The Effect of Nerolidol Renal Dysfunction following Ischemia–Reperfusion Injury in the Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Ischemia–Reperfusion Injury
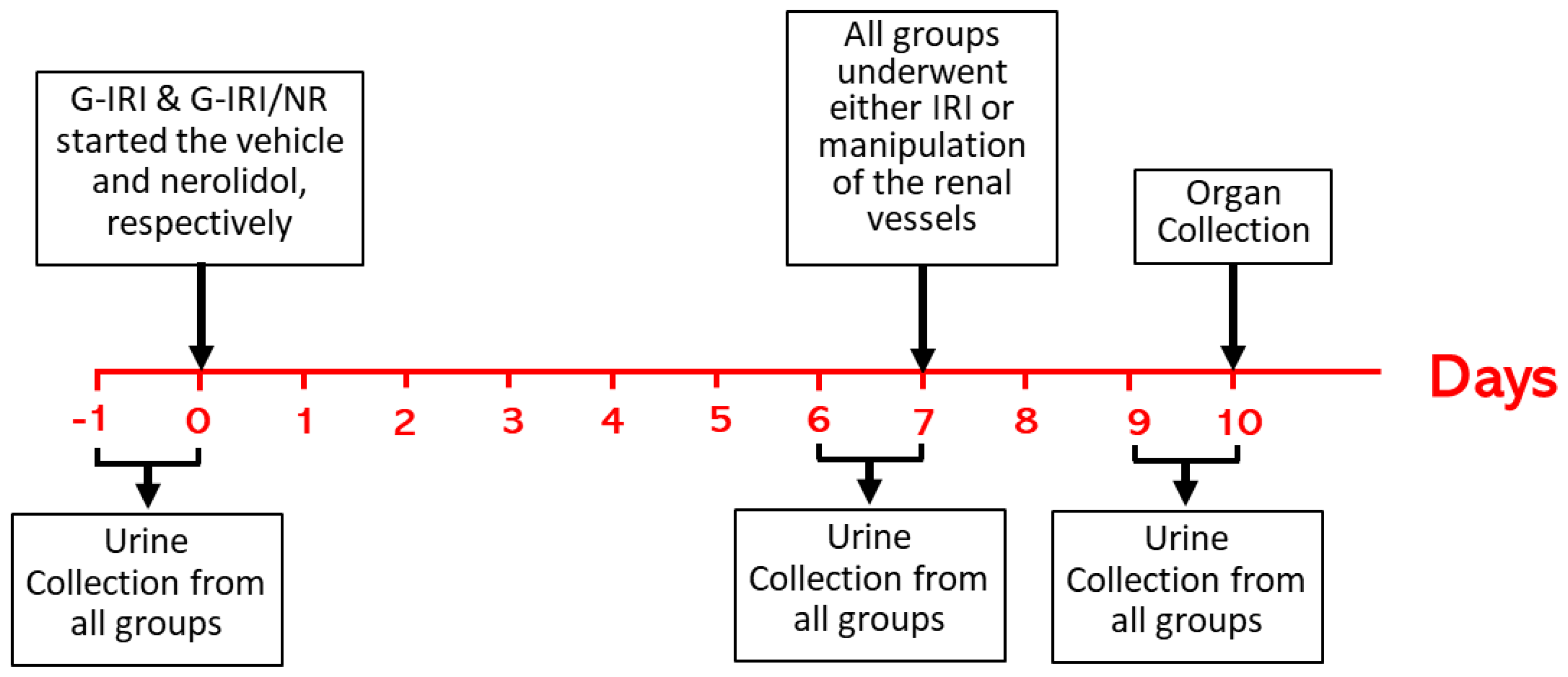
2.2. Experimental Protocol and Administration of Nerolidol and Vehicle
