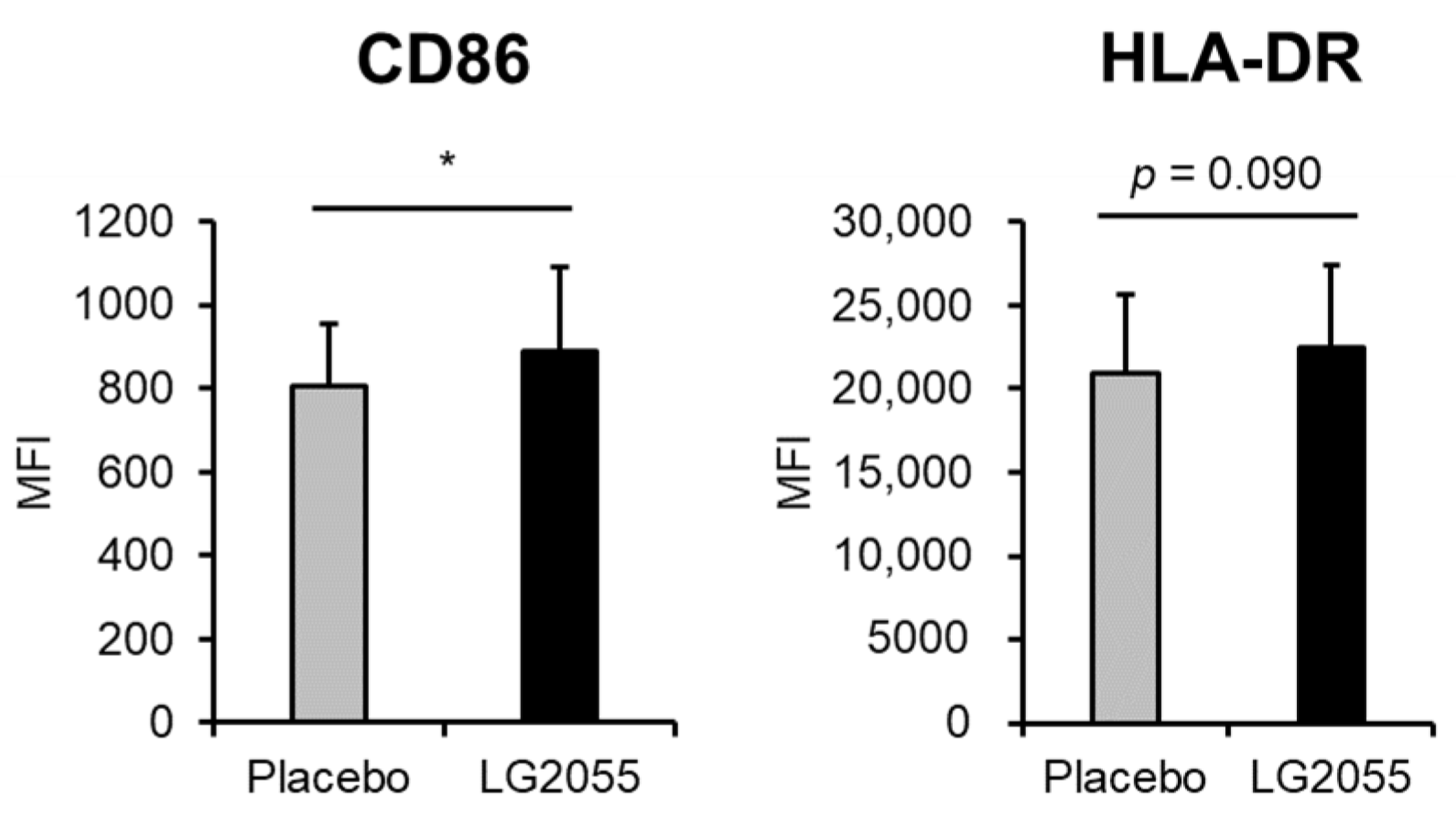
3.2.5. pDC Activity
3.2.6. Salivary sIgA, Serum IgA, Serum IgG Levels, and NK Cell Activity
3.2.7. Analysis of Fecal Microbiota
3.2.8. Stratified Analysis
3.2.9. Safety Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girard, M.P.; Tam, J.S.; Assossou, O.M.; Kieny, M.P. The 2009 A (H1N1) influenza virus pandemic: A review. Vaccine 2010, 28, 4895–4902. [Google Scholar] [CrossRef]
- Godbout, J.P.; Glaser, R. Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. J. Neuroimmune Pharmacol. 2006, 1, 421–427. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Dantzer, R.; Heijnen, C.J.; Kavelaars, A.; Laye, S.; Capuron, L. The neuroimmune basis of fatigue. Trends Neurosci. 2014, 37, 39–46. [Google Scholar] [CrossRef]
- Colonna, M.; Trinchieri, G.; Liu, Y.J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004, 12, 1219–1226. [Google Scholar] [CrossRef]
- Kubo, S.; Miyakawa, M.; Tada, A.; Oda, H.; Motobayashi, H.; Iwabuchi, S.; Tamura, S.; Tanaka, M.; Hashimoto, S. Lactoferrin and its digestive peptides induce interferon-α production and activate plasmacytoid dendritic cells ex vivo. Biometals 2023, 36, 563–573. [Google Scholar] [CrossRef]
- Jounai, K.; Ikado, K.; Sugimura, T.; Ano, Y.; Braun, J.; Fujiwara, D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS ONE 2012, 7, e32588. [Google Scholar] [CrossRef]
- Naito, T.; Morikawa, M.; Yamamoto-Fujimura, M.; Iwata, A.; Maki, A.; Kato-Nagaoka, N.; Oana, K.; Kiyoshima-Shibata, J.; Matsuura, Y.; Kaji, R.; et al. Diverse impact of a probiotic strain, Lacticaseibacillus paracasei Shirota, on peripheral mononuclear phagocytic cells in healthy Japanese office workers: A randomized, double-blind, controlled trial. Biosci. Microbiota Food Health 2023, 42, 65–72. [Google Scholar] [CrossRef]
- Tanaka, T.; Oe, M.; Yamashita, S.; Kimura, M.; Seino, S.; Kajiyama, D.; Okuyama, Y.; Matsuoka, R.; Morishita, R.; Tsukinoki, K. Immunomodulatory effect of acetic acid bacteria (Gluconacetobacter hansenii GK-1) on plasmacytoid dendritic cells-A double-blinded placebo-controlled study. Jpn. Pharmacol. Ther. 2022, 50, 2237–2248. [Google Scholar]
- Sasai, M.; Kano, C.; Sakano, K.; Nakamura, K.; Iwama, Y.; Sawada, D.; Hirota, T. The effect of Lactobacillus acidophilus L-92 on the subjective symptoms of physical condition and the immune parameters in healthy adults-Randamized, double-blind, placebo-controlled, parallel-group studies. Jpn. Pharmacol. Ther. 2021, 49, 1261–1271. [Google Scholar]
- Tanizawa, Y.; Tada, I.; Kobayashi, H.; Endo, A.; Maeno, S.; Toyoda, A.; Arita, M.; Nakamura, Y.; Sakamoto, M.; Ohkuma, M.; et al. Lactobacillus paragasseri sp. nov., a sister taxon of Lactobacillus gasseri, based on whole-genome sequence analyses. Int. J. Syst. Evol. Microbiol. 2018, 68, 3512–3517. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef]
