Fructose Diet-Associated Molecular Alterations in Hypothalamus of Adolescent Rats: A Proteomic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Protein Extraction
2.4. Proteomic Analysis
2.5. Western Blotting
2.6. Analysis of Tumor Necrosis Factor Alpha (TNF-alpha) and Interleukin 6 (IL-6)
2.7. Evaluation of Nitro-Tyrosine and Haptoglobin (Hpt)
2.8. Statistical Analysis
3. Results
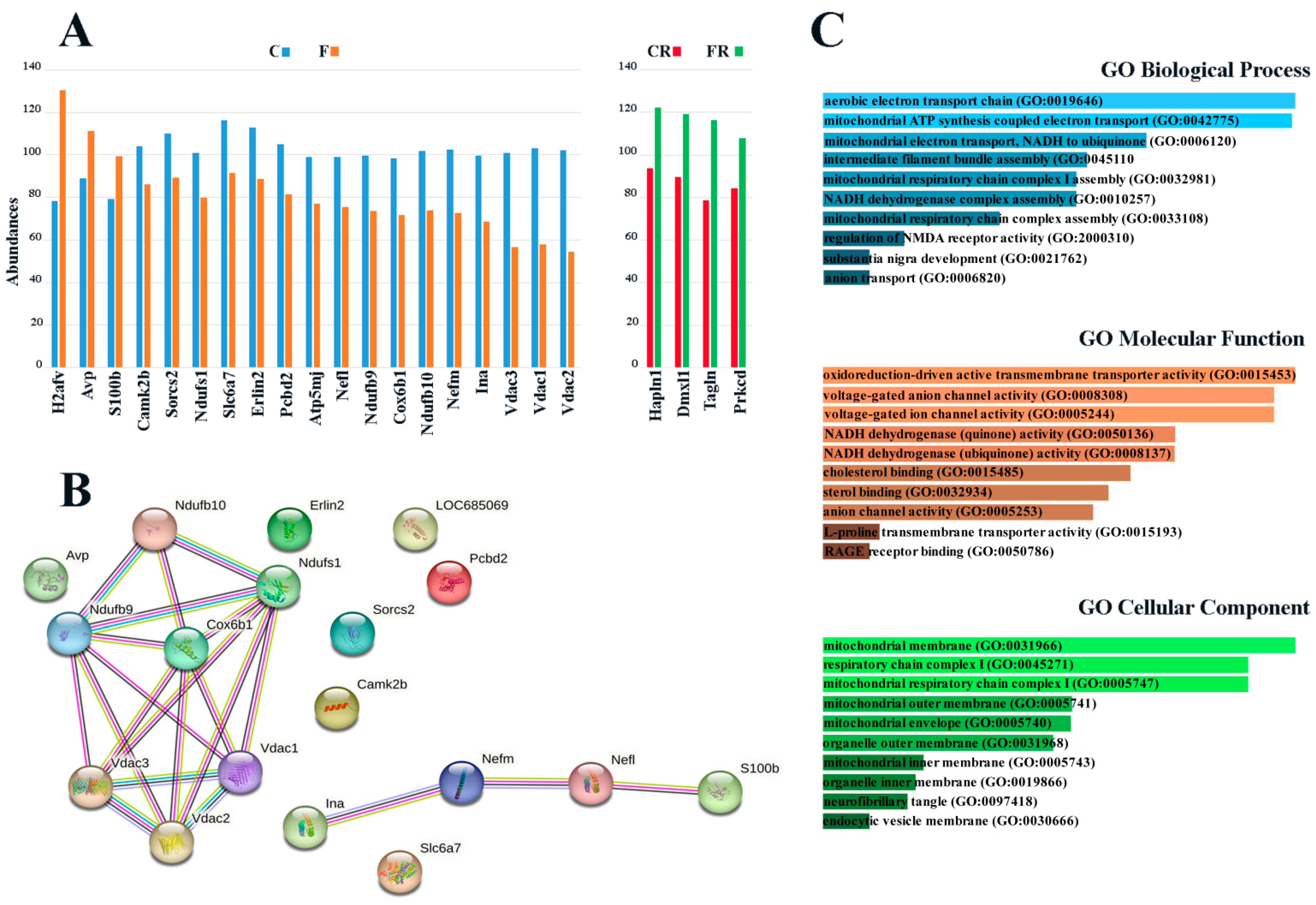
3.1. Identification of Fructose-Induced Hypothalamic Changes by Proteomic Analysis
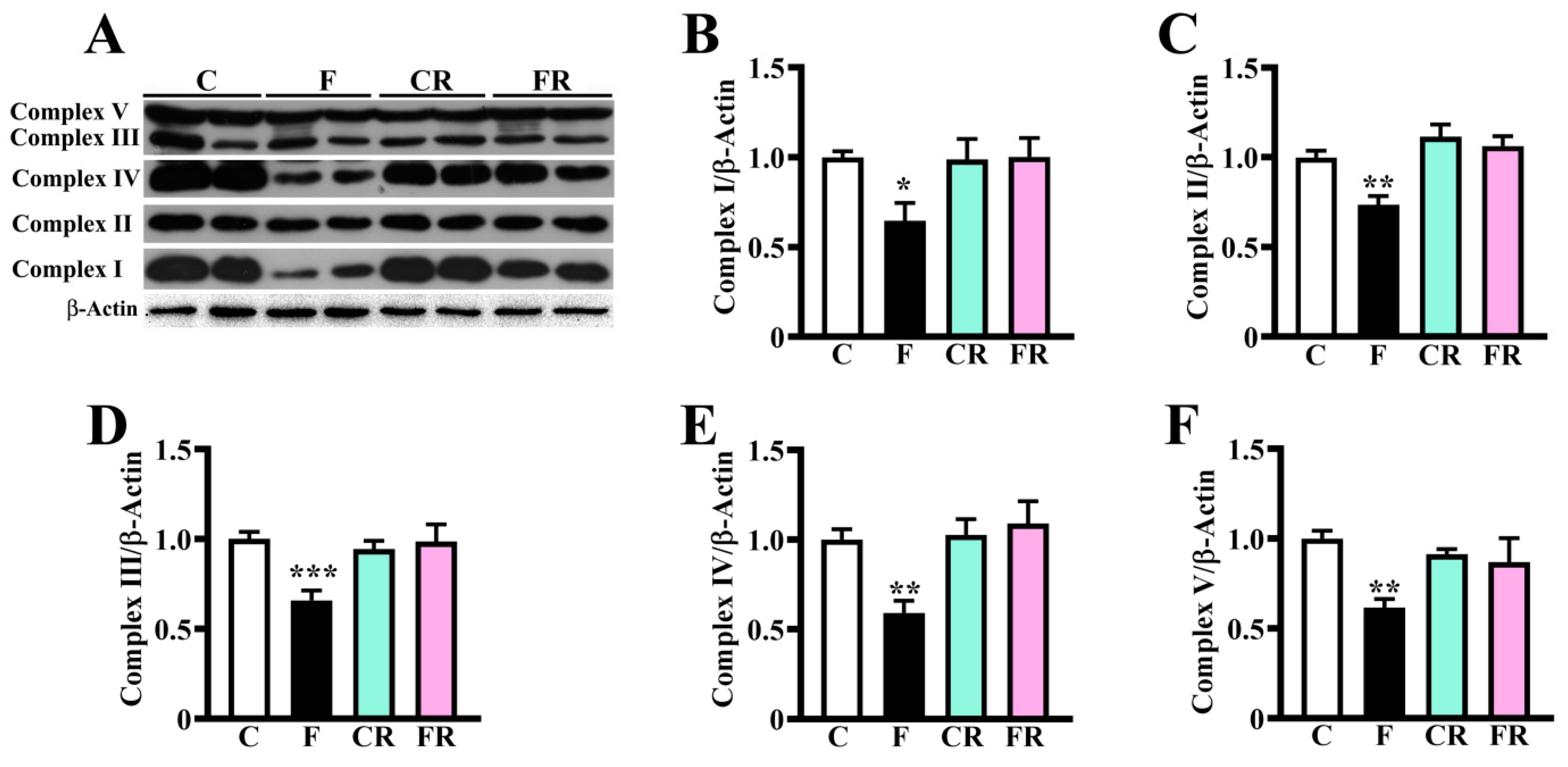
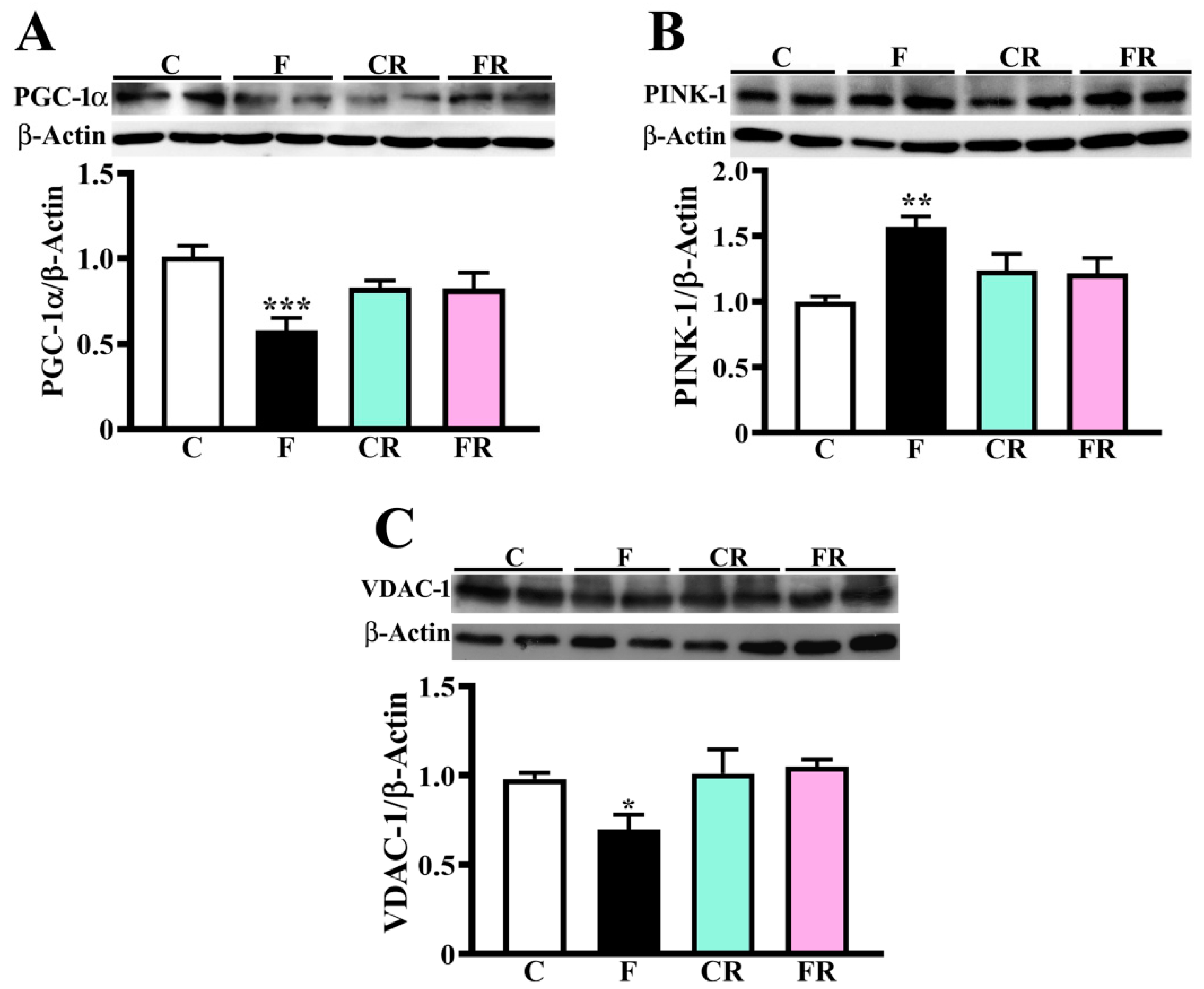
3.2. Reduced Amount of Mitochondrial Respiratory Complexes, PGC-1α and VDAC-1 and Higher Level of PINK-1 in Hypothalamus of Fructose-Fed Adolescent Rats
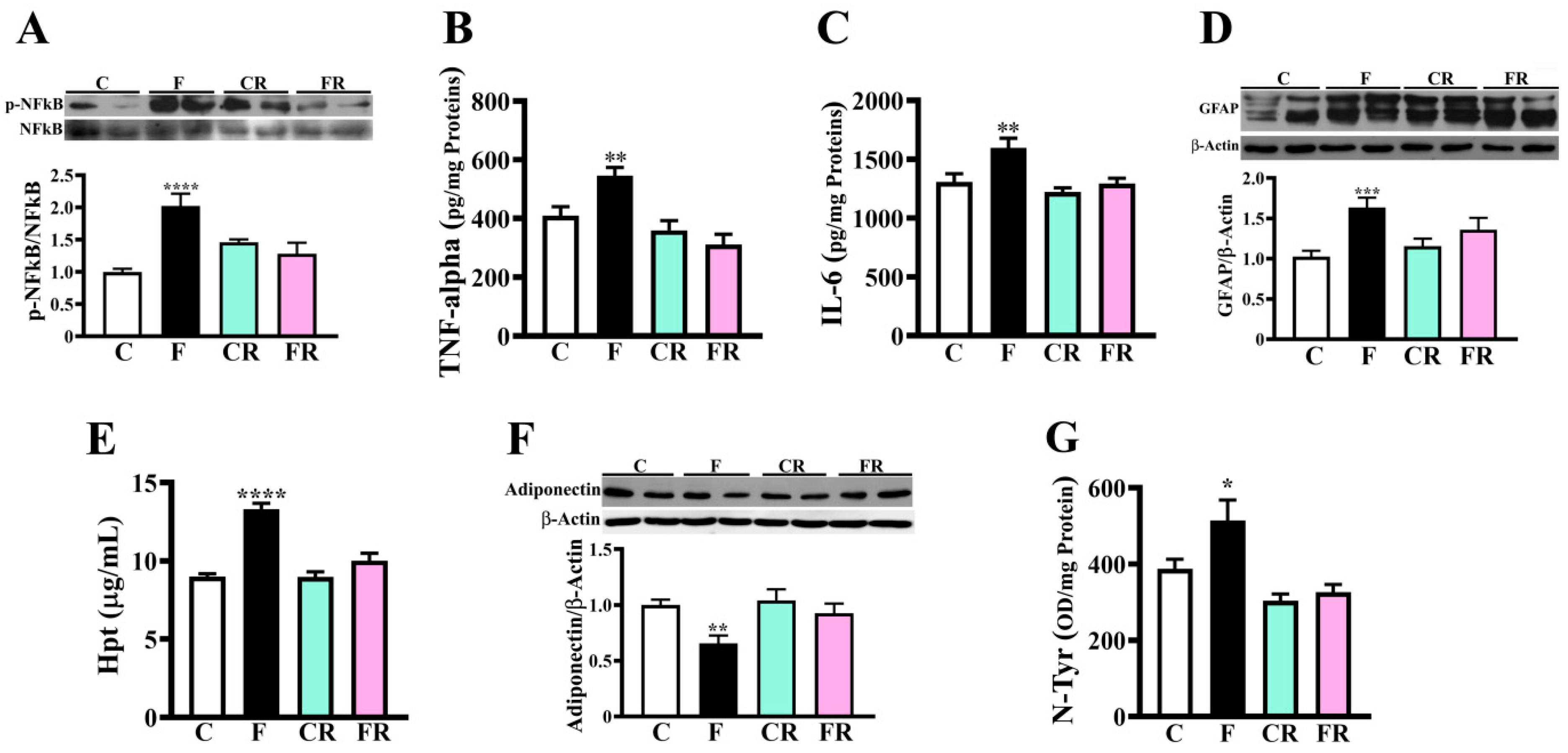
3.3. Increased Levels of Inflammatory and Oxidative Stress Markers in Hypothalamus of Fructose-Fed Adolescent Rats
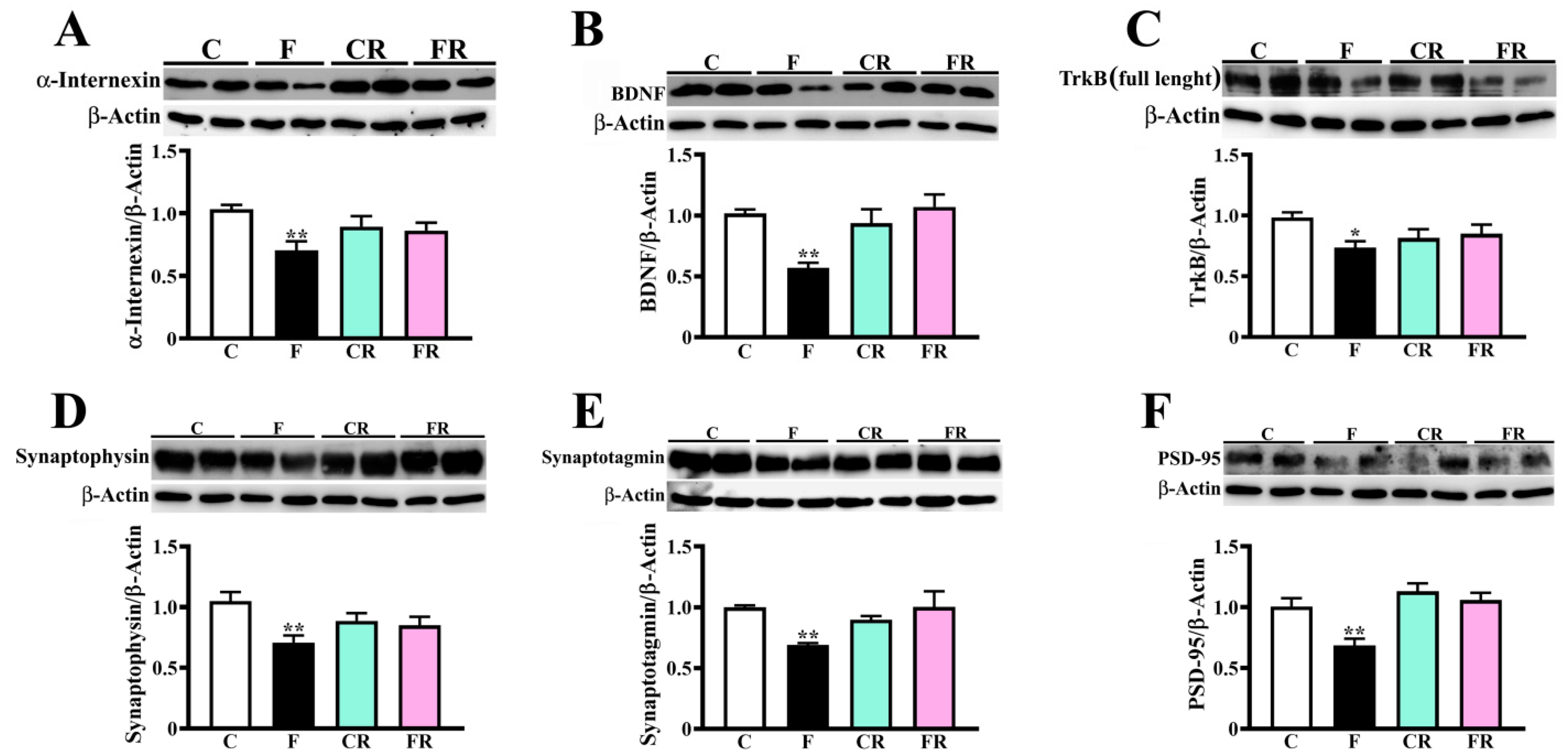
3.4. Decreased Amount of Neuronal Intermediate Filaments, BDNF, and Synaptic Markers in Hypothalamus of Fructose-Fed Adolescent Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.; Pellerin, L. Nutritional Impact on Metabolic Homeostasis and Brain Health. Front. Neurosci. 2022, 15, 767405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, K.; Chen, B.; Liu, R.; Cheng, S.; Zhang, Y.; Lu, J. Overnutrition Induced Cognitive Impairment: Insulin Resistance, Gut-Brain Axis, and Neuroinflammation. Front. Neurosci. 2022, 16, 884579. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Gomez-Pinilla, F.; Nagel, M.; Nakagawa, T.; Rodriguez-Iturbe, B.; Sanchez-Lozada, L.G.; Tolan, D.R.; Lanaspa, M.A. Cerebral Fructose Metabolism as a Potential Mechanism Driving Alzheimer’s Disease. Front Aging Neurosci. 2020, 12, 560865. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Cipolat, R.P.; Royes, L.F.F. Dietary fructose as a model to explore the influence of peripheral metabolism on brain function and plasticity. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166036. [Google Scholar] [CrossRef]
- Vos, M.B.; Kimmons, J.E.; Gillespie, C.; Welsh, J.; Blanck, H.M. Dietary fructose consumption among US children and adults: The third national health and nutrition examination survey. Medscape J. Med. 2008, 10, 160. [Google Scholar]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Mirtschink, P.; Jang, C.; Arany, Z.; Krek, W. Fructose metabolism, cardiometabolic risk, and the epidemic of coronary artery disease. Eur. Heart J. 2018, 39, 2497–2505. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Packard, C.J.; Borén, J. Dietary fructose and the metabolic syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, J.; Sun, Y.; Zhan, Q.; Zhang, D. High fructose diet: A risk factor for immune system dysregulation. Hum. Immunol. 2022, 83, 538–546. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Iossa, S.; Cigliano, L. Sweet but Bitter: Focus on Fructose Impact on Brain Function in Rodent Models. Nutrients 2020, 13, 1. [Google Scholar] [CrossRef]
- Cigliano, L.; Spagnuolo, M.S.; Crescenzo, R.; Cancelliere, R.; Iannotta, L.; Mazzoli, A.; Liverini, G.; Iossa, S. Short-Term Fructose Feeding Induces Inflammation and Oxidative Stress in the Hippocampus of Young and Adult Rats. Mol. Neurobiol. 2018, 55, 2869–2883. [Google Scholar] [CrossRef]