2.3. Experimental Groups
2.4. Sample Collection and Analysis
2.5. Gene Expression Analysis
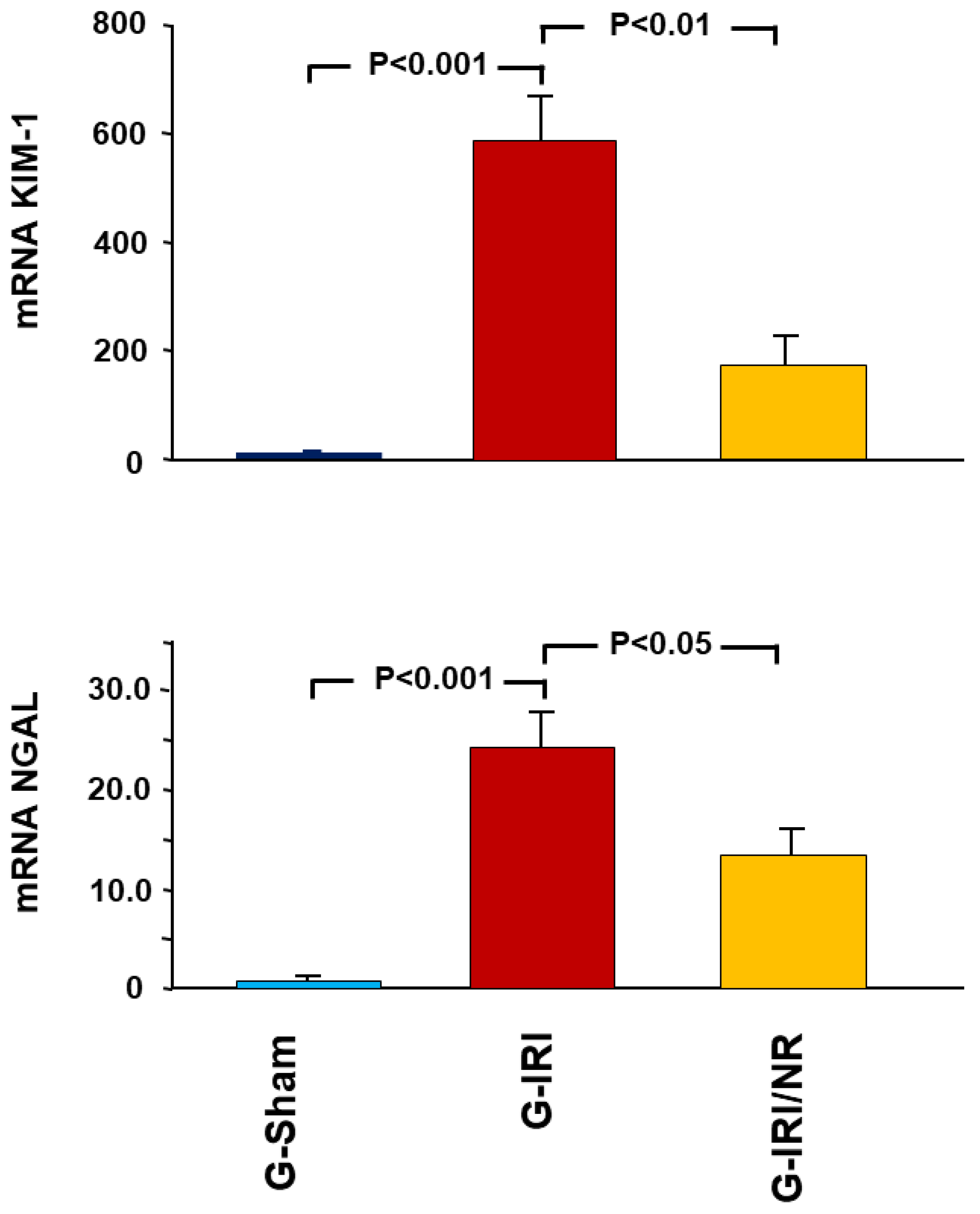
- Acute kidney injury markers, i.e., kidney injury molecule-1 (KIM1) and neutrophil gelatinase-associated lipocalin (NGAL);
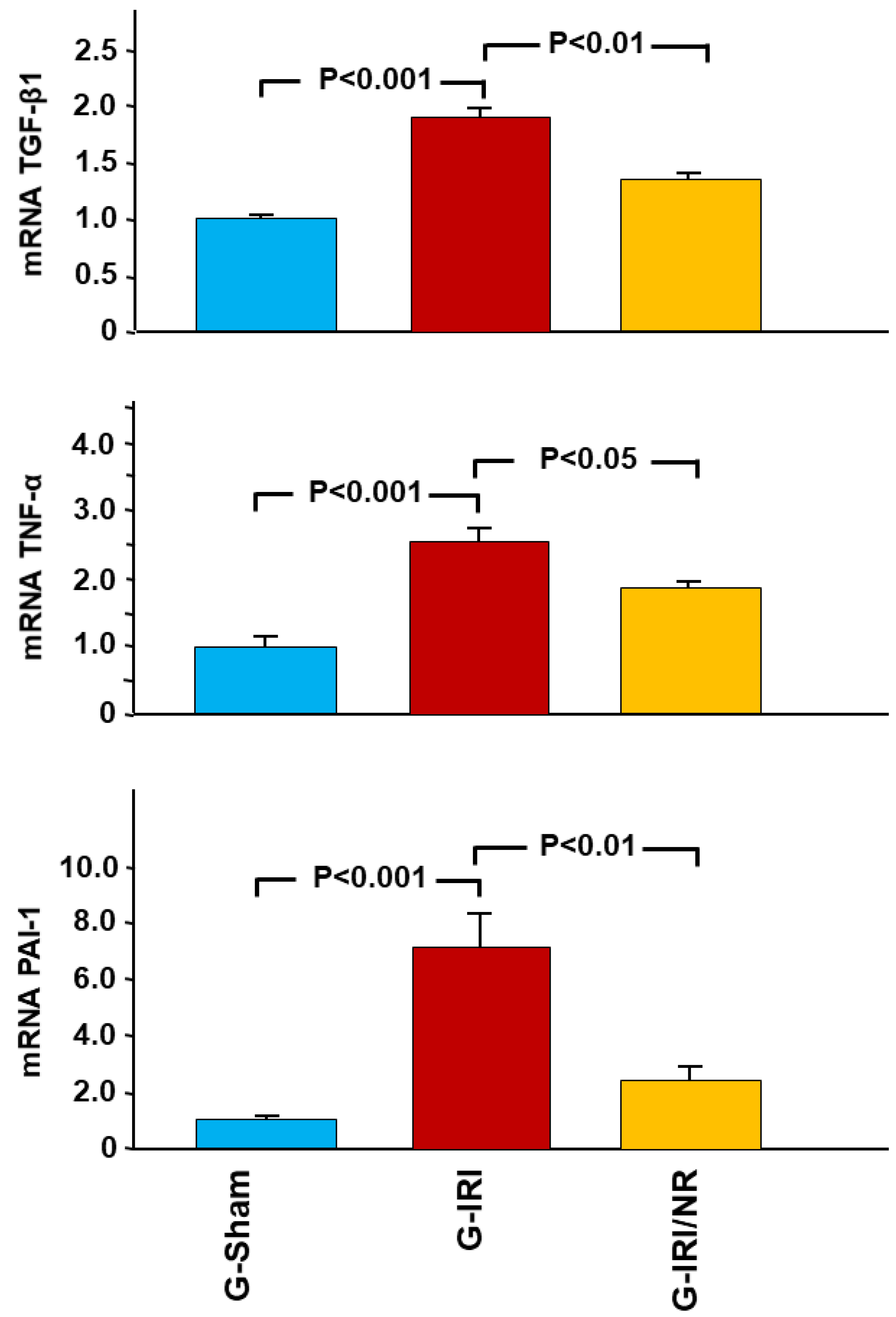
