Determinants of Z-Score of Bone Mineral Density among Premenopausal Saudi Females in Different Age Groups: A Cross Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Study Setting and Design
2.2. Sample Size
2.3. Exclusion Criteria
2.4. Data Collection
2.5. Measurement of Body Mass Index
2.6. Bone Mineral Density Measurement
2.7. Blood Sample Collection
2.8. Ethical Considerations
2.9. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMD | Bone Mineral Density |
| PBM | Peak Bone Mass |
| BMI | Body Mass Index |
| METs | Metabolic Equivalents |
| IQR | Interquartile Range |
| ISCD | International Society for Clinical Densitometry |
| QUS | Quantitative Ultrasound |
References
- Theintz, G.; Buchs, B.; Rizzoli, R.; Slosman, D.; Clavien, H.; Sizonenko, P.C.; Bonjour, J.P. Longitudinal monitoring of bone mass accumulation in healthy adolescents: Evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J. Clin. Endocrinol. Metab. 1992, 75, 1060–1065. [Google Scholar] [PubMed]
- Hernandez, C.J.; Beaupré, G.S.; Carter, D.R. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos. Int. 2003, 14, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Boreham, C.A.; McKay, H.A. Physical activity in childhood and bone health. Br. J. Sport. Med. 2011, 45, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Lang, T.F.; McMahon, D.J.; Liu, X.S.; Guo, X.E.; Zhang, C.; Stein, E.M.; Dempster, D.W.; Young, P.; Saeed, I.; et al. Central QCT reveals lower volumetric BMD and stiffness in premenopausal women with idiopathic osteoporosis, regardless of fracture history. J. Clin. Endocrinol. Metab. 2012, 97, 4244–4252. [Google Scholar] [CrossRef]
- Mäkitie, R.E.; Costantini, A.; Kämpe, A.; Alm, J.J.; Mäkitie, O. New insights into monogenic causes of osteoporosis. Front. Endocrinol. 2019, 10, 70. [Google Scholar]
- Karlsson, M.K.; Ahlborg, H.G.; Karlsson, C. Maternity and bone mineral density. Acta Orthop. 2005, 76, 2–13. [Google Scholar] [CrossRef]
- Shaffer, R.A.; Rauh, M.J.; Brodine, S.K.; Trone, D.W.; Macera, C.A. Predictors of stress fracture susceptibility in young female recruits. Am. J. Sport. Med. 2006, 34, 108–115. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Gordon, C.M.; Baim, S.; Leonard, M.B.; Bishop, N.J.; Bianchi, M.L.; Kalkwarf, H.J.; Langman, C.B.; Plotkin, H.; Rauch, F.; et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 2008, 43, 1115–1121. [Google Scholar] [CrossRef]
- Ardawi, M.S.; Maimany, A.A.; Bahksh, T.M.; Nasrat, H.A.; Milaat, W.A.; Al-Raddadi, R.M. Bone mineral density of the spine and femur in healthy Saudis. Osteoporos. Int. 2005, 16, 43–55. [Google Scholar]
- El-Desouki, M.I.; Sulimani, R.A. High prevalence of osteoporosis in Saudi men. Saudi Med. J. 2007, 28, 774–777. [Google Scholar]
- Sadat-Ali, M.; AlElq, A. Osteoporosis among male Saudi Arabs: A pilot study. Ann. Saudi Med. 2006, 26, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Ali, M.; AlZamami, J.F.; AlNaimi, S.N.; Al-Noaimi, D.A.; AlDakheel, D.A.; AlSayed, H.N.; Al-Turki, H.A.; AlOmran, A.S. Osteoporosis: Is the Prevalence Increasing in Saudi Arabia. Ann. Afr. Med. 2022, 21, 54–57. [Google Scholar] [CrossRef] [PubMed]
- National Plan for Osteoporosis Prevention and Management in the Kingdom of Saudi Arabia. 2018. Available online: https://www.moh.gov.sa/en/Ministry/MediaCenter/Publications/Documents/NPOPM (accessed on 9 September 2023).
- Hendrix, S.L. Menstrual History & Activity/Diet Questionnaire. West County Spine & Joint Chiropractic Clinic. 2010. Available online: http://www.westcospineandjoint.com/wp-content/uploads/2010/10/Menstrual-History.Activity.Diet-Questionnaire.2.24.15.pdf (accessed on 1 October 2020).