- Mazzoli, A.; Spagnuolo, M.S.; Nazzaro, M.; Gatto, C.; Iossa, S.; Cigliano, L. Fructose Removal from the Diet Reverses Inflammation, Mitochondrial Dysfunction, and Oxidative Stress in Hippocampus. Antioxidants 2021, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Bergamo, P.; Crescenzo, R.; Iannotta, L.; Treppiccione, L.; Iossa, S.; Cigliano, L. Brain Nrf2 pathway, autophagy, and synaptic function proteins are modulated by a short-term fructose feeding in young and adult rats. Nutr. Neurosci. 2020, 23, 309–320. [Google Scholar] [CrossRef]
- Van der Borght, K.; Köhnke, R.; Göransson, N.; Deierborg, T.; Brundin, P.; Erlanson-Albertsson, C.; Lindqvist, A. Reduced Neurogenesis in the Rat Hippocampus Following High Fructose Consumption. Regul. Pept. 2011, 167, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Konanur, V.R.; Taing, L.; Usui, R.; Kayser, B.D.; Goran, M.I.; Kanoski, S.E. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 2015, 25, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Ren, L.F.; Zhou, X.; Han, D.W. A high-fructose diet induces hippocampal insulin resistance and exacerbates memory deficits in male Sprague-Dawley rats. Nutr. Neurosci. 2015, 18, 323–328. [Google Scholar] [CrossRef]
- Harrell, C.S.; Zainaldin, C.; McFarlane, D.; Hyer, M.M.; Stein, D.; Sayeed, I.; Neigh, G.N. High-fructose diet during adolescent development increases neuroinflammation and depressive-like behavior without exacerbating outcomes after stroke. Brain Behav. Immun. 2018, 73, 340–351. [Google Scholar] [CrossRef]
- Clark, K.A.; Alves, J.M.; Jones, S.; Yunker, A.G.; Luo, S.; Cabeen, R.P.; Angelo, B.; Xiang, A.H.; Page, K.A. Dietary Fructose Intake and Hippocampal Structure and Connectivity during Childhood. Nutrients 2020, 12, 909. [Google Scholar] [CrossRef]
- Elmquist, J.K.; Coppari, R.; Balthasar, N.; Ichinose, M.; Lowell, B.B. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J. Comp. Neurol. 2005, 493, 63–71. [Google Scholar] [CrossRef]
- Marty, N.; Dallaporta, M.; Thorens, B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology 2007, 22, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.S.; Burgado, J.; Kelly, S.D.; Johnson, Z.P.; Neigh, G.N. High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats. Psychoneuroendocrinology 2015, 62, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Pan, Y.; Wang, R.; Kang, L.L.; Xue, Q.C.; Wang, X.N.; Kong, L.D. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats. J. Nutr. Biochem. 2014, 25, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, S.; Nestorov, J.; Matić, G.; Elaković, I. Chronic Stress Combined with a Fructose Diet Reduces Hypothalamic Insulin Signaling and Antioxidative Defense in Female Rats. Neuroendocrinology 2019, 108, 278–290. [Google Scholar] [CrossRef]
- Bermejo-Millo, J.C.; Guimarães, M.R.M.; de Luxán-Delgado, B.; Potes, Y.; Pérez-Martínez, Z.; Díaz-Luis, A.; Caballero, B.; Solano, J.J.; Vega-Naredo, I.; Coto-Montes, A. High-Fructose Consumption Impairs the Redox System and Protein Quality Control in the Brain of Syrian Hamsters: Therapeutic Effects of Melatonin. Mol. Neurobiol. 2018, 55, 7973–7986. [Google Scholar] [CrossRef]
- Li, J.M.; Ge, C.X.; Xu, M.X.; Wang, W.; Yu, R.; Fan, C.Y.; Kong, L.D. Betaine recovers hypothalamic neural injury by inhibiting astrogliosis and inflammation in fructose-fed rats. Mol. Nutr. Food Res. 2015, 59, 189–202. [Google Scholar] [CrossRef]