- Cytokines involved in the inflammation and fibrosis, i.e., tumor necrosis factor-α (TNFα), transforming growth factor-β (TGF-β1), interleukin-6 (IL-6), interleukin-1 beta (IL-1β) and plasminogen activator inhibitor-1 (PAI-1);
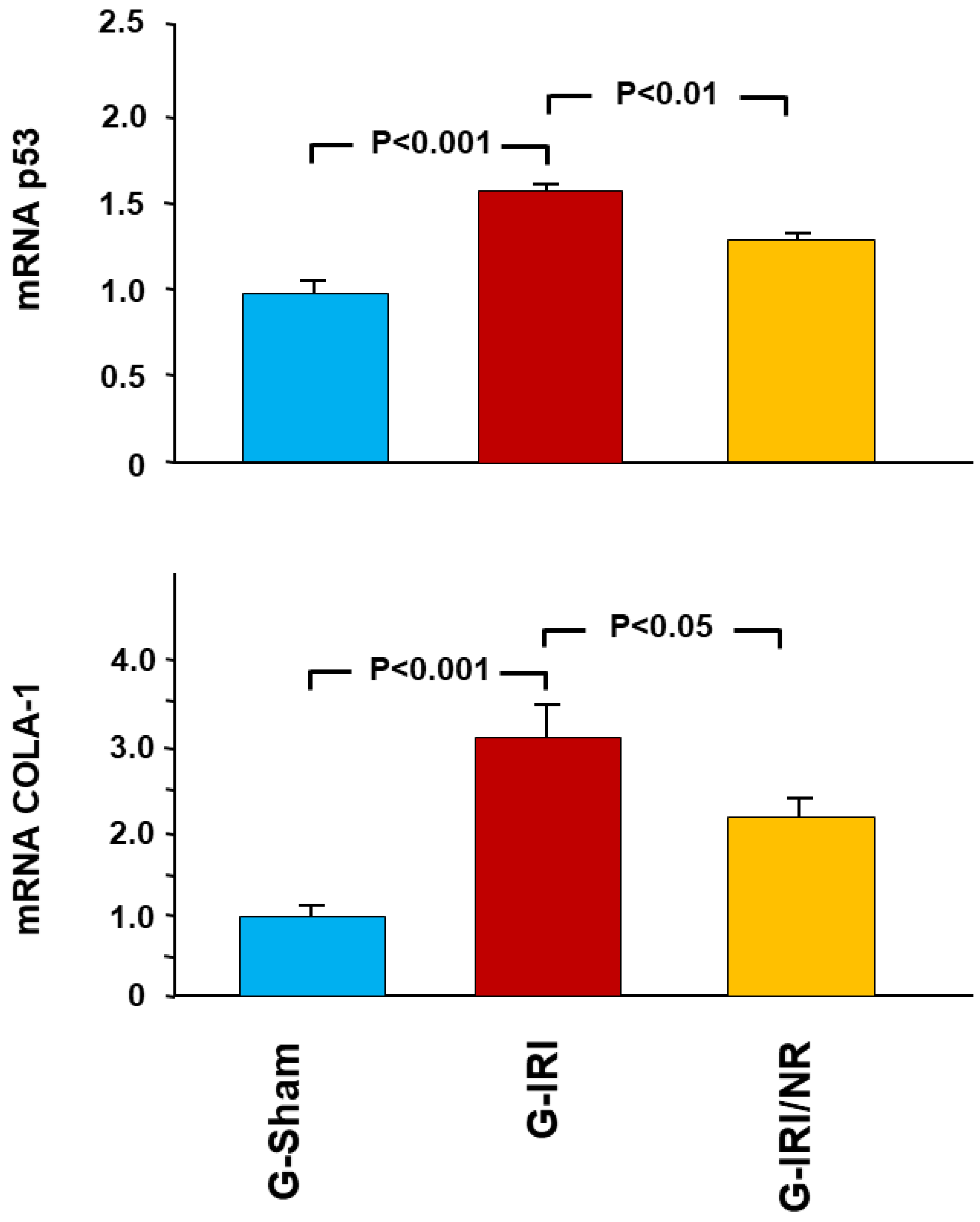
- The proapoptotic gene p53.
