Considerations of Low Carbohydrate Availability (LCA) to Relative Energy Deficiency in Sport (RED-S) in Female Endurance Athletes: A Narrative Review
Abstract
:1. Introduction
2. Low Energy Availability
2.1. Female Athlete Triad (Triad)
2.2. Relative Energy Deficiency in Sport (RED-S)
2.3. The Role of LCA in LEA
3. CHO Background
3.1. Physiological and Biochemical Role of Carbohydrates
3.2. Metabolism of CHO
3.3. Current CHO Recommendations
3.4. Prevalence & Current Intake of CHO
4. Low Carbohydrate Availability (LCA) Themes
4.1. Literature Search
4.1.1. Methodology of Search
4.1.2. Results of Search
4.2. Results
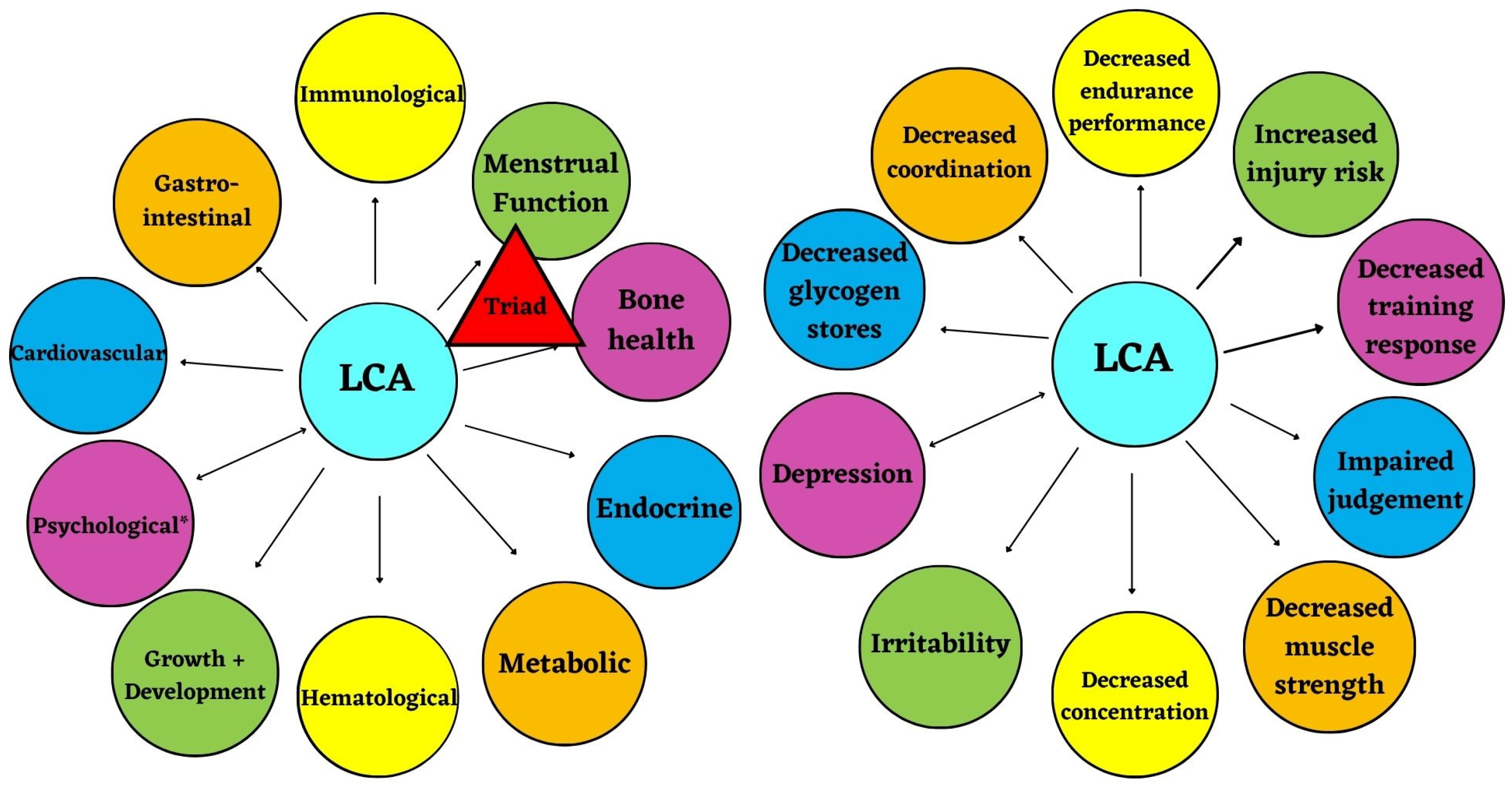
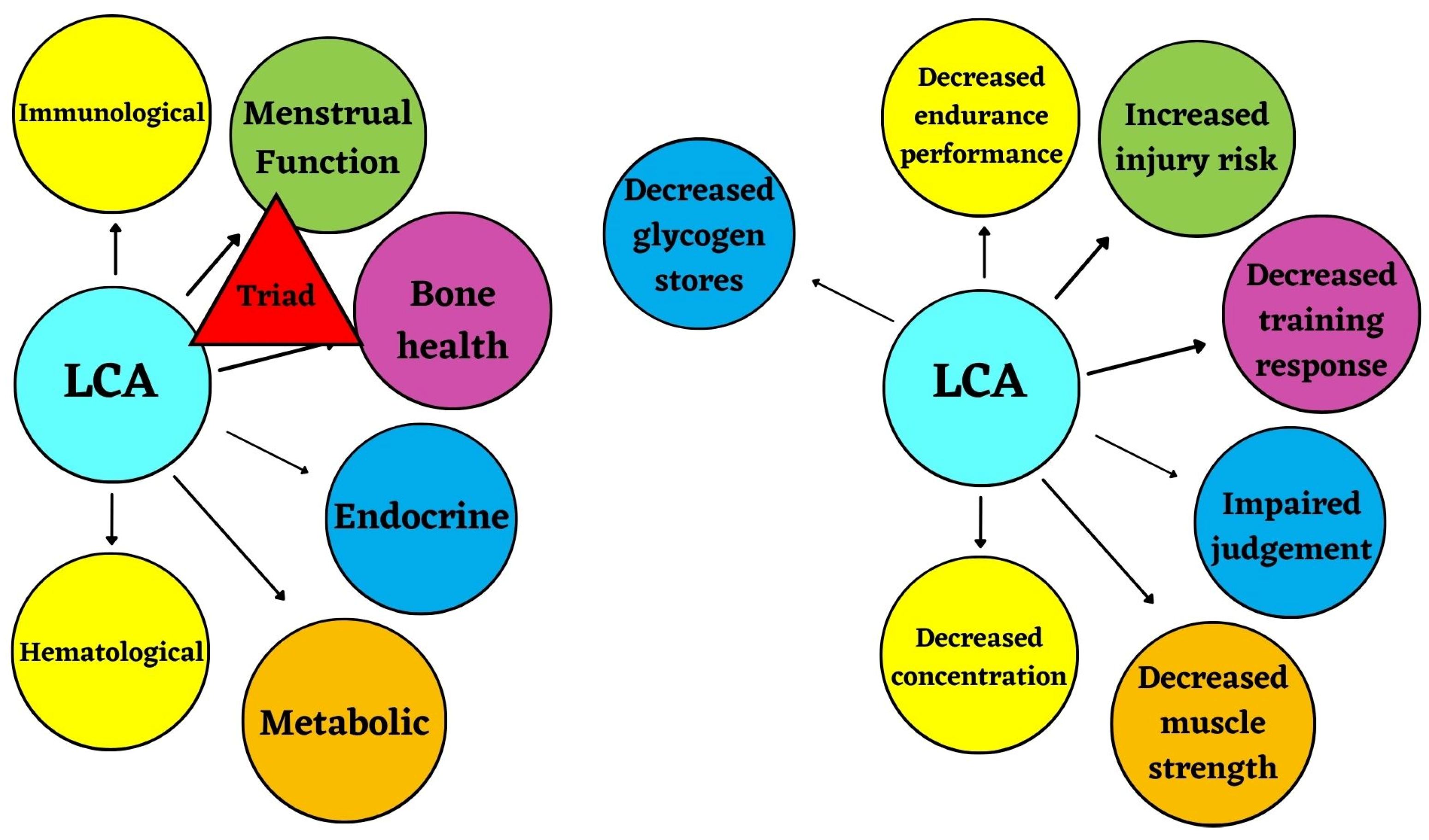
4.2.1. Health Consequences of LCA
4.2.2. Performance Consequences of LCA
5. Population Considerations
5.1. Male vs. Female Populations
5.2. Menstrual Cycle Considerations
5.3. Controversy of Low vs. High Carbohydrate Diets
6. Limitations
7. Future Directions
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grabmüllerová, A. Social Media and the Olympics: A Chance for Improving Gender Equality. Front. Sport Act. Living 2022, 4, 825440. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.; Bieuzen, F.; Bleakley, C. Where are all the female participants in Sports and Exercise Medicine research? Psychol. Soc. Sci. Humanit. 2014, 14, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Cowley, E.S.; Olenick, A.A.; McNulty, K.L.; Ross, E.Z. “Invisible Sportswomen”: The Sex Data Gap in Sport and Exercise Science Research. Women Sport Phys. Act. J. 2021, 29, 146–151. [Google Scholar] [CrossRef]
- Kuikman, M.A.; Smith, E.S.; Mckay, A.K.A.; Ackerman, K.E.; Harris, R.; Elliott-Sale, K.J.; Stellingwerff, T.; Burke, L.M. Fuelling the Female Athlete: Auditing her Representation in Studies of Acute Carbohydrate Intake for Exercise. Med. Sci. Sport Exerc. 2022, 55, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, B.; Ackerman, K.E. Recommendations and Nutritional Considerations for Female Athletes: Health and Performance. Sport Med. 2021, 51, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad—Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 47, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Logue, D.; Madigan, S.M.; Delahunt, E.; Heinen, M.; Mc Donnell, S.J.; Corish, C.A. Low Energy Availability in Athletes: A Review of Prevalence, Dietary Patterns, Physiological Health, and Sports Performance. Sports Med. 2018, 48, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef]