- Hamad, E.M.; Sato, M.; Uzu, K.; Yoshida, T.; Higashi, S.; Kawakami, H.; Kadooka, Y.; Matsuyama, H.; Abd El-Gawad, I.A.; Imaizumi, K. Milk fermented by Lactobacillus gasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats. Br. J. Nutr. 2009, 101, 716–724. [Google Scholar] [CrossRef]
- Sato, M.; Uzu, K.; Yoshida, T.; Hamad, E.M.; Kawakami, H.; Matsuyama, H.; Abd El-Gawad, I.A.; Imaizumi, K. Effects of milk fermented by Lactobacillus gasseri SBT2055 on adipocyte size in rats. Br. J. Nutr. 2008, 99, 1013–1017. [Google Scholar] [CrossRef]
- Nakagawa, H.; Shiozaki, T.; Kobatake, E.; Hosoya, T.; Moriya, T.; Sakai, F.; Taru, H.; Miyazaki, T. Effects and mechanisms of prolongevity induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 2016, 15, 227–236. [Google Scholar] [CrossRef]
- Kobatake, E.; Nakagawa, H.; Seki, T.; Miyazaki, T. Protective effects and functional mechanisms of Lactobacillus gasseri SBT2055 against oxidative stress. PLoS ONE 2017, 12, e0177106. [Google Scholar] [CrossRef]
- Ishida, Y.; Uenishi, K.; Suzuki, H.; Seto, Y.; Teshima, T.; Fujiwara, S. Milk fermented with yogurt cultures and Lactobacillus gasseri SBT2055 (LG2055: Strain Yukijirushi) compared with yogurt: Influence on intestinal microflora and bowel habits of healthy young women. Pharmacometrics 2001, 61, 203–213. [Google Scholar]
- Nakayama, Y.; Moriya, T.; Sakai, F.; Ikeda, N.; Shiozaki, T.; Hosoya, T.; Nakagawa, H.; Miyazaki, T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci. Rep. 2014, 4, 4638. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 4812. [Google Scholar] [CrossRef]
- Sakai, F.; Hosoya, T.; Ono-Ohmachi, A.; Ukibe, K.; Ogawa, A.; Moriya, T.; Kadooka, Y.; Shiozaki, T.; Nakagawa, H.; Nakayama, Y.; et al. Lactobacillus gasseri SBT2055 induces TGF-β expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS ONE 2014, 9, e105370. [Google Scholar] [CrossRef] [PubMed]
- Nishihira, J.; Moriya, T.; Sakai, F.; Kabuki, T.; Kawasaki, Y.; Nishimura, M. Lactobacillus gasseri SBT2055 stimulates immunoglobulin production and innate immunity after influenza vaccination in healthy adult volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. Funct. Foods Health Dis. 2016, 6, 544–568. [Google Scholar] [CrossRef]
- Kobatake, E.; Iwama, Y.; Arai, T.; Shioya, N.; Kise, M.; Kabuki, T. Intake of Lactobacillus paragasseri SBT2055 improves subjective symptoms of common cold during winter season in healthy adults: A randomized, double-blind, placebo-controlled parallel-group comparative study. Front. Nutr. 2022, 9, 1063584. [Google Scholar] [CrossRef]
