The Relationship among Bowel [18]F-FDG PET Uptake, Pathological Complete Response, and Eating Habits in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
- (a)
- willingness to participate to the study;
- (b)
- age ≥ 18 years old;
- (c)
- female gender;
- (d)
- histopathologically confirmed diagnosis of BC;
- (e)
- clinical stage T1c-T4, N0-N3, M0 at presentation;
- (f)
- Eastern Cooperative Oncology Group (ECOG) Performance Status 0–1;
- (g)
- baseline left ventricular ejection fraction ≥ 55%;
- (h)
- adequate hematologic, liver and hepatic function;
- (i)
- ability to give informed consent according to International Conference on Harmonization /European Union Good Clinical Practice, and national/local regulation.
- inability to respond to survey;
- prior history of invasive BC;
- stage IV BC;
- prior systemic therapy for BC;
- previous therapy with anthracyclines/taxanes for any malignancy;
- use of immunomodulatory agents at the time of enrolment/during the previous 2 months;
- use of antibiotics at the time of enrolment/during the previous month;
- history of other malignancy within 5 years prior to the enrolment;
- pregnancy/breastfeeding/intention of becoming pregnant during the study.
2.2. Survey Design
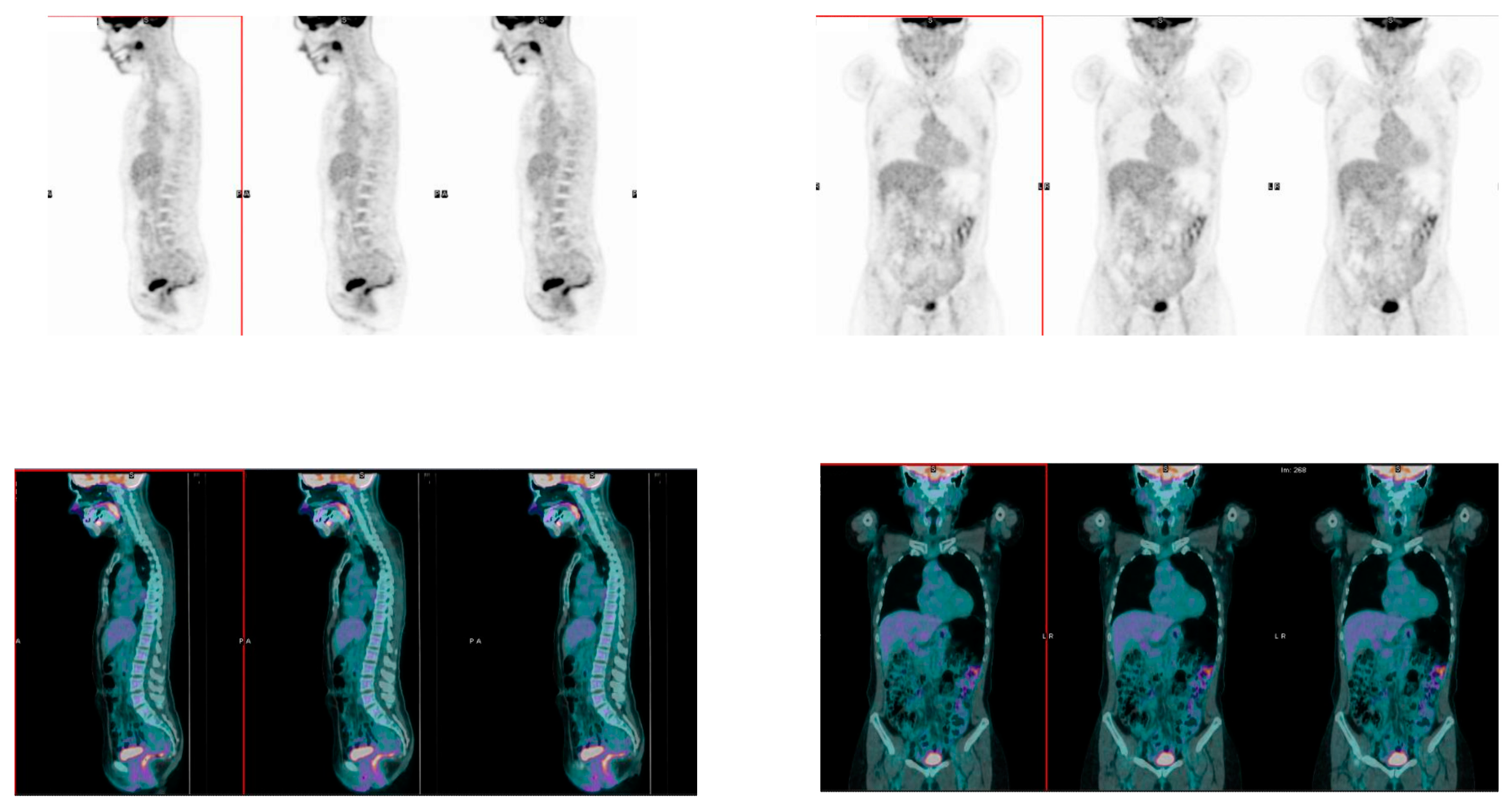
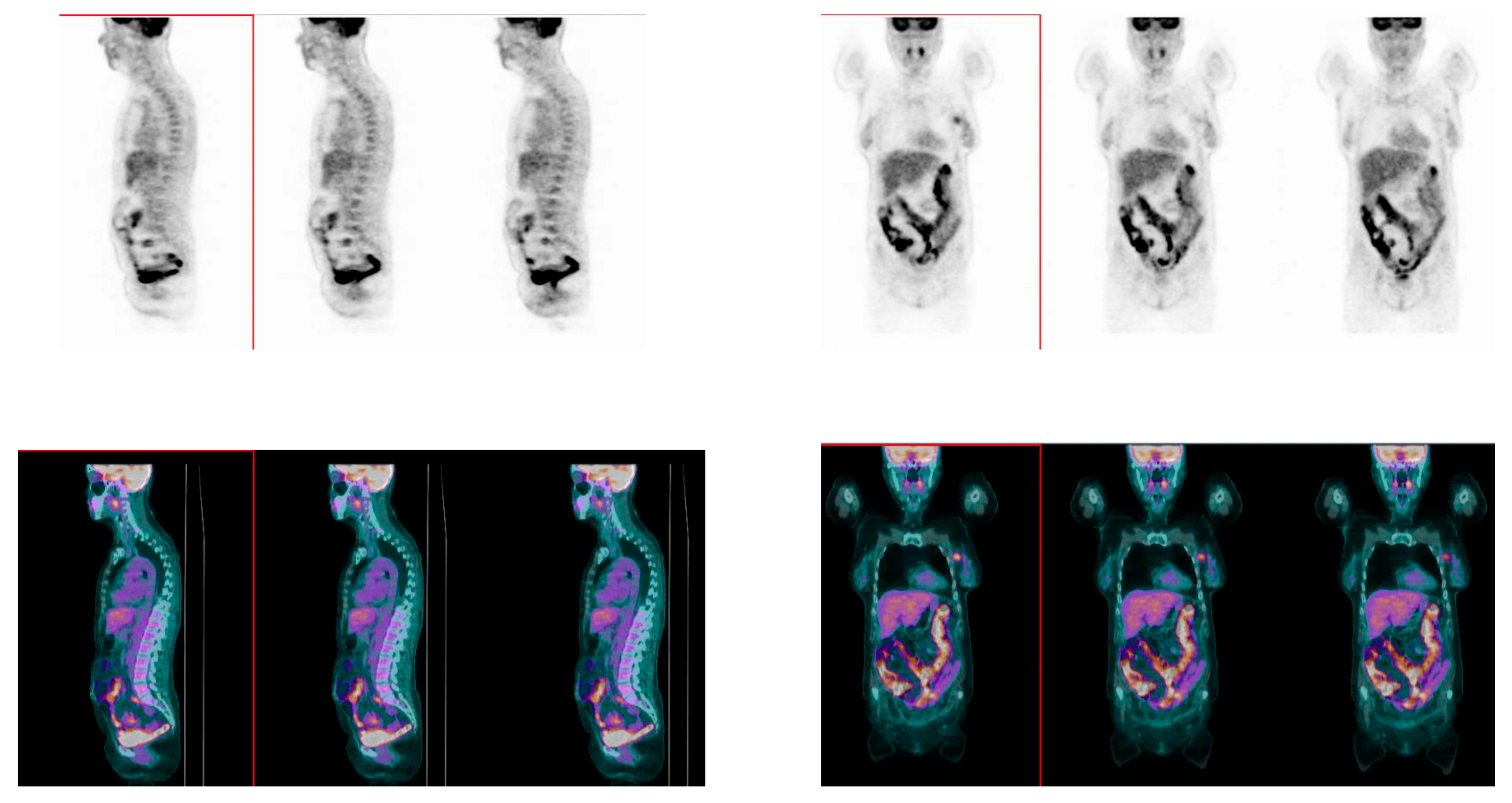
2.3. [18]F-FDG PET/CT Acquisition Protocol
2.4. Statistical Analysis
3. Results
3.1. Patients’ and Tumors’ Characteristics
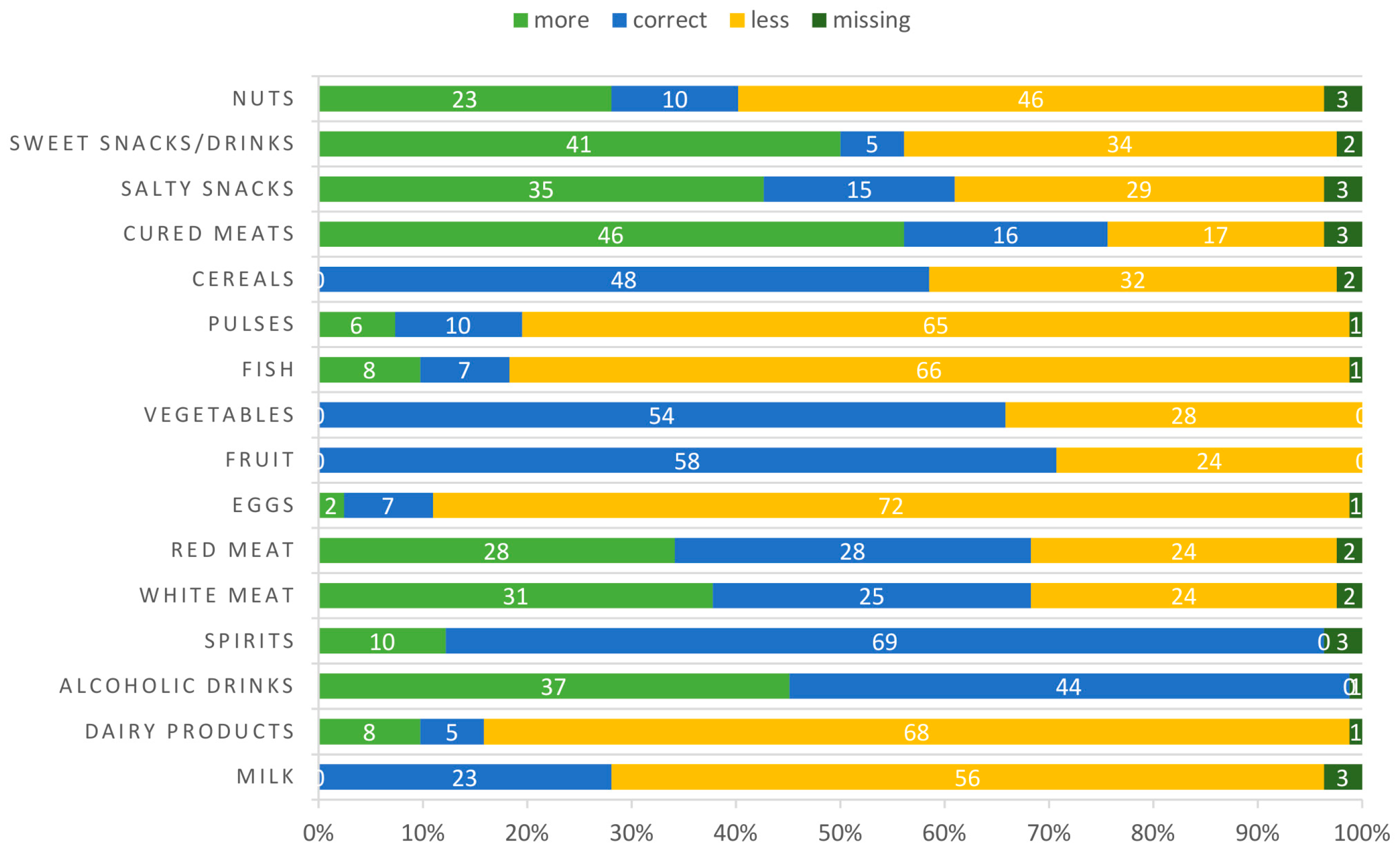
3.2. Eating Habits
3.3. Correlation between Eating and Exercise Habits and Bowel [18]F-FDG Uptake
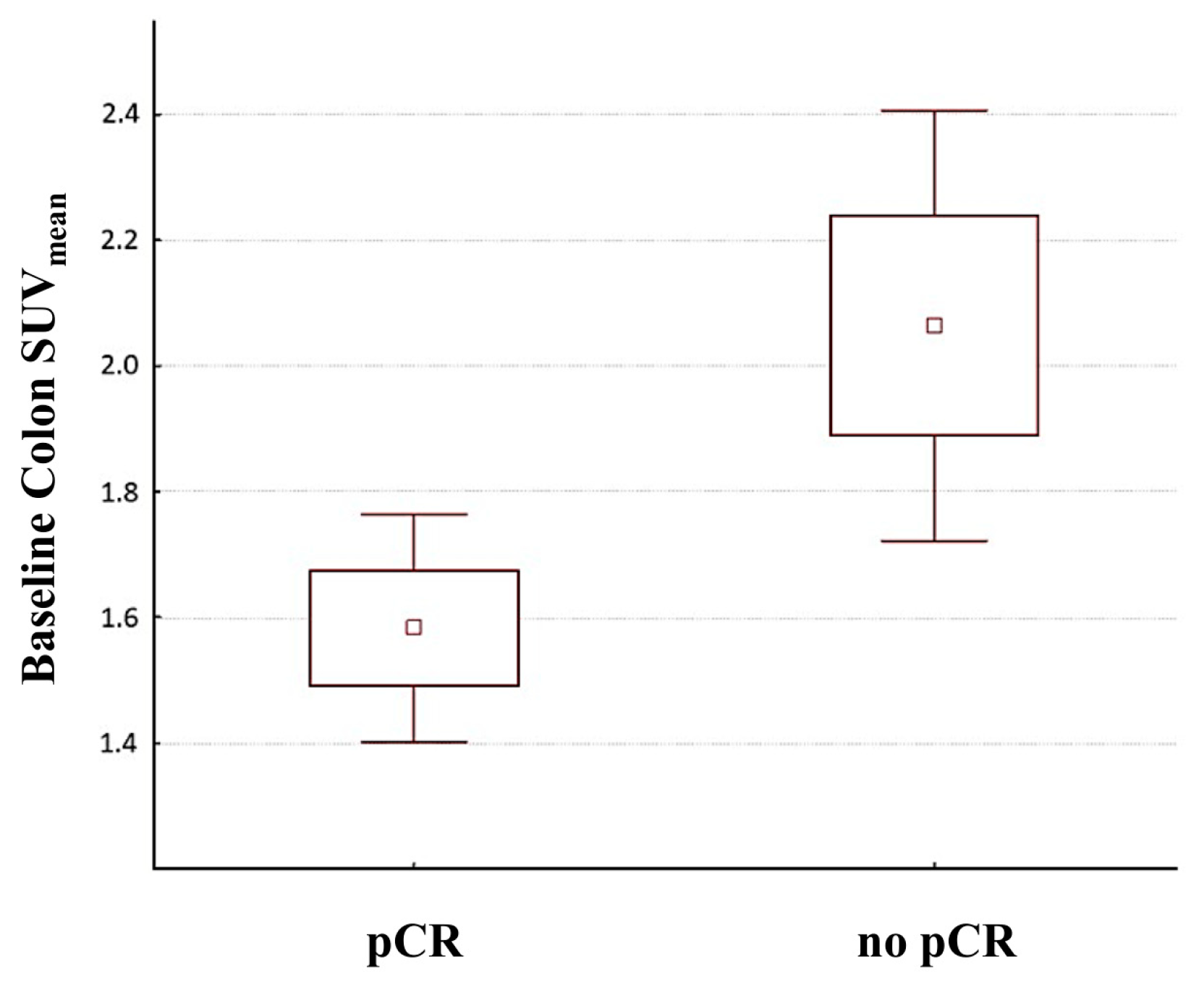
3.4. Association between Bowel [18]F-FDG Uptake and Response to Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Caprara, G.; Tieri, M.; Fabi, A.; Guarneri, V.; Falci, C.; Dieci, M.V.; Turazza, M.; Ballardini, B.; Bin, A.; Cinieri, S.; et al. Results of the ECHO (Eating habits CHanges in Oncologic patients) Survey: An Italian Cross-Sectional Multicentric Study to Explore Dietary Changes and Dietary Supplement Use; in Breast Cancer Survivors. Front. Oncol. 2021, 11, 705927. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Pan, K.; Neuhouser, M.L.; Manson, J.E.; Thomson, C.A.; Mossavar-Rahmani, Y.; Lane, D.S.; Johnson, K.C.; et al. Dietary Modification and Breast Cancer Mortality: Long-Term Follow-Up of the Women’s Health Initiative Randomized Trial. J. Clin. Oncol. 2020, 38, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise; Diet; and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2020, 18, JCO2200687. [Google Scholar] [CrossRef]