- De Souza, M.J.; Nattiv, A.; Joy, E.; Misra, M.; I Williams, N.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br. J. Sports Med. 2014, 48, 289. [Google Scholar] [CrossRef]
- Otis, C.L.; Drinkwater, B.; Johnson, M.; Loucks, A.; Wilmore, J. ACSM Position Stand: The Female Athlete Triad. Med. Sci. Sport Exerc. 1997, 29, i–ix. [Google Scholar] [CrossRef]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P. The Female Athlete Triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [CrossRef]
- Beals, K.A.; Manore, M.M. Disorders of the Female Athlete Triad Among Collegiate Athletes. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Carson, T.L.; West, B.T.; Sonneville, K.; Zernicke, R.F.; Clarke, P.; Harlow, S.; Karvonen-Gutierrez, C. Identifying latent classes of Relative Energy Deficiency in Sport (RED-S) consequences in a sample of collegiate female cross country runners. Br. J. Sports Med. 2022, 57, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Brook, E.M.; Tenforde, A.S.; Broad, E.M.; Matzkin, E.G.; Yang, H.Y.; Collins, J.E.; Blauwet, C.A. Low energy availability, menstrual dysfunction, and impaired bone health: A survey of elite para athletes. Scand. J. Med. Sci. Sport. 2019, 29, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Barrack, M.T.; Gibbs, J.C.; De Souza, M.J.; Williams, N.I.; Nichols, J.F.; Rauh, M.J.; Nattiv, A. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: A prospective multisite study of exercising girls and women. Am. J. Sports Med. 2014, 42, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Elliott-Sale, K.J.; Parsons, A.; Tang, J.C.; Greeves, J.P.; Fraser, W.D.; Sale, C. Effects of reduced energy availability on bone metabolism in women and men. Bone 2017, 105, 191–199. [Google Scholar] [CrossRef]
- Holtzman, B.; Tenforde, A.S.; Parziale, A.L.; Ackerman, K.E. Characterization of Risk Quantification Differences Using Female Athlete Triad Cumulative Risk Assessment and Relative Energy Deficiency in Sport Clinical Assessment Tool. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 569–575. [Google Scholar] [CrossRef]
- Hutson, M.J.; O’Donnell, E.; Brooke-Wavell, K.; Sale, C.; Blagrove, R.C. Effects of Low Energy Availability on Bone Health in Endurance Athletes and High-Impact Exercise as A Potential Countermeasure: A Narrative Review. Sport Med. 2021, 51, 391–403. [Google Scholar] [CrossRef]
- Stenqvist, T.B.; Melin, A.K.; Garthe, I.; Slater, G.; Paulsen, G.; Iraki, J.; Areta, J.; Torstveit, M.K. Prevalence of Surrogate Markers of Relative Energy Deficiency in Male Norwegian Olympic-Level Athletes. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 497–506. [Google Scholar] [CrossRef]