- Barrett, B.; Brown, R.L.; Mundt, M.P.; Thomas, G.R.; Barlow, S.K.; Highstrom, A.D.; Bahrainian, M. Validation of a short form Wisconsin upper respiratory symptom survey (WURSS-21). Health Qual. Life Outcomes 2009, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K.; Tanihata, Y.; Miura, Y.; Fujiwara, D.; Yamamoto, N. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Kanayama, M.; Haida, M.; Fujimoto, S.; Oroguchi, T.; Sata, K.; Mita, N.; Kutsuzawa, T.; Ikeuchi, M.; Kondo, M.; et al. Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza in healthy adults in a randomized controlled trial. J. Funct. Foods 2016, 24, 492–500. [Google Scholar] [CrossRef]
- Kawada, Y.; Naito, Y.; Andoh, A.; Ozeki, M.; Inoue, R. Effect of storage and DNA extraction method on 16S rRNA-profiled fecal microbiota in Japanese adults. J. Clin. Biochem. Nutr. 2019, 64, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Inoue, R.; Nakayama, K.; Inatomi, T. Inclusion of Bacillus amyloliquefaciens strain TOA5001 in the diet of broilers suppresses the symptoms of coccidiosis by modulating intestinal microbiota. Anim. Sci. J. 2018, 89, 679–687. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Krug, A.; Rothenfusser, S.; Hornung, V.; Jahrsdörfer, B.; Blackwell, S.; Ballas, Z.K.; Endres, S.; Krieg, A.M.; Hartmann, G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 2001, 31, 2154–2163. [Google Scholar] [CrossRef]
- Xu, N.; Yao, H.P.; Lv, G.C.; Chen, Z. Downregulation of TLR7/9 leads to deficient production of IFN-α from plasmacytoid dendritic cells in chronic hepatitis B. Inflamm. Res. 2012, 61, 997–1004. [Google Scholar] [CrossRef]
- Hamuro, K.; Saito, H.; Saito, T.; Kohda, N. Identification of antigens recognized by salivary IgA using microbial protein microarrays. Biosci. Microbiota Food Health 2022, 41, 177–184. [Google Scholar] [CrossRef]
- Saito, Y.; Fujii, M.; Watanabe, T.; Maruyama, K.; Kowatari, Y.; Ogata, H.; Kumagai, T. Randomized, double-blind, placebo-controlled, parallel-group study of the effect of Lactobacillus paracasei K71 intake on salivary release of secretory immunoglobulin A. Biosci. Microbiota Food Health 2017, 36, 55–63. [Google Scholar] [CrossRef]
- Neville, V.; Gleeson, M.; Folland, J.P. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med. Sci. Sport. Exerc. 2008, 40, 1228–1236. [Google Scholar] [CrossRef]
- Fahlman, M.M.; Engels, H.J. Mucosal IgA and URTI in American college football players: A year longitudinal study. Med. Sci. Sport. Exerc. 2005, 37, 374–380. [Google Scholar] [CrossRef]
- Tezuka, H.; Abe, Y.; Asano, J.; Sato, T.; Liu, J.; Iwata, M.; Ohteki, T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 2011, 34, 247–257. [Google Scholar] [CrossRef]
- Sugimura, T.; Jounai, K.; Ohshio, K.; Tanaka, T.; Suwa, M.; Fujiwara, D. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin. Immunol. 2013, 149, 509–518. [Google Scholar] [CrossRef]