- Mili, N.; Paschou, S.A.; Goulis, D.G.; Dimopoulos, M.A.; Lambrinoudaki, I.; Psaltopoulou, T. Obesity; metabolic syndrome; and cancer: Pathophysiological and therapeutic associations. Endocrine 2021, 74, 478–497. [Google Scholar] [CrossRef]
- Pedersini, R.; di Mauro, P.; Bosio, S.; Zanini, B.; Zanini, A.; Amoroso, V.; Turla, A.; Vassalli, L.; Ardine, M.; Monteverdi, S.; et al. Changes in eating habits and food preferences in breast cancer patients undergoing adjuvant chemotherapy. Sci. Rep. 2021, 11, 12975. [Google Scholar] [CrossRef]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer. 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Thomson, C.A. Diet and breast cancer: Understanding risks and benefits. Nutr. Clin. Pract. 2012, 27, 636–650. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Carayol, M.; Ninot, G.; Senesse, P.; Bleuse, J.P.; Gourgou, S.; Sancho-Garnier, H.; Sari, C.; Romieu, I.; Romieu, G.; Jacot, W. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: The “APAD1” randomized controlled trial. BMC Cancer. 2019, 19, 737. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention; Treatment and Recurrence. Nutrients. 2019, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Weltzien, E.; Kushi, L.H.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J. Clin. Oncol. 2009, 27, 919–926. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A. Diet and Prognosis in Women with Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Benz, M.R.; Krause, B.J.; Pomykala, K.L.; Buck, A.K.; Czernin, J. (18)F-FDG-PET/CT in evaluating response to therapy in solid tumors: Where we are and where we can go. Q. J. Nucl. Med. Mol. Imaging. 2011, 55, 620–632. [Google Scholar]