- Peklaj, E.; Reščič, N.; Koroušic´ Seljak, B.; Rotovnik Kozjek, N. Is RED-S in athletes just another face of malnutrition? Clin. Nutr. ESPEN 2022, 48, 298–307. [Google Scholar] [CrossRef]
- Hawley, J.A. Manipulating Carbohydrate Availability to Promote Training Adaptation. Sport Sci. Exch. 2014, 27, 1–7. [Google Scholar]
- Hawley, J.A.; Burke, L.M. Carbohydrate Availability and Training Adaptation: Effects on Cell Metabolism. Exerc. Sport Sci. Rev. 2010, 38, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Hoerner, N.R.; Gibbs, J.C.; Zinner, C.; Braun, H.; De Souza, M.J.; Schaenzer, W. Low energy availability in exercising men is associated with reduced leptin and insulin but not with changes in other metabolic hormones. J. Sports Sci. 2016, 34, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Ishibashi, A.; Tanabe, Y.; Iwayama, K.; Kamei, A.; Takahashi, H.; Goto, K. Muscle Glycogen Content during Endurance Training under Low Energy Availability. Med. Sci. Sport Exerc. 2020, 52, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.C.; Stuart-Hill, L.; Martin, S.; Gaul, C. Nutrition Status of Junior Elite Canadian Female Soccer Athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.L.; Albracht-Schulte, K.; Robert-McComb, J.J. Micronutrient deficiency in athletes and inefficiency of supplementation: Is low energy availability a culprit? PharmaNutrition 2020, 14, 100229. [Google Scholar] [CrossRef]
- McHaffie, S.J.; Langan-Evans, C.; Morehen, J.C.; Strauss, J.A.; Areta, J.L.; Rosimus, C.; Evans, M.; Elliott-Sale, K.J.; Cronin, C.J.; Morton, J.P. Carbohydrate fear, skinfold targets and body image issues: A qualitative analysis of player and stakeholder perceptions of the nutrition culture within elite female soccer. Sci. Med. Footb. 2022, 6, 675–685. [Google Scholar] [CrossRef]
- Harris, A. Longitudinal Assessment of Dietary Intake in Collegiate Cross Country Runners Throughout a Competitive Season. Bachelor’s Honors Thesis, Florida State University, Tallahassee, FL, USA, 2021. [Google Scholar]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.-C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef]
- Alghannam, A.F.; Ghaith, M.M.; Alhussain, M.H. Regulation of Energy Substrate Metabolism in Endurance Exercise. Int. J. Environ. Res. Public Health 2021, 18, 4963. [Google Scholar] [CrossRef]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Charlton, B.T.; Forsyth, S.; Clarke, D.C. Low Energy Availability and Relative Energy Deficiency in Sport: What Coaches Should Know. Int. J. Sport. Sci. Coach. 2022, 17, 445–460. [Google Scholar] [CrossRef]