- Lu, W.; Xu, Y.; Shao, X.; Gao, F.; Li, Y.; Hu, J.; Zuo, Z.; Shao, X.; Zhou, L.; Zhao, Y.; et al. Uric acid produces an inflammatory response through activation of NF-κB in the hypothalamus: Implications for the pathogenesis of metabolic disorders. Sci. Rep. 2015, 5, 12144. [Google Scholar] [CrossRef]
- Cargnin-Carvalho, A.; de Mello, A.H.; Bressan, J.B.; Backes, K.M.; Uberti, M.F.; Fogaça, J.B.; da Rosa Turatti, C.; Cavalheiro, E.K.F.F.; Vilela, T.C.; Rezin, G.T. Can fructose influence the development of obesity mediated through hypothalamic alterations? J. Neurosci. Res. 2020, 98, 1662–1668. [Google Scholar] [CrossRef]
- Bauer, L.L.; Murphy, M.R.; Wolf, B.W.; Fahey, G.C., Jr. Estimates of starch digestion in the rat small intestine differ from those obtained using in vitro time-sensitive starch fractionation assays. J. Nutr. 2003, 133, 2256–2261. [Google Scholar] [CrossRef]
- Sasaki, T.; Sotome, I.; Okadome, H. In vitro starch digestibility and in vivo glucose response of gelatinized potato starch in the presence of non-starch polysaccharides. Starch 2015, 67, 415–423. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Maresca, B.; Mollica, M.P.; Cavaliere, G.; Cefaliello, C.; Trinchese, G.; Esposito, M.G.; Scudiero, R.; Crispino, M.; Abrescia, P.; et al. Haptoglobin increases with age in rat hippocampus and modulates Apolipoprotein E mediated cholesterol trafficking in neuroblastoma cell lines. Front. Cell Neurosci. 2014, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Visconti, S.; D’Ambrosio, C.; Fiorillo, A.; Arena, S.; Muzi, C.; Zottini, M.; Aducci, P.; Marra, M.; Scaloni, A.; Camoni, L. Overexpression of 14-3-3 proteins enhances cold tolerance and increases levels of stress-responsive proteins of Arabidopsis plants. Plant Sci. 2019, 289, 110215. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Pallottini, V.; Mazzoli, A.; Iannotta, L.; Tonini, C.; Morone, B.; Ståhlman, M.; Crescenzo, R.; Strazzullo, M.; Iossa, S.; et al. A Short-Term Western Diet Impairs Cholesterol Homeostasis and Key Players of Beta Amyloid Metabolism in Brain of Middle-Aged Rats. Mol. Nutr. Food Res. 2020, 64, e2000541. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Maresca, B.; La Marca, V.; Carrizzo, A.; Veronesi, C.; Cupidi, C.; Piccoli, T.; Maletta, R.G.; Bruni, A.C.; Abrescia, P.; et al. Haptoglobin interacts with apolipoprotein E and beta-amyloid and influences their crosstalk. ACS Chem. Neurosci. 2014, 5, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Spagnuolo, M.S.; Cancelliere, R.; Iannotta, L.; Mazzoli, A.; Gatto, C.; Iossa, S.; Cigliano, L. Effect of Initial Aging and High-Fat/High-Fructose Diet on Mitochondrial Bioenergetics and Oxidative Status in Rat Brain. Mol. Neurobiol. 2019, 56, 7651–7663. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Donizetti, A.; Iannotta, L.; Aliperti, V.; Cupidi, C.; Bruni, A.C.; Cigliano, L. Brain-derived neurotrophic factor modulates cholesterol homeostasis and Apolipoprotein E synthesis in human cell models of astrocytes and neurons. J. Cell Physiol. 2018, 233, 6925–6943. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Mollica, M.P.; Maresca, B.; Cavaliere, G.; Cefaliello, C.; Trinchese, G.; Scudiero, R.; Crispino, M.; Cigliano, L. High fat diet and inflammation—Modulation of haptoglobin level in rat brain. Front. Cell Neurosci. 2015, 9, 479. [Google Scholar] [CrossRef]
- Mazzoli, A.; Spagnuolo, M.S.; Gatto, C.; Nazzaro, M.; Cancelliere, R.; Crescenzo, R.; Iossa, S.; Cigliano, L. Adipose Tissue and Brain Metabolic Responses to Western Diet-Is There a Similarity between the Two? Int. J. Mol. Sci. 2020, 21, 786. [Google Scholar] [CrossRef]