- Procollagen type-1 (COLA-1); and
- The antioxidant enzymes glutathione peroxidase (GPX-1) and glutathione-disulfide reductase (GSR).
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Histological Studies
3. Statistical Analysis
4. Results
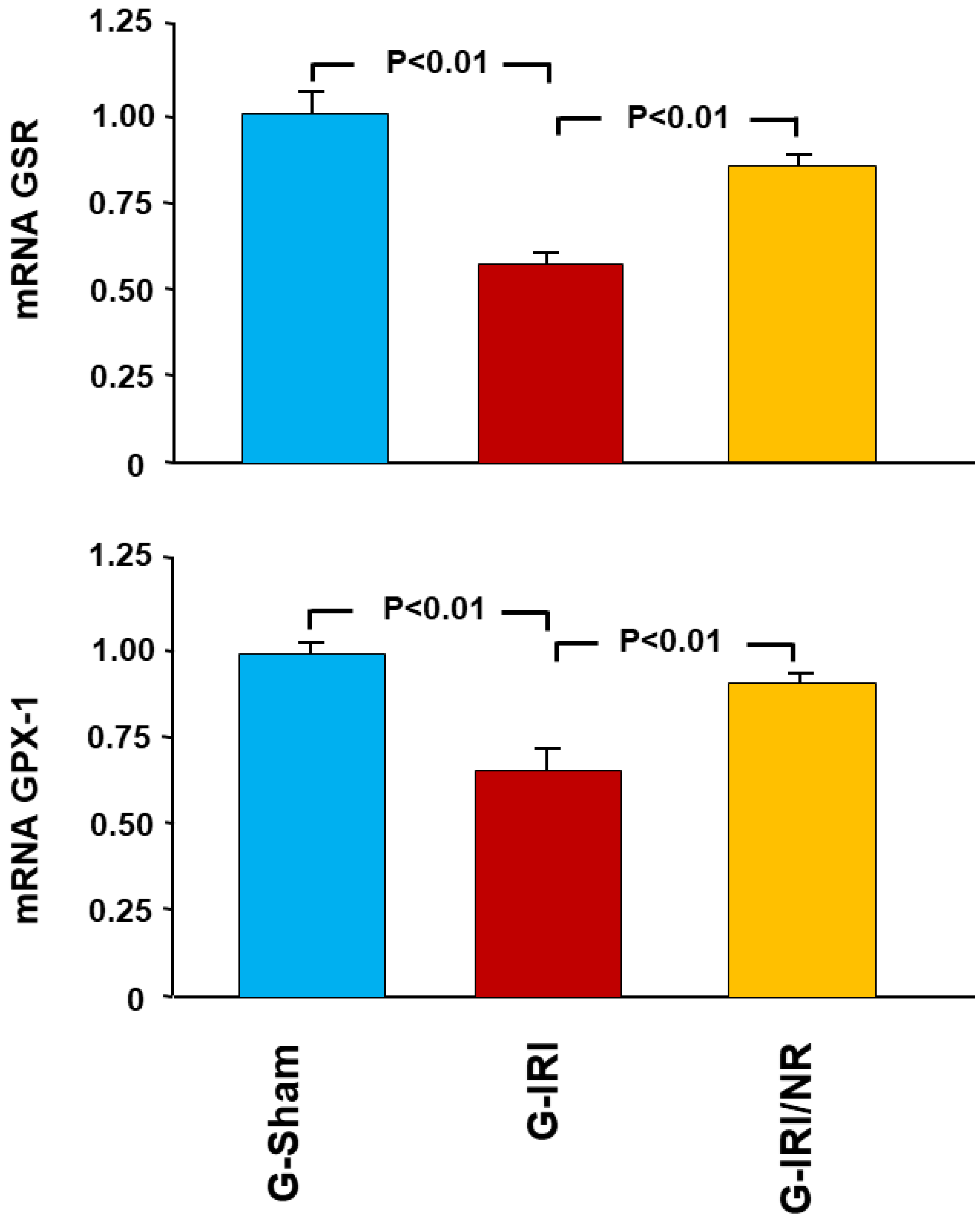
4.1. Gene Expression Analysis Results
4.2. Enzyme-Linked Immunosorbent Assay (ELISA) Results
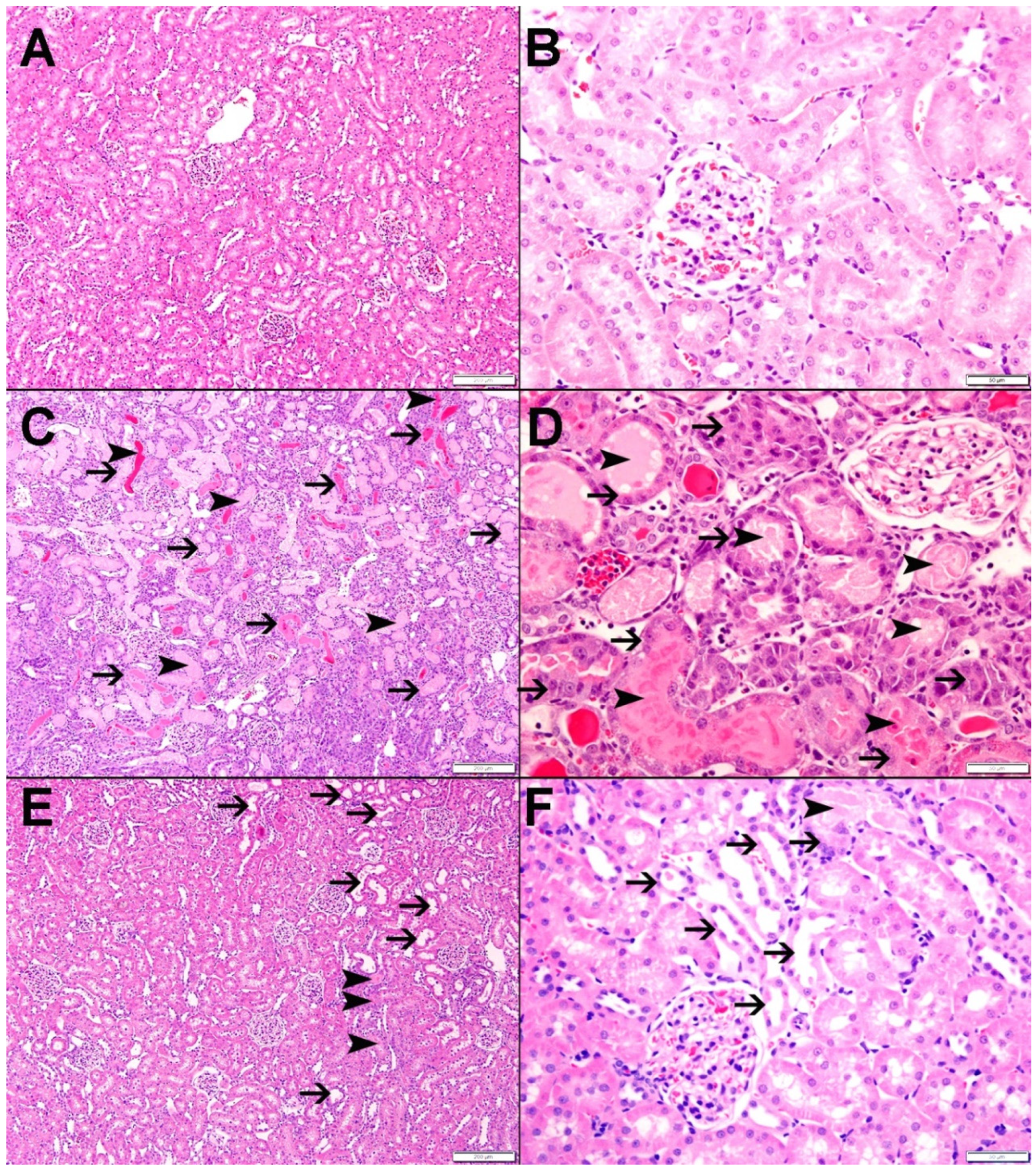
4.3. Histological Studies
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Weight, S.C.; Bell, P.R.; Nicholson, M.L. Renal ischaemia-reperfusion injury. Br. J. Surg. 1996, 83, 162–170. [Google Scholar] [PubMed]
- Myers, B.D.; Miller, D.C.; Mehigan, J.T.; Robertson, C.R.; Derby, G.; Spencer, R.; Friedman, S. Nature of the renal injury following total renal ischemia in man. J. Clin. Investig. 1984, 73, 329–341. [Google Scholar] [CrossRef]
- Hammad, F.T.; Davis, G.; Zhang, X.Y.; Wheatley, A.M. Endotelin ETA and ETB receptor antagonism during cold preservation in renal transplantation. Transplantation 2001, 71, 619–627. [Google Scholar] [CrossRef]
- Hammad, F.T.; Wheatley, A.M.; Davis, G. Role of endothelin ET(A) receptor antagonism in the post-transplant renal response to angiotensin II in the rat. Exp. Physiol. 2001, 86, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Goes, N.; Urmson, J.; Ramassar, V.; Halloran, P. Ischemic acute tubular necrosis induces an extensive local cytokine response. Evidence for induction of interferon-gamma, transforming growth factor-beta 1, granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-10. Transplantation 1995, 59, 565–572. [Google Scholar] [CrossRef]
- Petersen, N.S.; Mitchell, H.K. Recovery of protein synthesis after heat shock: Prior heat treatment affects the ability of cells to translate mRNA. Proc. Natl. Acad. Sci. USA 1981, 78, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Troppmann, C.; Gillingham, K.J.; Benedetti, E.; Almond, P.S.; Gruessner, R.W.; Najarian, J.S.; Matas, A.J. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. Transplantation 1995, 59, 962–968. [Google Scholar] [CrossRef]
- Unal, S.; Ozmen, S.; DemIr, Y.; Yavuzer, R.; LatIfoğlu, O.; Atabay, K.; Oguz, M. The Effect of Gradually Increased Blood Flow on Ischemia-Reperfusion Injury. Ann. Plast. Surg. 2001, 47, 412–416. [Google Scholar] [CrossRef]
- Yang, C.W.; Kim, B.S.; Kim, J.; Ahn, H.J.; Park, J.H.; Jin, D.C.; Kim, Y.S.; Bang, B.K. Preconditioning with sodium arsenite inhibits apoptotic cell death in rat kidney with ischemia/reperfusion or cyclosporine-induced Injuries. The possible role of heat-shock protein 70 as a mediator of ischemic tolerance. Nephron Exp. Nephrol. 2001, 9, 284–294. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Palmeira, C.M.; Oliveira, M.M.; Santos, D.; Simões, A.M.; Rocha, S.M.; Coimbra, M.A.; Peixoto, F. Nerolidol effects on mitochondrial and cellular energetics. Toxicol. Vitr. 2012, 26, 189–196. [Google Scholar] [CrossRef]