- Viner, R.T.; Harris, M.; Berning, J.R.; Meyer, N.L. Energy availability and dietary patterns of adult male and female competitive cyclists with lower than expected bone mineral density. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Mckay, A.K.A.; Peeling, P.; Pyne, D.B.; Welvaert, M.; Tee, N.; Leckey, J.J.; Sharma, A.P.; Ross, M.L.R.; Garvican-Lewis, L.A.; Swinkels, D.W.; et al. Chronic Adherence to a Ketogenic Diet Modifies Iron Metabolism in Elite Athletes. Med. Sci. Sports Exerc. 2019, 51, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Mckay, A.K.A.; Peeling, P.; Pyne, D.B.; Tee, N.; Whitfield, J.; Sharma, A.P.; Heikura, I.A.; Burke, L.M. Six Days of Low Carbohydrate, Not Energy Availability, Alters the Iron and Immune Response to Exercise in Elite Athletes. Med. Sci. Sports Exerc. 2022, 54, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Macdermid, P.W.; Stannard, S.R. A Whey-Supplemented, High-Protein Diet Versus a High-Carbohydrate Diet: Effects on Endurance Cycling Performance. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.M.; Sale, C.; Fraser, W.; Tang, J.; Shepherd, S.O.; Strauss, J.A.; Close, G.L.; Cocks, M.; Louis, J.; Pugh, J.; et al. Post-exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: Implications for training adaptation. J. Physiol. 2019, 597, 4779–4796. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.; Elliott-Sale, K.J.; Currell, K.; Tang, J.; Fraser, W.D.; Sale, C. The effect of postexercise carbohydrate and protein ingestion on bone metabolism. Med. Sci. Sports Exerc. 2017, 49, 1209–1218. [Google Scholar] [CrossRef]
- Heikura, I.A.; Burke, L.M.; Hawley, J.A.; Ross, M.L.; Garvican-Lewis, L.; Sharma, A.P.; McKay, A.K.A.; Leckey, J.J.; Welvaert, M.; McCall, L.; et al. A Short-Term Ketogenic Diet Impairs Markers of Bone Health in Response to Exercise. Front. Endocrinol. 2020, 10, 880. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Acute dietary carbohydrate manipulation and the subsequent inflammatory and hepcidin responses to exercise. Eur. J. Appl. Physiol. 2015, 115, 2521–2530. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Sim, M.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Seven days of high carbohydrate ingestion does not attenuate post-exercise IL-6 and hepcidin levels. Eur. J. Appl. Physiol. 