- Aquilano, K.; Sciarretta, F.; Turchi, R.; Li, B.H.; Rosina, M.; Ceci, V.; Guidobaldi, G.; Arena, S.; D’Ambrosio, C.; Audano, M.; et al. Low-protein/high-carbohydrate diet induces AMPK-dependent canonical and non-canonical thermogenesis in subcutaneous adipose tissue. Redox Biol. 2020, 36, 101633. [Google Scholar] [CrossRef]
- Li, Z.; Adams, R.M.; Chourey, K.; Hurst, G.B.; Hettich, R.L.; Pan, C. Systematic comparison of label-free, metabolic labeling, and isobaric chemical labeling for quantitative proteomics on LTQ Orbitrap Velos. J. Proteome Res. 2012, 11, 1582–1590. [Google Scholar] [CrossRef]
- Megger, D.A.; Pott, L.L.; Ahrens, M.; Padden, J.; Bracht, T.; Kuhlmann, K.; Eisenacher, M.; Meyer, H.E.; Sitek, B. Comparison of label-free and label-based strategies for proteome analysis of hepatoma cell lines. Biochim. Biophys. Acta 2014, 1844, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 2002, 286, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wareski, P.; Vaarmann, A.; Choubey, V.; Safiulina, D.; Liiv, J.; Kuum, M.; Kaasik, A. PGC-1α and PGC-1Β Regulate Mitochondrial Density in Neurons. J. Biol. Chem. 2009, 284, 21379–21385. [Google Scholar] [CrossRef]
- Martinez-Redondo, V.; Pettersson, A.T.; Ruas, J.L. The hitchhiker’s guide to PGC-1alpha isoform structure and biological functions. Diabetologia 2015, 58, 1969–1977. [Google Scholar] [CrossRef]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Camara, A.K.S.; Zhou, Y.; Wen, P.C.; Tajkhorshid, E.; Kwok, W.M. Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target. Front. Physiol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Van Eldik, L.J.; Wainwright, M.S. The Janus face of glial-derived S100B: Beneficial and detrimental functions in the brain. Rest. Neurol. Neurosci. 2003, 21, 97–108. [Google Scholar]
- Ponath, G.; Schettler, C.; Kaestner, F.; Voigt, B.; Wentker, D.; Arolt, V.; Rothermundt, M. Autocrine S100B effects on astrocytes are mediated via RAGE. J. Neuroimmunol. 2007, 184, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, R.; Giambanco, I.; Donato, R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol. Aging 2010, 31, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, S.; Xu, A.; George, J. The multifaceted and controversial immunometabolic actions of adiponectin. Trends Endocrinol. Metab. 2014, 25, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Bloemer, J.; Pinky, P.D.; Govindarajulu, M.; Hong, H.; Judd, R.; Amin, R.H.; Moore, T.; Dhanasekaran, M.; Reed, M.N.; Suppiramaniam, V. Role of adiponectin in central nervous system disorders. Neural. Plast. 2018, 2018, 4593530. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Liem, R.K.H.; Messing, A. Dysfunctions of neuronal and glial intermediate filaments in disease. J. Clin. Investig. 2009, 119, 1814–1824. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Glerup, S.; Bolcho, U.; Mølgaard, S.; Bøggild, S.; Vaegter, C.B.; Smith, A.H.; Nieto-Gonzalez, J.L.; Ovesen, P.L.; Pedersen, L.F.; Fjorback, A.N.; et al. SorCS2 is required for BDNF-dependent plasticity in the hippocampus. Mol. Psychiatry 2016, 21, 1740–1751. [Google Scholar] [CrossRef]
- Meng, Q.; Ying, Z.; Noble, E.; Zhao, Y.; Agrawal, R.; Mikhail, A.; Zhuang, Y.; Tyagi, E.; Zhang, Q.; Lee, J.H.; et al. Systems nutrigenomics reveals brain gene networks linking metabolic and brain disorders. EBioMedicine 2016, 7, 157–166. [Google Scholar] [CrossRef]