- Kubota, K. From tumor biology to clinical Pet: A review of positron emission tomography (PET) in oncology. Ann. Nucl. Med. 2001, 15, 471–486. [Google Scholar] [CrossRef]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The Evolving Role of FDG-PET/CT in the Diagnosis; Staging; and Treatment of Breast Cancer. Mol. Imaging Biol. 2019, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, M.H.; Uenzen, M.; Helisch, A.; Dahmen, A.; Mudter, J.; Goetz, M.; Schreckenberger, M.; Galle, P.R.; Bartenstein, P.; Neurath, M.F. 18F-Fluorodeoxyglucose positron-emission tomography (PET) can be used to assess inflammation non-invasively in Crohn’s disease. Dig. Dis. Sci. 2012, 57, 2658–2668. [Google Scholar] [CrossRef]
- Rubin, D.T.; Surma, B.L.; Gavzy, S.J.; Schnell, K.M.; Bunnag, A.P.; Huo, D.; Appelbaum, D.E. Positron emission tomography (PET) used to image subclinical inflammation associated with ulcerative colitis (UC) in remission. Inflamm. Bowel Dis. 2009, 15, 750–755. [Google Scholar] [CrossRef][Green Version]
- Sena, Y.; Matsumoto, S.; Silman, C.; Otsuka, K.; Kiyota, T. Physiological 18F-FDG uptake in the normal adult anal canal: Evaluation by PET/CT. Ann. Nucl. Med. 2020, 34, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- CREA Centro di Ricerca Alimenti e Nutrizione. Linee Guida Per Una Sana Alimentazione; Centro di Ricerca Alimenti e Nutrizione: Rome, Italy, 2018; ISBN 978-88-96597-01-9. Available online: https://www.crea.gov.it/web/alimenti-e-nutrizione/-/linee-guidaper-una-sana-alimentazione-2018 (accessed on 1 September 2022).
- De Sanctis, R.; Agostinetto, E.; Masci, G.; Ferraro, E.; Losurdo, A.; Viganò, A.; Antunovic, L.; Zuradelli, M.; Torrisi, R.M.C.; Santoro, A. Predictive Factors of Eribulin Activity in Metastatic Breast Cancer Patients. Oncology 2018, 94, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Viganò, A.; Savastano, E.; Petolicchio, B.; Toscano, M.; De Sanctis, R.; Maestrini, I.; Di Piero, V. A Study of Clinical Features and Risk Factors of Self-Referring Emergency Department Headache Patients: A Comparison with Headache Center Outpatients. Eur. Neurol. 2020, 83, 34–40. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, R.; Viganò, A.; Giuliani, A.; Gronchi, A.; De Paoli, A.; Navarria, P.; Quagliuolo, V.; Santoro, A.; Colosimo, A. Unsupervised versus Supervised Identification of Prognostic Factors in Patients with Localized Retroperitoneal Sarcoma: A Data Clustering and Mahalanobis Distance Approach. Biomed. Res. Int. 2018, 2018, 2786163. [Google Scholar] [CrossRef]