2016, 116, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Fensham, N.C.; Heikura, I.A.; McKay, A.K.A.; Tee, N.; Ackerman, K.E.; Burke, L.M. Short-Term Carbohydrate Restriction Impairs Bone Formation at Rest and During Prolonged Exercise to a Greater Degree than Low Energy Availability. J. Bone Miner. Res. 2022, 37, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Heikura, I.A.; Stellingwerff, T.; Areta, J.L. Low energy availability in female athletes: From the lab to the field. Eur. J. Sport Sci. 2022, 22, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Hilton, L.K.; Loucks, A.B. Low energy availability, not exercise stress, suppresses the diurnal rhythm of leptin in healthy young women. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E43–E49. Available online: http://www.ajpendo.org (accessed on 14 September 2022). [CrossRef] [PubMed]
- Mata, F.; Valenzuela, P.L.; Gimenez, J.; Tur, C.; Ferreria, D.; Domínguez, R.; Sanchez-Oliver, A.J.; Sanz, J.M.M. Carbohydrate availability and physical performance: Physiological overview and practical recommendations. Nutrients 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Bytomski, J.R. Fueling for Performance. Sports Health 2018, 10, 47–53. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine, Academy of Nutrition and Dietetics, Dietitians of Canada. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Beermann, B.L.; Lee, D.G.; Almstedt, H.C.; McCormack, W.P. Nutritional Intake and Energy Availability of Collegiate Distance Runners. J. Am. Coll. Nutr. 2020, 39, 747–755. [Google Scholar] [CrossRef]
- Condo, D.; Lohman, R.; Kelly, M.; Carr, A. Nutritional Intake, Sports Nutrition Knowledge and Energy Availability in Female Australian Rules Football Players. Nutrients 2019, 11, 971. [Google Scholar] [CrossRef]
- Nunes, C.L.; Matias, C.N.; Santos, D.A.; Morgado, J.P.; Monteiro, C.P.; Sousa, M.; Minderico, C.S.; Rocha, P.M.; St-Onge, M.-P.; Sardinha, L.B.; et al. Characterization and comparison of nutritional intake between preparatory and competitive phase of highly trained athletes. Medicina 2018, 54, 41. [Google Scholar] [CrossRef]
- Heikura, I.A.; Stellingwerff, T.; Mero, A.A.; Uusitalo, A.L.T.; Burke, L.M. A mismatch between athlete practice and current sports nutrition guidelines among elite female and male middle- and long-distance athletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Sale, C.; Varley, I.; Jones, T.W.; James, R.M.; Tang, J.C.Y.; Fraser, W.D.; Greeves, J.P. Effect of carbohydrate feeding on the bone metabolic response to running. J. Appl. Physiol. 