- Raieli, S.; Trichot, C.; Korniotis, S.; Pattarini, L.; Soumelis, V. TLR1/2 orchestrate human plasmacytoid predendritic cell response to gram+ bacteria. PLoS Biol. 2019, 17, e3000209. [Google Scholar] [CrossRef]
- Al-Hassany, L.; Haas, J.; Piccininni, M.; Kurth, T.; Maassen Van Den Brink, A.; Rohmann, J.L. Giving Researchers a Headache—Sex and Gender Differences in Migraine. Front. Neurol. 2020, 11, 549038. [Google Scholar] [CrossRef]
- Oniszczenko, W. Affective Temperaments and Meteoropathy Among Women: A Cross-sectional Study. PLoS ONE 2020, 15, e0232725. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chen, Y.T.; Ou, S.M.; Li, S.Y.; Yang, A.C.; Tang, C.H.; Wang, S.J. Temperature variation and the incidence of cluster headache periods: A nationwide population study. Cephalalgia 2014, 34, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Oe, M.; Kimura, M.; Okuyama, Y.; Seino, S.; Kajiyama, D.; Matsuoka, R.; Masuda, Y.; Tsukinoki, K. Improving Effect of Acetic Acid Bacteria (Gluconacetobacter hansenii GK-1) on sIgA and Physical Conditions in Healthy People: Double-Blinded Placebo-Controlled Study. Food Nutr. Sci. 2022, 13, 541–557. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanayama, M.; Fujii, T.; Fujiwara, D.; Sugimura, K. Effects of the Beverage Containing Lactococcus lactis subsp. lactis JCM5805 on Anti–viral Immune Responses and Maintenance of Physical Conditions -A Randomized, Double–blind, Placebo–controlled, Parallel–group Trial. Jpn. Pharmacol. Ther. 2015, 43, 1465–1472. [Google Scholar]
- Fujiwara, S.; Seto, Y.; Kimura, A.; Hashiba, H. Establishment of orally-administered Lactobacillus gasseri SBT2055SR in the gastrointestinal tract of humans and its influence on intestinal microflora and metabolism. J. Appl. Microbiol. 2001, 90, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Fujita, T.; Suzuki, Y.; Benno, Y. Monitoring and survival of Lactobacillus gasseri SBT2055 in the human intestinal tract. Microbiol. Immunol. 2006, 50, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Kawai, J.; Mori, K.; Hartanto, T.; Komatsu, K.; Kudo, T.; Fukuda, S. Dietary supplement of mushrooms promotes SCFA production and moderately associates with IgA production: A pilot clinical study. Front. Nutr. 2023, 9, 1078060. [Google Scholar] [CrossRef] [PubMed]
- Shirouchi, B.; Nagao, K.; Umegatani, M.; Shiraishi, A.; Morita, Y.; Kai, S.; Yanagita, T.; Ogawa, A.; Kadooka, Y.; Sato, M. Probiotic Lactobacillus gasseri SBT2055 improves glucose tolerance and reduces body weight gain in rats by stimulating energy expenditure. Br. J. Nutr. 2016, 116, 451–458. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Holmes, I.; Harris, K.; Quince, C. Dirichlet multinomial mixtures: Generative models for microbial metagenomics. PLoS ONE 2012, 7, e30126. [Google Scholar] [CrossRef]