- Zhang, D.M.; Jiao, R.Q.; Kong, L.D. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef]
- Sindhunata, D.P.; Meijnikman, A.S.; Gerdes, V.E.A.; Nieuwdorp, M. Dietary fructose as a metabolic risk factor. Am. J. Physiol. Cell Physiol. 2022, 323, C847–C856. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.; Lallès, J.P.; Malbert, C.H.; Val-Laillet, D. Dietary sugars: Their detection by the gut-brain axis and their peripheral and central effects in health and diseases. Eur. J. Nutr. 2015, 54, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.C.; Martin, R.J.; Whitney, M.L.; Edwards, G.L. Intracerebroventricular injection of fructose stimulates feeding in rats. Nutr Neurosci. 2002, 5, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Wolfgang, M.; Tokutake, Y.; Chohnan, S.; Lane, M.D. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc. Natl. Acad. Sci. USA 2008, 105, 16871–16875. [Google Scholar] [CrossRef]
- Havel, P.J. Glucose but not fructose infusion increases circulating leptin in proportion to adipose stores in Rhesus monkeys. Exp. Clin. Endocrinol. Diabetes 1997, 105, 37–38. [Google Scholar] [CrossRef]
- Teff, K.L.; Elliott, S.S.; Tschop, M.; Kieffer, T.J.; Rader, D.; Heiman, M.; Townsend, R.R.; Keim, N.L.; D’Alessio, D.; Havel, P.J. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef]
- Figlewicz, D.P.; Bennett, J.; Evans, S.B.; Kaiyala, K.; Sipols, A.J.; Benoit, S.C. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav. Neurosci. 2004, 118, 479–487. [Google Scholar] [CrossRef]
- Ochoa, M.; Malbert, C.H.; Lallès, J.P.; Bobillier, E.; Val-Laillet, D. Effects of chronic intake of starch-, glucose- and fructose containing diets on eating behaviour in adult minipigs. Appl. Anim. Behav. Sci. 2014, 157, 61–71. [Google Scholar] [CrossRef]
- Shapiro, A.; Mu, W.; Roncal, C.; Cheng, K.Y.; Johnson, R.J.; Scarpace, P.J. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1370–R1375. [Google Scholar] [CrossRef]
- Bursać, B.N.; Vasiljević, A.D.; Nestorović, N.M.; Veličković, N.A.; Vojnović Milutinović, D.D.; Matić, G.M.; Djordjevic, A.D. High-fructose diet leads to visceral adiposity and hypothalamic leptin resistance in male rats-Do glucocorticoids play a role? J. Nutr. Biochem. 2014, 25, 446–455. [Google Scholar] [CrossRef]
- Lindqvist, A.; Baelemans, A.; Erlanson-Albertsson, C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul. Pept. 2008, 150, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Kong, L.D. High fructose diet-induced metabolic syndrome: Pathophysiological mechanism and treatment by traditional Chinese medicine. Pharmacol. Res. 2018, 130, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Exp. Med. Biol. 2012, 942, 3–37. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Zhou, Z.; Hagopian, K.; López-Domínguez, J.A.; Kim, K.; Jasoliya, M.; Roberts, M.N.; Cortopassi, G.A.; Showalter, M.R.; Roberts, B.S.; González-Reyes, J.A.; et al. A ketogenic diet impacts markers of mitochondrial mass in a tissue specific manner in aged mice. Aging 2021, 13, 7914–7930. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Maldonado, E.N.; Krelin, Y. Vdac1 at the crossroads of cell metabolism, apoptosis and cell stress. Cell Stress 2017, 1, 11–36. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Albensi, B.C. Dysfunction of mitochondria: Implications for Alzheimer’s disease. Int. Rev. Neurobiol. 2019, 145, 13–27. [Google Scholar] [CrossRef]