- Lapczynski, A.; Bhatia, S.; Letizia, C.; Api, A. Fragrance material review on nerolidol (isomer unspecified). Food Chem. Toxicol. 2008, 46, S247–S250. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbck, S.; Averbck, D.; Ldaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Asaikumar, L.; Vennila, L.; Akila, P.; Sivasangari, S.; Kanimozhi, K.; Premalatha, V.; Sindhu, G. Preventive effect of nerolidol on isoproterenol induced myocardial damage in Wistar rats: Evidences from biochemical and histopathological studies. Drug Dev. Res. 2019, 80, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Pahwa, P.; Goel, R.K. Protective Effect of Nerolidol Against Pentylenetetrazol-Induced Kindling, Oxidative Stress and Associated Behavioral Comorbidities in Mice. Neurochem. Res. 2016, 41, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.D.N.; De Almeida, A.A.C.; Oliveira, J.D.S.; Dos Santos, P.S.; De Sousa, D.P.; De Freitas, R.M. Antioxidant Effects of Nerolidol in Mice Hippocampus After Open Field Test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef]
- Krist, S.; Banovac, D.; Tabanca, N.; Wedge, D.E.; Gochev, V.K.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial Activity of Nerolidol and its Derivatives against Airborne Microbes and Further Biological Activities. Nat. Prod. Commun. 2015, 10, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, D.; Bao, Y.; Shi, Y.; Cui, Y.; Guo, M. Nerolidol Protects Against LPS-induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Signaling. Phytother. Res. 2017, 31, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Türkmen, N.B.; Yüce, H.; Taşlidere, A.; Şahin, Y.; Çiftçi, O. The Ameliorate Effects of Nerolidol on Thioasteamide-induced Oxidative Damage in Heart and Kidney Tissue. Turk. J. Pharm. Sci. 2022, 19, 1–8. [Google Scholar] [CrossRef]
- Hammad, F.T.; Al-Salam, S.; AlZaabi, S.S.; Alfalasi, M.M.; Hammad, A.F.; Yasin, J.; Lubbad, L. The effect of neprilysin and renin inhibition on the renal dysfunction following ischemia-reperfusion injury in the rat. Physiol. Rep. 2021, 9, e14723. [Google Scholar] [CrossRef]
- Klopell, F.C.; Lemos, M.; Sousa, J.P.B.; Comunello, E.; Maistro, E.L.; Bastos, J.K.; de Andrade, S.F. Nerolidol, an Antiulcer Constituent from the Essential Oil of Baccharis dracunculifolia DC (Asteraceae). Z. Nat. C J. Biosci. 2007, 62, 537–542. [Google Scholar] [CrossRef]