- Alessiani, M.; Petolicchio, B.; De Sanctis, R.; Squitieri, M.; Di Giambattista, R.; Puma, M.; Franzese, C.; Toscano, M.; Derchi, C.C.; Gilliéron, E.; et al. A Propensity Score Matching Study on the Effect of OnabotulinumtoxinA Alone versus Short-Term Psychodynamic Psychotherapy Plus Drug-of-Choice as Preventive Therapy in Chronic Migraine: Effects and Predictive Factors. Eur. Neurol. 2022, 85, 453–459. [Google Scholar] [CrossRef]
- Clotas, C.; Serral, G.; Vidal Garcia, E.; Puigpinós-Riera, R.; DAMA Cohort Group. Dietary changes and food habits: Social and clinical determinants in a cohort of women diagnosed with breast cancer in Barcelona (DAMA cohort). Cancer Causes Control 2021, 32, 1355–1364. [Google Scholar] [CrossRef]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.; Magness, S.; Jobin, C.; Lund, P.K. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat; Western-Style Diet; Systemic Inflammation; and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients. 2021, 13, 2795. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr. 2007, 137, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.P.; Sinaiko, A.R. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J. Am. Diet Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Moore, M.A.; Kono, S. Impact of C-reactive protein on disease risk and its relation to dietary factors. Asian Pac. J. Cancer Prev. 2007, 8, 167–177. [Google Scholar] [PubMed]
- Watzl, B.; Kulling, S.E.; Möseneder, J.; Barth, S.W.; Bub, A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy; nonsmoking men. Am. J. Clin. Nutr. 2005, 82, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Steffen, L.M.; Mayer-Davis, E.J.; Jenny, N.S.; Jiang, R.; Herrington, D.M.; Jacobs, D.R., Jr. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2006, 83, 1369–1379. [Google Scholar] [CrossRef]
- Moasses-Ghafari, B.; Fallahi, B.; Esfehani, A.F.; Eftekhari, M.; Rahmani, K.; Eftekhari, A.; Geramifar, P. Effect of Diet on Physiologic Bowel 18F-FDG Uptake. J. Nucl. Med. Technol. 2021, 49, 241–245. [Google Scholar] [CrossRef]
- Fallahi, B.; Moasses-Ghafari, B.; Fard-Esfahani, A.; Geramifar, P.; Beiki, D.; Emami-Ardekani, A.; Eftekhari, M. Factors influencing the pattern and intensity of myocardial 18F-FDG uptake in oncologic PET-CT imaging. Iran J. Nucl. Med. 2017, 25, 52–61. [Google Scholar]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef]