- Bianchi, R.; Kastrisianaki, E.; Giambanco, I.; Donato, R. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J. Biol. Chem. 2011, 286, 7214–7226. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.; Won, S.J.; Basu, J.; Kapfhamer, D.; Swanson, R.A. Microglial activation induced by the alarmin S100B is regulated by poly (ADP-ribose) polymerase-1. Glia 2016, 64, 1869–1878. [Google Scholar] [CrossRef]
- Rahman, M.H.; Kim, M.S.; Lee, I.K.; Yu, R.; Suk, K. Corrigendum: Interglial Crosstalk in Obesity-Induced Hypothalamic Inflammation. Front. Neurosci. 2019, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Bhusal, A.; Lee, W.H.; Lee, I.K.; Suk, K. Hypothalamic inflammation and malfunctioning glia in the pathophysiology of obesity and diabetes: Translational significance. Biochem. Pharmacol. 2018, 153, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Spezani, R.; da Silva, R.R.; Martins, F.F.; de Souza Marinho, T.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Intermittent fasting, adipokines, insulin sensitivity, and hypothalamic neuropeptides in a dietary overload with high-fat or high-fructose diet in mice. J. Nutr. Biochem. 2020, 83, 108419. [Google Scholar] [CrossRef] [PubMed]
- Raja Gopal Reddy, M.; Jeyakumar, S.M.; Vajreswari, A. Consumption of vitamin A-deficient diet elevates endoplasmic reticulum stress marker and suppresses high fructose-induced orexigenic gene expression in the brain of male Wistar rats. Nutr. Neurosci. 2022, 25, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A.; Gatto, C.; Crescenzo, R.; Cigliano, L.; Iossa, S. Prolonged Changes in Hepatic Mitochondrial Activity and Insulin Sensitivity by High Fructose Intake in Adolescent Rats. Nutrients 2021, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Samant, N.P.; Gupta, G.L. Adiponectin: A potential target for obesity-associated Alzheimer’s disease. Metab. Brain Dis. 2021, 36, 1565–1572. [Google Scholar] [CrossRef]
- Chan, K.-H.; Lam, K.S.-L.; Cheng, O.-Y.; Kwan, J.S.-C.; Ho, P.W.-L.; Cheng, K.K.-Y.; Chung, S.K.; Ho, J.W.-M.; Guo, V.Y.; Xu, A. Adiponectin is protective against oxidative stress induced cytotoxicity in amyloid-beta neurotoxicity. PLoS ONE 2012, 7, e52354. [Google Scholar] [CrossRef]
- Ng, R.C.-L.; Chan, K.-H. Potential Neuroprotective Effects of Adiponectin in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 592. [Google Scholar] [CrossRef]
- Lee, H.; Tu, T.H.; Park, B.S.; Yang, S.; Kim, J.G. Adiponectin Reverses the Hypothalamic Microglial Inflammation during Short-Term Exposure to Fat-Rich Diet. Int. J. Mol. Sci. 2019, 20, 5738. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.E.; Lowe, C.; Legler, K.; Benzler, J.; Boucsein, A.; Böttiger, G.; Grattan, D.R.; Williams, L.M.; Tups, A.; Grattan, D. Central Adiponectin Acutely Improves Glucose Tolerance in Male Mice. Endocrinology 2014, 155, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Czerwinska, J.; Bogacka, I.; Chojnowska, K.; Smolinska, N.; Dobrzyn, K.; Kiezuna, M.; Zaobidna, E.; Myszczynski, K.; Nowakowski, J.J.; et al. Sex- and season-dependent differences in the expression of adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) in the hypothalamic-pituitaryadrenal axis of the Eurasian beaver (Castor fiber L.). Gen. Comp. Endocrinol. 2020, 298, 113575. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.; Smolinska, N.; Maleszka, A.; Kiezun, M.; Dobrzyn, K.; Czerwinska, J.; Szeszko, K.; Nitkiewicz, A. Expression of Adiponectin and its Receptors in the Porcine Hypothalamus During the Oestrous Cycle. Reprod. Domest. Anim. 2014, 49, 378–386. [Google Scholar] [CrossRef]