- Available online: https://www.brockvillegeneralhospital.ca/en/patient-care/resources/BMD-Patient-Questionnaire.pdf (accessed on 9 September 2023).
- Al-Hazzaa, H.M.; Al-Sobayel, H.I.; Musaiger, A.O. Convergent validity of the Arab Teens Lifestyle Study (ATLS) physical activity questionnaire. Int. J. Environ. Res. Public Health 2011, 8, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Ridley, K.; Ainsworth, B.E.; Olds, T.S. Development of a compendium of energy expenditures for youth. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings. Available online: http://www.fao.org/3/i9940en/I9940EN.pdf (accessed on 20 June 2019).
- World Health Organization (WHO). WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level: Summary Meeting Report. 2020. Available online: https://www.who.int/chp/topics/Osteoporosis.pdf (accessed on 21 August 2020).
- Hollis, B.W.; Wagner, C.L. Normal serum vitamin D levels. N. Engl. J. Med. 2005, 352, 515–516. [Google Scholar]
- Troy, K.L.; Mancuso, M.E.; Butler, T.A.; Johnson, J.E. Exercise early and often: Effects of physical activity and exercise on women’s bone health. Int. J. Environ. Res. Public Health 2018, 15, 878. [Google Scholar] [CrossRef]
- Baxter-Jones, A.D.; Kontulainen, S.A.; Faulkner, R.A.; Bailey, D.A. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone 2008, 43, 1101–1107. [Google Scholar] [CrossRef]
- Nilsson, M.; Ohlsson, C.; Mellström, D.; Lorentzon, M. Previous sport activity during childhood and adolescence is associated with increased cortical bone size in young adult men. J. Bone Miner. Res. 2009, 24, 125–133. [Google Scholar] [CrossRef]
- Karlsson, M.K.; Nordqvist, A.; Karlsson, C. Physical activity increases bone mass during growth. Food Nutr. Res. 2008, 52, 1871. [Google Scholar] [CrossRef]
- Mazocco, L.; Chagas, P. Association between body mass index and osteoporosis in women from northwestern Rio Grande do Sul. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 299–305. [Google Scholar]
- Al-Tohamy, M.; El-Lebedy, D.; Adel Abdelhalim, D.; Amin, D.; Megahed, S.; Khalil, A. Bone Health and its Relation to Energy Intake, Fat Mass, and its Distribution. Pak. J. Biol. Sci. 2020, 23, 1075–1085. [Google Scholar]
- Madeira, E.; Mafort, T.T.; Madeira, M.; Guedes, E.P.; Moreira, R.O.; de Mendonça, L.M.; Lima, I.C.B.; de Pinho, P.R.A.; Lopes, A.J.; Farias, M.L.F. Lean mass as a predictor of bone density and microarchitecture in adult obese individuals with metabolic syndrome. Bone 2014, 59, 89–92. [Google Scholar] [PubMed]