- Chatauret, N.; Badet, L.; Barrou, B.; Hauet, T. Ischemia-reperfusion: From cell biology to acute kidney injury. Front. Biosci. 2014, 24, S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, K.; Wada, T.; Kaneko, S.; Murphy, P.M. Roles of chemokines in renal ischemia/reperfusion injury. Front. Biosci. 2008, 13, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Al-Salam, S.; Lubbad, L. Curcumin Provides Incomplete Protection of the Kidney in Ischemia Reperfusion Injury. Physiol. Res. 2012, 61, 503–511. [Google Scholar] [CrossRef]
- Hammad, F.T.; Al-Salam, S.; Lubbad, L. Does aliskiren protect the kidney following ischemia reperfusion injury? Physiol. Res 2013, 62, 681–690. [Google Scholar] [CrossRef]
- Lerman, L.; Textor, S.C. Pathophysiology of ischemic nephropathy. Urol. Clin. N. Am. 2001, 28, 793–803. [Google Scholar] [CrossRef]
- Spurgeon, K.R.; Donohoe, D.L.; Basile, D.P. Transforming growth factor-beta in acute renal failure: Receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am. J. Physiol. Ren. Physiol. 2005, 288, F568–F577. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Moreira, K.L.; Schafer, A.S.; Cossetin, L.F.; Da, S.A.; Da Veiga, M.L.; Da Rocha, M.I.U.; Stefani, L.M.; et al. Nerolidol-loaded nanospheres prevent behavioral impairment via ameliorating Na(+), K(+)-ATPase and AChE activities as well as reducing oxidative stress in the brain of Trypanosoma evansi-infected mice. Naunyn Schmiedebergs. Arch. Pharm. 2017, 390, 139–148. [Google Scholar] [CrossRef]
- Dobashi, K.; Ghosh, B.; Orak, J.; Singh, I.; Singh, A. Kidney ischemia-reperfusion: Modulation of antioxidant defenses. Mol. Cell. Biochem. 2000, 205, 1–11. [Google Scholar] [CrossRef]
- Jiang, G.P.; Liao, Y.J.; Huang, L.L.; Zeng, X.J.; Liao, X.H. Effects and molecular mechanism of pachymic acid on ferroptosis in renal ischemia reperfusion injury. Mol. Med. Rep. 2020, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- AlAsmari, A.F.; Ali, N.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; Almoqbel, D.; AlSwayyed, M.; Alshammari, A.; Alanazi, M.M.; Alhoshani, A.; et al. Geraniol Ameliorates Doxorubicin-Mediated Kidney Injury through Alteration of Antioxidant Status, Inflammation, and Apoptosis: Potential Roles of NF-κB and Nrf2/Ho-1. Nutrients 2022, 14, 1620. [Google Scholar] [CrossRef]
- Hammad, F.T.; Al Salam, S.; Nemmar, A.; Ali, M.; Lubbad, L. The Effect of Arabic Gum on Renal Function in Reversible Unilateral Ureteric Obstruction. Biomolecules 2019, 9, 25. [Google Scholar] [CrossRef]
- Speeckaert, M.M.; Speeckaert, R.; Laute, M.; Vanholder, R.; Delanghe, J.R. Tumor Necrosis Factor Receptors: Biology and Therapeutic Potential in Kidney Diseases. Am. J. Nephrol. 2012, 36, 261–270. [Google Scholar] [CrossRef]