- Marsh, A.; Radford-Smith, G.; Banks, M.; Lord, A.; Chachay, V. Dietary intake of patients with inflammatory bowel disease aligns poorly with traditional Mediterranean diet principles. Nutr. Diet. 2022, 79, 229–237. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef]
- Illescas, O.; Rodríguez-Sosa, M.; Gariboldi, M. Mediterranean Diet to Prevent the Development of Colon Diseases: A Meta-Analysis of Gut Microbiota Studies. Nutrients 2021, 13, 2234. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Longo, S.; Stefanelli, G.; Viscido, A.; Latella, G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients 2021, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity; stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Abedpoor, N.; Taghian, F.; Hajibabaie, F. Physical activity ameliorates the function of organs via adipose tissue in metabolic diseases. Acta Histochem. 2022, 124, 151844. [Google Scholar] [CrossRef] [PubMed]
- Hajibabaie, F.; Abedpoor, N.; Assareh, N.; Tabatabaiefar, M.A.; Shariati, L.; Zarrabi, A. The Importance of SNPs at miRNA Binding Sites as Biomarkers of Gastric and Colorectal Cancers: A Systematic Review. J. Pers. Med. 2022, 12, 456. [Google Scholar] [CrossRef]
- Abedpoor, N.; Taghian, F.; Hajibabaie, F. Cross Brain-Gut Analysis Highlighted Hub Genes and LncRNA Networks Differentially Modified During Leucine Consumption and Endurance Exercise in Mice with Depression-Like Behaviors. Mol. Neurobiol. 2022, 59, 4106–4123. [Google Scholar] [CrossRef]
- Kang, J.Y.; Kim, H.N.; Chang, Y.; Yun, Y.; Ryu, S.; Shin, H.; Kim, H.L. Gut microbiota and physiologic bowel 18F-FDG uptake. EJNMMI Res. 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Kim, H.N.; Bang, J.I.; Lim, W.; Moon, B.I.; Paik, N.S.; Kim, B.S.; Kim, H.L. Physiologic intestinal 18F-FDG uptake is associated with alteration of gut microbiota and proinflammatory cytokine levels in breast cancer. Sci. Rep. 2019, 9, 18273. [Google Scholar] [CrossRef] [PubMed]
- Kumral, D.; Zfass, A.M. Gut Movements: A Review of the Physiology of Gastrointestinal Transit. Dig. Dis. Sci. 2018, 63, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut microbiome-micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins? Biofactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Barleben, A.; Mills, S. Anorectal anatomy and physiology. Surg. Clin. N. Am. 2010, 90, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef]