- Parker, S.E.; Troisi, R.; Wise, L.A.; Palmer, J.R.; Titus-Ernstoff, L.; Strohsnitter, W.C.; Hatch, E.E. Menarche, menopause, years of menstruation, and the incidence of osteoporosis: The influence of prenatal exposure to diethylstilbestrol. J. Clin. Endocrinol. Metab. 2014, 99, 594–601. [Google Scholar] [PubMed]
- Ferrari, S.; Bianchi, M.L.; Eisman, J.A.; Foldes, A.J.; Adami, S.; Wahl, D.A.; Stepan, J.J.; de Vernejoul, M.-C.; Kaufman, J.-M.; IOF Committee of Scientific Advisors Working Group on Osteoporosis Pathophysiology. Osteoporosis in young adults: Pathophysiology, diagnosis, and management. Osteoporos. Int. 2012, 23, 2735–2748. [Google Scholar]
- Kang, H.; Chen, Y.M.; Han, G.; Huang, H.; Chen, W.Q.; Wang, X.; Zhu, Y.Y.; Xiao, S.M. Associations of age, BMI, and years of menstruation with proximal femur strength in Chinese postmenopausal women: A cross-sectional study. Int. J. Environ. Res. Public Health 2016, 13, 157. [Google Scholar]
- Ensom, M.H.; Liu, P.Y.; Stephenson, M.D. Effect of pregnancy on bone mineral density in healthy women. Obstet. Gynecol. Surv. 2002, 57, 99–111. [Google Scholar] [CrossRef]
- Akil, S.; Al-Mohammed, H.; Al-Batati, N.; Tirsen, M.; Al-Otaibi, A.; AlZahrani, A.; Bakhder, D.; AlSubaie, R.; AbuAlsaud, S. Quantitative ultrasound assessment of the effect of parity on bone mineral density in females. BMC Womens Health 2021, 21, 380. [Google Scholar]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Brincat, M.; Erel, C.T.; Tremollieres, F.; Gambacciani, M.; Lambrinoudaki, I.; Moen, M.H.; Schenck-Gustafsson, K.; Vujovic, S.; Rozenberg, S.; et al. European menopause and andropause society position statement: Vitamin D and postmenopausal health. Maturitas 2012, 71, 83–88. [Google Scholar]
- Van den Heuvel, E.G.H.M.; Steijns, J.M.J.M. Dairy products and bone health: How strong is the scientific evidence? Nutr. Res. Rev. 2018, 31, 164–178. [Google Scholar]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, physical activity, and dietary supplementation to prevent bone mineral density loss: A food pyramid. Nutrients 2021, 14, 74. [Google Scholar] [PubMed]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, Z.A.; Sultan, I.E.; Guraya, S.S.; Al-Zalabani, A.H.; Khoshhal, K.I. Low