- Ding, Y.; Choi, M.E. Regulation of autophagy by TGF-beta: Emerging role in kidney fibrosis. Semin. Nephrol 2014, 34, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chorostowska-Wynimko, J.; Swiercz, R.; Skrzypczak-Jankun, E.; Wojtowicz, A.; Selman, S.H.; Jankun, J. A novel form of the plasminogen activator inhibitor created by cysteine mutations extends its half-life: Relevance to cancer and angiogenesis. Mol. Cancer Ther. 2003, 2, 19–28. [Google Scholar]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell. Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.I.; Tang, S.C.; Lai, K.N.; Leung, J.C. Kidney injury molecule-1: More than just an injury marker of tubular epithelial cells? J. Cell. Physiol. 2013, 228, 228–917. [Google Scholar] [CrossRef]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of Neutrophil Gelatinase-Associated Lipocalin as a Novel Early Urinary Biomarker for Ischemic Renal Injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Comper, W.D.; Hilliard, L.M.; Nikolic-Paterson, D.J.; Russo, L.M. Disease-dependent mechanisms of albuminuria. Am. J. Physiol. Physiol. 2008, 295, F1589–F1600. [Google Scholar] [CrossRef]
- Eppel, G.A.; Osicka, T.M.; Pratt, L.M.; Jablonski, P.; Howden, B.O.; Glasgow, E.F.; Comper, W.D. The return of glomerular-filtered albumin to the rat renal vein. Kidney Int. 1999, 55, 1861–1870. [Google Scholar] [CrossRef]
- Osicka, T.M.; Strong, K.J.; Nikolic-Paterson, D.; Atkins, R.C.; Jerums, G.; Comper, W.D. Renal processing of serum proteins in an albumin-deficient environment: An in vivo study of glomerulonephritis in the Nagase analbuminaemic rat. Nephrol. Dial. Transplant. 2004, 19, 320–328. [Google Scholar] [CrossRef] [PubMed]


| KIM-1 (NM_173149.2) | Forward | GCCTGGAATAATCACACTGTAAG |
| Reverse | GCAACGGACATGCCAACATAG | |
| Probe | d FAM-TCCCTTTGAGGAAGCCGCAGA-BHQ-1 | |
| Lipocalin 2 (LCN2) (NM_130741.1) | Forward | CTGTTCCCACCGACCAATGC |
| Reverse | CCACTGCACATCCCAGTCA | |
| Probe | FAM-TGACAACTGAACAGACGGTGAGCG-BHQ-1 | |
| TNF-α (NM_012675.3) | Forward | CTCACACTCAGATCATCTTCTC |
| Reverse | CCGCTTGGTGGTTTGCTAC | |
| Probe | FAM-CTCGAGTGACAAGCCCGTAGCC-BHQ-1 | |
| TGF-β1 NM_012620.1 | Forward | GTGGCTGAACCAAGGAGACG |
| Reverse | CGTGGAGTACATTATCTTTGCTGTC | |
| Probe | FAM-ACAGGGCTTTCGCTTCAGTGCTC-BHQ-1 | |
| PAI-1 (NM_012620.1) | Forward | GGCACAATCCAACAGAGACAA |
| Reverse | GGCTTCTCATCCCACTCTCAAG | |
| Probe | FAM-CCTCTTCATGGGCCAGCTGATGG-BHQ-1 | |
| IL-6 (NM_012589.2) | Forward | TCACAGAGGATACCACCCACAACA |
| Reverse | CACAAGTCCGGAGAGGAGAC | |
| Probe | FAM-TCAGAATTGCCATTGCACAACTCT-BHQ-1 | |
| IL-1β (NM_031512.2) | Forward | ATGCCTCGTGCTGTCTGACC |
| Reverse | GCTCATGGAGAATACCACTTGTTGG | |
| Probe | FAM-AGCTGAAAGCTCTCCACCTCAATGGA-BHQ-1 | |
| p53 (NM_030989.3) | Forward | CGAGATGTTCCGAGAGCTGAATG |
| Reverse | GTCTTCGGGTAGCTGGAGTG | |
| Probe | FAM-CCTTGGAATTAAAGGATGCCCGTGC-BHQ-1 | |
| COL1A (NM_053304.1) | Forward | CTGACTGGAAGAGCGGAGAGT |