- Basu, S.; Chen, W.; Tchou, J.; Mavi, A.; Cermik, T.; Czerniecki, B.; Schnall, M.; Alavi, A. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: A potentially useful method for disease characterization. Cancer 2008, 112, 995–1000. [Google Scholar] [CrossRef]
- Williams, J.M.; Rani, S.D.; Li, X.; Arlinghaus, L.R.; Lee, T.C.; MacDonald, L.R.; Partridge, S.C.; Kang, H.; Whisenant, J.G.; Abramson, R.G.; et al. Comparison of prone versus supine 18F-FDG-PET of locally advanced breast cancer: Phantom and preliminary clinical studies. Med. Phys. 2015, 42, 3801–3813. [Google Scholar] [CrossRef]
- Jeong, Y.; Baek, S.; Park, J.W.; Joo, J.H.; Kim, J.S.; Lee, S.W. Lymph node standardized uptake values at pre-treatment 18F-fluorodeoxyglucose positron emission tomography as a valuable prognostic factor for distant metastasis in nasopharyngeal carcinoma. Br. J. Radiol. 2017, 90, 20160239. [Google Scholar] [CrossRef]
- Husi, K.; Pinczés, L.I.; Fejes, Z.; Nagy, B., Jr.; Illés, Á.; Miltényi, Z. Combined prognostic role of TARC and interim 18F-FDG PET/CT in patients with Hodgkin lymphoma-real world observational study. Hell. J. Nucl. Med. 2022, 25, 125–131. [Google Scholar] [CrossRef]
- Guzmán Ortiz, S.; Mucientes Rasilla, J.; Vargas Núñez, J.A.; Royuela, A.; Rodríguez Carrillo, J.L.; Dotor de Lama, A.; Navarro Matilla, M.B.; Mitjavila Casanovas, M. Evaluation of the prognostic value of the metabolic volumetric parameters calculated with 18F-FDG PET/CT and its value added to the molecular characteristics in patients with diffuse large B-cell lymphoma. Rev. Esp. Med. Nucl. Imagen. Mol. 2022, 41, 215–222. [Google Scholar] [CrossRef]
- Wu, J.; Deng, H.; Zhong, H.; Wang, T.; Rao, Z.; Wang, Y.; Chen, Y.; Zhang, C. Comparison of 68Ga-FAPI and 18F-FDG PET/CT in the Evaluation of Patients With Newly Diagnosed Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 924223. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xu, P.; Cheng, S.; Lin, M.; Zhong, H.; Li, W.; Huang, H.; Ouyang, B.; Yi, H.; Chen, J.; et al. Comparison of Nasopharyngeal MR; 18 F-FDG PET/CT; and 18 F-FDG PET/MR for Local Detection of Natural Killer/T-Cell Lymphoma; Nasal Type. Front. Oncol. 2020, 10, 576409. [Google Scholar] [CrossRef] [PubMed]