- Abgrall, A.; Poizat, G.; Prevost, M.; Riffault, L.; De La Barrera, L.; Hanine, R.; Djordjevic, K.; Benomar, Y.; Taouis, M. Evidence for the Neuronal Expression and Secretion of Adiponectin. Cells 2022, 11, 2725. [Google Scholar] [CrossRef]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. Br. J. Pharm. 2012, 165, 313–327. [Google Scholar] [CrossRef]
- Qiu, G.; Wan, R.; Hu, J.; Mattson, M.P.; Spangler, E.; Liu, S.; Yau, S.S.Y.; Lee, T.M.C.; Gleichmann, M.; Ingram, D.K.; et al. Adiponectin protects rat hippocampal neurons against excitotoxicity. Age 2011, 33, 155–165. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; Hoo, R.L.C.; Ching, Y.P.; Christie, B.R.; Lee, T.M.C.; Xu, A.; So, K.-F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef]
- Zhu, Q.; Couillard-Despres, S.; Julien, J.P. Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Exp. Neurol. 1997, 148, 299–316. [Google Scholar] [CrossRef]
- Yum, S.W.; Zhang, J.; Mo, K.; Li, J.; Scherer, S.S. A novel recessive Nefl mutation causes a severe, early-onset axonal neuropathy. Ann. Neurol. 2009, 66, 759–770. [Google Scholar] [CrossRef]
- Yuan, A.; Sershen, H.; Veeranna; Basavarajappa, B.S.; Kumar, A.; Hashim, A.; Berg, M.; Lee, J.-H.; Sato, Y.; Rao, M.V.; et al. Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol. Psychiatry 2015, 20, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Sershen, H.; Veeranna; Basavarajappa, B.S.; Kumar, A.; Hashim, A.; Berg, M.; Lee, J.-H.; Sato, Y.; Rao, M.V.; et al. Functions of neurofilaments in synapses. Mol. Psychiatry 2015, 20, 915. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef] [PubMed]
- Puram, S.V.; Kim, A.H.; Ikeuchi, Y.; Wilson-Grady, J.T.; Merdes, A.; Gygi, S.P.; Azad, B.A. Unique CaMKIIβ Signaling Pathway at the Centrosome Regulates Dendrite Patterning in the Brain. Nat. Neurosci. 2011, 14, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Hisatsune, C.; Miyamoto, H.; Ogawa, N.; Mikoshiba, K. Regulation of spinogenesis in mature Purkinje cells via mGluR/PKC-mediated phosphorylation of CaMKII. Proc. Natl. Acad. Sci. USA 2017, 114, E5256–E5265. [Google Scholar] [CrossRef]
- Sałaciak, K.; Koszałka, A.; Żmudzka, E.; Pytka, K. The Calcium/Calmodulin-Dependent Kinases II and IV as Therapeutic Targets in Neurodegenerative and Neuropsychiatric Disorders. Int. J. Mol. Sci. 2021, 22, 4307. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Mazzoli, A.; Nazzaro, M.; Troise, A.D.; Gatto, C.; Tonini, C.; Colardo, M.; Segatto, M.; Scaloni, A.; Pallottini, V.; et al. Long-Lasting Impact of Sugar Intake on Neurotrophins and Neurotransmitters from Adolescence to Young Adulthood in Rat Frontal Cortex. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, J.; Milner, T.A.; Vonsattel, J.G.; Palko, M.E.; Tessarollo, L.; Hempstead, B.L. SorCS2-mediated NR2A trafficking regulates motor deficits in Huntington’s disease. JCI Insight 2017, 2, e88995. [Google Scholar] [CrossRef]
- Yoshii, A.; Constantine-Paton, M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat. Neurosci. 2007, 10, 702–711. [Google Scholar] [CrossRef]