| Reverse | CCTGTCTCCATGTTGCAGTAGAC | |
| Probe | FAM-ACTGGATCGACCCTAACCAAGGC-BHQ-1 | |
| GSR NM_053906.2 | Forward | CATCCCTACCGTGGTCTTCAG |
| Reverse | ATGGACGGCTTCATCTTCAGT | |
| Probe | FAM-CCACCCGCCTATCGGGACAGT-BHQ-1 | |
| GPx-1 NM_030826.4 | Forward | GTGCTGCTCATTGAGAATGTCG |
| Reverse | TCATTCTTGCCATTCTCCTGATG | |
| Probe | FAM-TCCCTCTGAGGCACCACGAC-BHQ-1 | |
| PPIA (NM_017101.1) | Forward | GCGTCTGCTTCGAGCTGT |
| Reverse | CACCCTGGCACATGAATCC | |
| Probe | Quasar 670-TGCAGACAAAGTTCCAAAGACAGCA-BHQ-2 |
| Group | ||||
|---|---|---|---|---|
| G-Sham | G-IRI | G-IRI/NR | ||
| S. Creatinine (mg/dL) | Basal | 0.31 ± 0.02 | 0.30 ± 0.02 | 0.32 ± 0.02 |
| Pre-IRI | 0.29 ± 0.01 | 0.32 ± 0.05 | 0.33 ± 0.02 | |
| Post-IRI | 0.29 ± 0.02 | 1.51 ± 0.48 * | 0.37 ± 0.02 *,$ | |
| S. Urea (mg/dL) | Basal | 28.9 ± 1.4 | 29.0 ± 1.4 | 31.1 ± 2.8 |
| Pre-IRI | 26.6 ± 0.8 | 30.7 ± 2.8 | 28.8 ± 2.3 | |
| Post-IRI | 25.4 ± 0.6 | 82.2 ± 19.8 * | 31.7 ± 2.1 $ | |
| Creatinine Clearance (mL/min) | Basal | 62.3 ± 4.3 | 64.7 ± 9.1 | 63.5 ± 5.7 |
| Pre-IRI | 72.7 ± 3.4 | 69.9 ± 6.6 | 67.0 ± 5.1 | |
| Post-IRI | 76.4 ± 3.0 | 33.5 ± 7.8 * | 74.1 ± 2.8 $ | |
| (1) | ||||
| $ | Group | |||
|---|---|---|---|---|
| * | G-Sham | G-IRI | G-IRI/NR | |
| 24-h Urinary Albumin (µg) | Basal | 0.073 ± 0.005 | 0.078 ± 0.013 | 0.073 ± 0.009 |
| Pre-IRI | 0.077 ± 0.006 | 0.079 ± 0.009 | 0.079 ± 0.006 | |
| Post-IRI | 0.069 ± 0.005 | 0.783 ± 0.111 * | 0.570 ± 0.078 *,$ | |
| Albumin/Creatinine Ratio | Basal | 13.9 ± 1.1 | 13.1 ± 2.1 | 13.6 ± 1.7 |
| Pre-IRI | 13.9 ± 0.9 | 13.7 ± 2.2 | 13.8 ± 0.8 | |
| Post-IRI | 12.4 ± 0.9 | 166.1 ± 27.0 * | 88.8 ± 11.0 *,$ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, F.T.; Al-Salam, S.; Ahmad, R.; Yasin, J.; Hammad, A.F.; Rasheed, J.A.; Lubbad, L. The Effect of Nerolidol Renal Dysfunction following Ischemia–Reperfusion Injury in the Rat. Nutrients 2023, 15, 455. https://doi.org/10.3390/nu15020455
Hammad FT, Al-Salam S, Ahmad R, Yasin J, Hammad AF, Rasheed JA, Lubbad L. The Effect of Nerolidol Renal Dysfunction following Ischemia–Reperfusion Injury in the Rat. Nutrients. 2023; 15(2):455. https://doi.org/10.3390/nu15020455
Chicago/Turabian StyleHammad, Fayez T., Suhail Al-Salam, Rahaf Ahmad, Javed Yasin, Awwab F. Hammad, Jasmine Abdul Rasheed, and Loay Lubbad. 2023. "The Effect of Nerolidol Renal Dysfunction following Ischemia–Reperfusion Injury in the Rat" Nutrients 15, no. 2: 455. https://doi.org/10.3390/nu15020455
APA StyleHammad, F. T., Al-Salam, S., Ahmad, R., Yasin, J., Hammad, A. F., Rasheed, J. A., & Lubbad, L. (2023). The Effect of Nerolidol Renal Dysfunction following Ischemia–Reperfusion Injury in the Rat. Nutrients, 15(2), 455. https://doi.org/10.3390/nu15020455