- Kampe, K.K.; Rotermund, R.; Tienken, M.; Thomalla, G.; Regier, M.; Klutmann, S.; Kluge, S. Diagnostic Value of Positron Emission Tomography Combined with Computed Tomography for Evaluating Critically Ill Neurological Patients. Front. Neurol. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Eugène, T.; Couec, M.L.; Strullu, M.; Frampas, E.; Campion, L.; Kraeber-Bodéré, F.; Bodet-Milin, C. Prognostic Value and Clinical Impact of (18)FDG-PET in the Management of Children with Burkitt Lymphoma after Induction Chemotherapy. Front. Med. 2014, 1, 54. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.R.; Qu, M.M.; Zhai, Y.N.; Feng, W.; Gao, Y.; Lei, J.Q. Diagnostic role of 18F-FDG PET/MRI in the TNM staging of breast cancer: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4328–4337. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, H.N.; Yun, Y.; Kim, Y.; Ha, A.N.; Kim, H.L.; Kim, B.S. Background Intestinal 18F-FDG Uptake Is Related to Serum Lipid Profile and Obesity in Breast Cancer Patients. PLoS ONE 2015, 10, e0141473. [Google Scholar] [CrossRef]
| Patients (n = 82) | ||
|---|---|---|
| n | % | |
| Age | ||
| <50 | 46 | 56.1 |
| 50–64 | 29 | 35.4 |
| ≥65 | 7 | 8.5 |
| Smoke | ||
| no | 53 | 64.6 |
| yes | 15 | 18.3 |
| former | 14 | 17.1 |
| Menopause | ||
| no | 47 | 57.3 |
| yes | 28 | 34.1 |
| peri | 7 | 8.5 |
| BMI | ||
| <18.5 | 4 | 4.9 |
| 18.5–24.9 | 50 | 61.0 |
| 25–29.9 | 25 | 30.5 |
| ≥30 | 3 | 3.7 |
| Comorbidies | ||
| None | 54 | 65.9 |
| Intestinal | 8 | 9.8 |
| Others | 17 | 20.7 |
| Intestinal + others | 3 | 3.7 |
| no pCR (n = 45) | pCR (n = 37) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Stage | ||||
| IA | 2 | 4.4 | 6 | 16.2 |
| IIA | 17 | 37.8 | 19 | 51.4 |
| IIB | 18 | 40.0 | 9 | 24.3 |
| IIIA | 6 | 13.3 | 3 | 8.1 |
| IIIB | 1 | 2.2 | 0 | 0 |
| IIIC | 1 | 2.2 | 0 | 0 |
| Grade | ||||
| G1 | 0 | 0 | 0 | 0 |
| G2 | 16 | 35.6 | 9 | 24.3 |
| G3 | 29 | 64.4 | 28 | 75.7 |
| Subtype | ||||
| Luminal A | 1 | 2.2 | 0 | 0 |
| Luminal B | 3 | 6.7 | 4 | 10.8 |
| HER2+ | 27 | 60.0 | 18 | 48.6 |
| TNBC | 14 | 31.1 | 15 | 40.5 |
| Weekly Frequency Consumption Median | |
|---|---|
| Milk | 1 (0–7) |
| Dairy products | 2 (0–7) |
| Alcoholic drinks | 0 (0–7) |
| Spirits | 0 (0–2) |
| White meat | 2 (0–6) |
| Red meat | 1 (0–3) |
| Eggs | 1 (0–5) |
| Fruit | 7 (0–7) |