- Takagi, T.; Inoue, R.; Oshima, A.; Sakazume, H.; Ogawa, K.; Tominaga, T.; Mihara, Y.; Sugaya, T.; Mizushima, K.; Uchiyama, K.; et al. Typing of the Gut Microbiota Community in Japanese Subjects. Microorganisms 2022, 10, 664. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Antunes, K.H.; Fachi, J.L.; de Paula, R.; da Silva, E.F.; Pral, L.P.; Dos Santos, A.Á.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 3273. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]

| Active Capsules | Placebo Capsules | ||
|---|---|---|---|
| Energy | kcal | 3.6 | 3.7 |
| Protein | g | 0.06 | 0.01 |
| Fat | g | 0.03 | 0.03 |
| Sugar | g | 0.8 | 0.9 |
| Sodium | mg | 2.2 | 0.3 |
| LG2055 | cfu | >1 × 109 | not detected |
| LG2055 Group | Placebo Group | p-Value | ||
|---|---|---|---|---|
| n = 100 | n = 100 | |||
| Sex | male/female | 52/48 | 55/45 | 0.671 |
| Age | years | 47.0 ± 9.4 | 47.4 ± 8.9 | 0.758 |
| Height | cm | 165.4 ± 7.0 | 165.0 ± 8.7 | 0.775 |
| Weight | kg | 61.0 ± 10.4 | 61.3 ± 11.4 | 0.815 |
| BMI | kg/m2 | 22.2 ± 2.9 | 22.4 ± 3.2 | 0.614 |
| Symptoms | Group | n | With Symptoms | Without Symptoms | p-Value | ||
|---|---|---|---|---|---|---|---|
| Cumulative Days | Ratio | Cumulative Days | Ratio | ||||
| Runny nose | LG2055 | 7981 | 2674 | 33.5% | 5307 | 66.5% | <0.001 * |
| Placebo | 8065 | 2968 | 36.8% | 5097 | 63.2% | ||
| Plugged nose | LG2055 | 7981 | 1793 | 22.5% | 6188 | 77.5% | 0.005 * |
| Placebo | 8065 | 1965 | 24.4% | 6100 | 75.6% | ||
| Sneezing | LG2055 | 7981 | 1539 | 19.3% | 6442 | 80.7% | <0.001 * |
| Placebo | 8065 | 2067 | 25.6% | 5998 | 74.4% | ||
| Sore throat | LG2055 | 7981 | 652 | 8.2% | 7329 | 91.8% | 0.002 * |
| Placebo | 8065 | 769 | 9.5% | 7296 | 90.5% | ||
| Hoarseness | LG2055 | 7981 | 244 | 3.1% | 7737 | 96.9% | <0.001 * |
| Placebo | 8065 | 516 | 6.4% | 7549 | 93.6% | ||
| Cough | LG2055 | 7981 | 963 | 12.1% | 7018 | 87.9% | 0.964 |
| Placebo | 8065 | 975 | 12.1% | 7090 | 87.9% | ||
| Headache | LG2055 | 7981 | 969 | 12.1% | 7012 | 87.9% | <0.001 * |
| Placebo | 8065 | 755 | 9.4% | 7310 | 90.6% | ||
| Feeling tired | LG2055 | 7981 | 1483 | 18.6% | 6498 | 81.4% | <0.001 * |
| Placebo | 8065 | 1271 | 15.8% | 6794 | 84.2% | ||
| Chill | LG2055 | 7981 | 216 | 2.7% | 7765 | 97.3% | <0.001 * |
| Placebo | 8065 | 400 | 5.0% | 7665 | 95.0% | ||
| Fever | LG2055 | 7981 | 103 | 1.3% | 7878 | 98.7% | 0.244 |
| Placebo | 8065 | 88 | 1.1% | 7977 | 98.9% | ||
| Group | n | Bad | Good | p-Value | ||
|---|---|---|---|---|---|---|
| Cumulative Days | Ratio | Cumulative Days | Ratio | |||
| LG2055 | 7981 | 4789 | 60.0% | 3192 | 40.0% | <0.001 * |
| Placebo | 8065 | 5145 | 63.8% | 2920 | 36.2% | |
| Symptoms | Group | n | With Symptoms | Without Symptoms | p-Value | ||
|---|---|---|---|---|---|---|---|
| Cumulative Days | Ratio | Cumulative Days | Ratio | ||||
| Runny nose | LG2055 | 4789 | 1701 | 35.5% | 3088 | 64.5% | <0.001 * |