- Hu, X.; Ballo, L.; Pietila, L.; Viesselmann, C.; Ballweg, J.; Lumbard, D.; Stevenson, M.; Merriam, E.; Dent, E.W. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J. Neurosci. 2011, 31, 15597–15603. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, R.; Yang, J.L.; Kamimura, N.; Son, T.G.; Ouyang, X.; Luo, Y.; Okun, E.; Mattson, M.P. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012, 3, 1250. [Google Scholar] [CrossRef] [PubMed]

| Ingredients (g/100 g) | Control Diet | Fructose Diet |
|---|---|---|
| Standard Chow a | 50.5 | 50.5 |
| Sunflower Oil | 1.5 | 1.5 |
| Casein | 9.2 | 9.2 |
| Alphacel | 9.8 | 9.8 |
| Cornstarch | 20.4 | - |
| Fructose | - | 20.4 |
| Water | 6.4 | 6.4 |
| AIN-76 mineral mix | 1.6 | 1.6 |
| AIN-76 vitamin mix | 0.4 | 0.4 |
| Choline | 0.1 | 0.1 |
| Methionine | 0.1 | 0.1 |
| Energy content and composition | ||
| Gross Energy Density (kJ/g) | 17.2 | 17.2 |
| ME content (kJ/g) b | 11.1 | 11.1 |
| Proteins (% ME) | 29.0 | 29.0 |
| Lipids (% ME) | 10.6 | 10.6 |
| Carbohydrates (% ME) | 60.4 | 60.4 |
| Of which: | ||
| Fructose | - | 30.0 |
| Starch | 52.8 | 22.8 |
| Sugars | 7.6 | 7.6 |
| Primary Antibody | Secondary Antibody | |
|---|---|---|
| GFAP | Cell Signalling Technology; 1:1000 a | GAR-HRP IgG; :100,000 f |
| Synaptophysin | Merk-Millipore; 1:100,000 b | GAR-HRP IgG; 1:35,000 a |
| Synaptotagmin I | Cell Signalling Technology; 1:1000 c | GAR-HRP IgG; :200,000 c |
| PSD-95 | Cell Signalling Technology; 1:1000 c | GAR-HRP IgG; 1:60,000 c |
| BDNF | Abcam, Cambridge, UK (EPR1292); 1:2000 d | GAR-HRP IgG; 1:180,000 a |
| PGC-1α | Merk-Millipore; 1:2000 b | GAR-HRP IgG; 1:40,000 b |
| TrkB | Santa Cruz Biotechnology; 1:2000 d | GAR-HRP IgG; :100,000 d |
| VDAC 1 | Santa Cruz Biotechnology; 1:500 d | GAM-HRP IgG; :50,000 b |
| α-internexin | Santa Cruz Biotechnology; 1:500 d | GAM-HRP IgG; :70,000 b |
| PINK1 | Santa Cruz Biotechnology; 1:500 d | GAM-HRP IgG; 1:40,000 b |
| OXPHOS | Abcam, Cambridge, UK; 1:400 b | GAM-HRP IgG; 1:70,000–1:200,000 a |
| pNFkB | Santa Cruz Biotechnology; 1:200 d | GAM-HRP IgG; 1:50,000 b |
| NFkB | Santa Cruz Biotechnology; 1:500 b | GAM-HRP IgG; 1:15,000 b |
| β-Actin | Sigma-Aldrich; 1:1000 e | GAM-HRP IgG; 1:30,000 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, C.; Cigliano, L.; Mazzoli, A.; Matuozzo, M.; Nazzaro, M.; Scaloni, A.; Iossa, S.; Spagnuolo, M.S. Fructose Diet-Associated Molecular Alterations in Hypothalamus of Adolescent Rats: A Proteomic Approach. Nutrients 2023, 15, 475. https://doi.org/10.3390/nu15020475
D’Ambrosio C, Cigliano L, Mazzoli A, Matuozzo M, Nazzaro M, Scaloni A, Iossa S, Spagnuolo MS. Fructose Diet-Associated Molecular Alterations in Hypothalamus of Adolescent Rats: A Proteomic Approach. Nutrients. 2023; 15(2):475. https://doi.org/10.3390/nu15020475
Chicago/Turabian StyleD’Ambrosio, Chiara, Luisa Cigliano, Arianna Mazzoli, Monica Matuozzo, Martina Nazzaro, Andrea Scaloni, Susanna Iossa, and Maria Stefania Spagnuolo. 2023. "Fructose Diet-Associated Molecular Alterations in Hypothalamus of Adolescent Rats: A Proteomic Approach" Nutrients 15, no. 2: 475. https://doi.org/10.3390/nu15020475
APA StyleD’Ambrosio, C., Cigliano, L., Mazzoli, A., Matuozzo, M., Nazzaro, M., Scaloni, A., Iossa, S., & Spagnuolo, M. S. (2023). Fructose Diet-Associated Molecular Alterations in Hypothalamus of Adolescent Rats: A Proteomic Approach. Nutrients, 15(2), 475. https://doi.org/10.3390/nu15020475