| Vegetables | 7 (1–7) |
| Fish | 1.5 (0–6) |
| Pulses | 1 (0–7) |
| Cereals | 7 (0–7) |
| Cured meats | 2 (0–5) |
| Salty snacks | 1 (0–7) |
| Sweet snacks/drinks | 2.5 (0–7) |
| Nuts | 1 (0–7) |
| Patients (n = 82) | ||
|---|---|---|
| n | % | |
| Exercise weekly frequency | ||
| 0 | 37 | 45.1 |
| 1–3 | 26 | 31.7 |
| >3 | 17 | 20.7 |
| missing | 2 | 2.4 |
| Colon SUVmean | Rectum SUVmean | |||
|---|---|---|---|---|
| r | p | r | p | |
| Pro-inflammatory drinks | +0.33 | <0.01 | +0.14 | 0.51 |
| Pro-inflammatory foods | +0.25 | 0.04 | +0.02 | 0.86 |
| Anti-inflammatory foods | −0.21 | 0.05 | −0.23 | 0.04 |
| Physical activity | −0.20 | 0.46 | −0.11 | 0.64 |
| Smoke | −0.30 | 0.81 | −0.07 | 0.96 |
| BMI | +0.17 | 0.79 | +0.15 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiberio, P.; Antunovic, L.; Gaudio, M.; Viganò, A.; Pastore, M.; Miggiano, C.; Jacobs, F.; Benvenuti, C.; Farina, E.; Chiti, A.; et al. The Relationship among Bowel [18]F-FDG PET Uptake, Pathological Complete Response, and Eating Habits in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Nutrients 2023, 15, 211. https://doi.org/10.3390/nu15010211
Tiberio P, Antunovic L, Gaudio M, Viganò A, Pastore M, Miggiano C, Jacobs F, Benvenuti C, Farina E, Chiti A, et al. The Relationship among Bowel [18]F-FDG PET Uptake, Pathological Complete Response, and Eating Habits in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Nutrients. 2023; 15(1):211. https://doi.org/10.3390/nu15010211
Chicago/Turabian StyleTiberio, Paola, Lidija Antunovic, Mariangela Gaudio, Alessandro Viganò, Manuela Pastore, Chiara Miggiano, Flavia Jacobs, Chiara Benvenuti, Elisabetta Farina, Arturo Chiti, and et al. 2023. "The Relationship among Bowel [18]F-FDG PET Uptake, Pathological Complete Response, and Eating Habits in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy" Nutrients 15, no. 1: 211. https://doi.org/10.3390/nu15010211
APA StyleTiberio, P., Antunovic, L., Gaudio, M., Viganò, A., Pastore, M., Miggiano, C., Jacobs, F., Benvenuti, C., Farina, E., Chiti, A., Santoro, A., & De Sanctis, R. (2023). The Relationship among Bowel [18]F-FDG PET Uptake, Pathological Complete Response, and Eating Habits in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Nutrients, 15(1), 211. https://doi.org/10.3390/nu15010211