| Placebo | 4621 | 2020 | 43.7% | 2601 | 56.3% | ||
| Plugged nose | LG2055 | 4789 | 1078 | 22.5% | 3711 | 77.5% | <0.001 * |
| Placebo | 4621 | 1302 | 28.2% | 3319 | 71.8% | ||
| Sneezing | LG2055 | 4789 | 940 | 19.6% | 3849 | 80.4% | <0.001 * |
| Placebo | 4621 | 1341 | 29.0% | 3280 | 71.0% | ||
| Sore throat | LG2055 | 4789 | 405 | 8.5% | 4384 | 91.5% | 0.427 |
| Placebo | 4621 | 370 | 8.0% | 4251 | 92.0% | ||
| Hoarseness | LG2055 | 4789 | 165 | 3.4% | 4624 | 96.6% | <0.001 * |
| Placebo | 4621 | 246 | 5.3% | 4375 | 94.7% | ||
| Cough | LG2055 | 4789 | 637 | 13.3% | 4152 | 86.7% | 0.307 |
| Placebo | 4621 | 582 | 12.6% | 4039 | 87.4% | ||
| Headache | LG2055 | 4789 | 595 | 12.4% | 4194 | 87.6% | <0.001 * |
| Placebo | 4621 | 425 | 9.2% | 4196 | 90.8% | ||
| Feeling tired | LG2055 | 4789 | 743 | 15.5% | 4046 | 84.5% | 0.314 |
| Placebo | 4621 | 752 | 16.3% | 3869 | 83.7% | ||
| Chill | LG2055 | 4789 | 86 | 1.8% | 4703 | 98.2% | <0.001 * |
| Placebo | 4621 | 252 | 5.5% | 4369 | 94.5% | ||
| Fever | LG2055 | 4789 | 60 | 1.3% | 4729 | 98.7% | 0.503 |
| Placebo | 4621 | 51 | 1.1% | 4570 | 98.9% | ||
| Group | n | Bad | Good | p-Value | ||
|---|---|---|---|---|---|---|
| Cumulative Days | Ratio | Cumulative Days | Ratio | |||
| LG2055 | 4789 | 2779 | 58.0% | 2010 | 42.0% | <0.001 * |
| Placebo | 4621 | 2915 | 63.1% | 1706 | 36.9% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobatake, E.; Iwama, Y.; Arai, T.; Tsukisaka, Y.; Kabuki, T. Lactobacillus paragasseri SBT2055 Activates Plasmacytoid Dendritic Cells and Improves Subjective Symptoms of Common Cold in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Parallel-Group Comparative Trial. Nutrients 2023, 15, 4458. https://doi.org/10.3390/nu15204458
Kobatake E, Iwama Y, Arai T, Tsukisaka Y, Kabuki T. Lactobacillus paragasseri SBT2055 Activates Plasmacytoid Dendritic Cells and Improves Subjective Symptoms of Common Cold in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Parallel-Group Comparative Trial. Nutrients. 2023; 15(20):4458. https://doi.org/10.3390/nu15204458
Chicago/Turabian StyleKobatake, Eiji, Yoshitaka Iwama, Toshinobu Arai, Yuki Tsukisaka, and Toshihide Kabuki. 2023. "Lactobacillus paragasseri SBT2055 Activates Plasmacytoid Dendritic Cells and Improves Subjective Symptoms of Common Cold in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Parallel-Group Comparative Trial" Nutrients 15, no. 20: 4458. https://doi.org/10.3390/nu15204458
APA StyleKobatake, E., Iwama, Y., Arai, T., Tsukisaka, Y., & Kabuki, T. (2023). Lactobacillus paragasseri SBT2055 Activates Plasmacytoid Dendritic Cells and Improves Subjective Symptoms of Common Cold in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Parallel-Group Comparative Trial. Nutrients, 15(20), 4458. https://doi.org/10.3390/nu15204458