2015, 119, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Appaneal, R.N.; Hughes, D.; Vlahovich, N.; Waddington, G.; Burke, L.M.; Drew, M. Prevalence of impaired physiological function consistent with Relative Energy Deficiency in Sport (RED-S): An Australian elite and pre-elite cohort. Br. J. Sports Med. 2021, 55, 38–45. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.A.; Pyne, D.B.; Burke, L.M.; Peeling, P. Iron metabolism: Interactions with energy and carbohydrate availability. Nutrients 2020, 12, 3692. [Google Scholar] [CrossRef]
- Petkus, D.L.; Murray-Kolb, L.E.; De Souza, M.J. The Unexplored Crossroads of the Female Athlete Triad and Iron Deficiency: A Narrative Review. Sport Med. 2017, 47, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Lynne Walker, J.; Heigenhauser, G.J.F.; Hultman, E.; Spriet, L.L.; Lynne, J. Dietary carbohydrate, muscle glycogen content, and endurance performance in well-trained women. J. Appl. Physiol. 2000, 88, 2151–2158. Available online: http://www.jap.org (accessed on 14 September 2022). [CrossRef] [PubMed]
- Mclay, R.T.; Thomson, C.D.; Williams, S.M.; Rehrer, N.J. Carbohydrate Loading and Female Endurance Athletes: Effect of Menstrual-Cycle Phase. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 189–205. [Google Scholar] [CrossRef]
- James, A.P.; Lorraine, M.; Cullen, D.; Goodman, C.; Dawson, B.; Palmer, N.T.; Fournier, P.A. Muscle glycogen supercompensation: Absence of a gender-related difference. Eur. J. Appl. Physiol. 2001, 85, 533–538. [Google Scholar] [CrossRef]
- Paul, D.R.; Mulroy, S.M.; Horner, J.A.; Jacobs, K.A.; Lamb, D.R. Carbohydrate-Loading During the Follicular Phase of the Menstrual Cycle: Effects on Muscle Glycogen and Exercise Performance. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 430–441. [Google Scholar] [CrossRef]
- Randell, R.K.; Spriet, L.L. Nutritional Factors That Affect Fat Oxidation Rates During Exercise. Sport Sci. Exch. 2020, 33, 1–5. [Google Scholar]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef]
- Burke, L. Low carb high fat (LCHF) diets for athletes—Third time lucky? J. Sci. Med. Sport. 2017, 20, S1. [Google Scholar] [CrossRef]
- Seid, H.; Rosenbaum, M. Low Carbohydrate and Low-Fat Diets: What We Don’t Know and Why we Should Know It. Nutrients 2019, 11, 2749. [Google Scholar] [CrossRef]
- McSwiney, F.T.; Doyle, L.; Plews, D.J.; Zinn, C. Impact Of Ketogenic Diet On Athletes: Current Insights. Open Access J. Sport Med. 2019, 10, 171–183. [Google Scholar] [CrossRef]
- Burke, L.M.; Sharma, A.P.; Heikura, I.A.; Forbes, S.F.; Holloway, M.; McKay, A.K.A.; Bone, J.L.; Leckey, J.J.; Welvaert, M.; Ross, M.L. Crisis of confidence averted: Impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS ONE 2020, 15, e0235592. [Google Scholar] [CrossRef]
| Source | Energy Intake or Energy Availability in Female Athletes | Carbohydrate Intake in Female Athletes |
|---|---|---|
| American College of Sports Medicine (ACSM), Academy of Nutrition and Dietetics (AND), and Dietitians of Canada (DC) | Adequate EA ≥ 45 kcal/kg FFM/day LEA < 30 kcal/kg FFM/day | 6–10 g CHO/kg BW/day (athletes) |
| Recommendations and Nutritional Considerations for Female Athletes: Health and Performance | Adequate EA ≥ 45 kcal/kg FFM/day Subclinical LEA 30–45 kcal/kg FFM/day LEA < 30 kcal/kg FFM/day | 7–10 g CHO/kg BW/day |
| International Olympic Committee (IOC) | Adequate EA ≥ 45 kcal/kg FFM/day Subclinical LEA 30–45 kcal/kg FFM/day LEA < 30 kcal/kg FFM/day | 5–7 g CHO/kg BW/day (moderate exercise program, ~1 h/day); or 6–10 g CHO/kg BW/day (endurance program, moderate to high intensity, 1–3 h/day) |
| International Society of Sports Nutrition (ISSN) | N/A | 5–8 g CHO/kg BW/day (moderate- to high-intensity volume, 2–3 h/day, 5–6 times a week); or 8–10 g CHO/kg BW/day (high volume, intense exercise, 3–6 h/day, 1–2 sessions, 5–6 times a week) |
| 2020–2025 Dietary Guidelines for Americans Advisory Committee | EI: 2000–2400 kcal/day (females, moderately active to active, aged 18–50 years) | 45–65% of total calories from carbohydrates (females, aged 18–50 years) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodge, M.T.; Ward-Ritacco, C.L.; Melanson, K.J. Considerations of Low Carbohydrate Availability (LCA) to Relative Energy Deficiency in Sport (RED-S) in Female Endurance Athletes: A Narrative Review. Nutrients 2023, 15, 4457. https://doi.org/10.3390/nu15204457
Lodge MT, Ward-Ritacco CL, Melanson KJ. Considerations of Low Carbohydrate Availability (LCA) to Relative Energy Deficiency in Sport (RED-S) in Female Endurance Athletes: A Narrative Review. Nutrients. 2023; 15(20):4457. https://doi.org/10.3390/nu15204457
Chicago/Turabian StyleLodge, Melissa T., Christie L. Ward-Ritacco, and Kathleen J. Melanson. 2023. "Considerations of Low Carbohydrate Availability (LCA) to Relative Energy Deficiency in Sport (RED-S) in Female Endurance Athletes: A Narrative Review" Nutrients 15, no. 20: 4457. https://doi.org/10.3390/nu15204457
APA StyleLodge, M. T., Ward-Ritacco, C. L., & Melanson, K. J. (2023). Considerations of Low Carbohydrate Availability (LCA) to Relative Energy Deficiency in Sport (RED-S) in Female Endurance Athletes: A Narrative Review. Nutrients, 15(20), 4457. https://doi.org/10.3390/nu15204457