bone mineral density among young healthy adult Saudi women. Prevalence and associated factors in the age group of 20 to 36 years. Saudi Med. J. 2016, 37, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Al-Habdan, I.M.; Sadat-Ali, M.; Al-Muhanna, F.A.; Al-Elq, A.H.; Al-Mulhim, A.A. Bone mass measurement using quantitative ultrasound in healthy Saudi women. A cross-sectional screening. Saudi Med. J. 2009, 30, 1426–1431. [Google Scholar]
- Mahboub, S.M.; Al-Muammar, M.N.; Elareefy, A.A. Evaluation of the prevalence and correlated factors for decreased bone mass density among pre- and post-menopausal educated working women in Saudi Arabia. J. Health Popul. Nutr. 2014, 32, 513–519. [Google Scholar]
- Albaik, M.; Khan, J.A.; Sindi, I.; Akesson, K.E.; McGuigan, F.E.A. Bone mass in Saudi women aged 20–40 years: The association with obesity and vitamin D deficiency. Arch. Osteoporos. 2022, 17, 123. [Google Scholar]
- Kanis, J.A.; Delmas, P.; Burckhardt, P.; Cooper, C.; Torgerson, D.O. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos. Int. 1997, 7, 390–406. [Google Scholar] [CrossRef]
- Khosla, S.; Lufkin, E.G.; Hodgson, S.F.; Fitzpatrick, L.; Melton, L. Epidemiology and clinical features of osteoporosis in young individuals. Bone 1994, 15, 551–555. [Google Scholar]
- Hans, D.; Baim, S. Quantitative ultrasound (QUS) in the management of osteoporosis and assessment of fracture risk. J. Clin. Densitom. 2017, 20, 322–333. [Google Scholar] [PubMed]
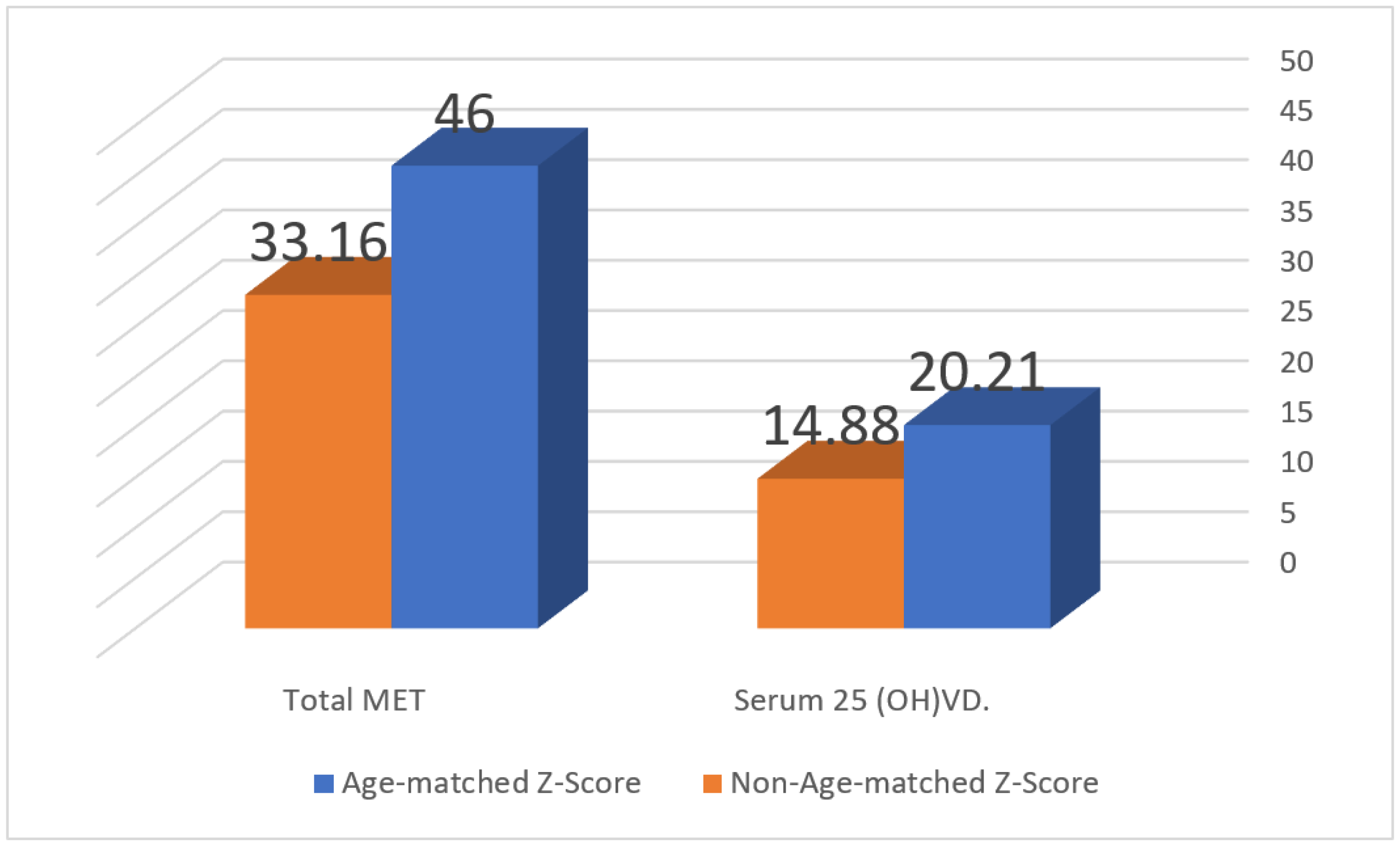
| Total N = 886 | Females ≤ 30 Years N = 605 (68.3%) | Females > 30 Years N = 281 (31.7%) | p Value | ||
|---|---|---|---|---|---|
| Age: Median (IQR): Years | 24 (12) | 22 (4) | 36 (8) | <0.001 | |
| Education | Below college education | 335 (37.8%) | 191 (32.3%) | 144 (48.8%) | <0.001 |
| Undergraduate student | 211 (23.8%) | 197 (33.3%) | 14 (4.7%) | ||
| College graduate | 340 (38.4%) | 203 (34.3%) | 137 (46.4%) | ||
| Occupation | Student | 594 (67%) | 580 (95.9%) | 14 (5%) | <0.001 |
| Housewife | 128 (14.4%) | 3 (0.5%) | 125 (44.5%) | ||
| Employee | 164 (18.5%) | 22 (3.6%) | 142 (50.5%) | ||
| Marital status | Single | 533 (64.7%) | 474 (88.3%) | 59 (20.6%) | <0.001 |
| Married/previously married | 291 (35.3%) | 63 (11.7%) | 228 (79.4%) | ||
| Menarche: Median age (IQR) | 13 (2) | 13 (2) | 13 (2) | 0.231 | |
| Irregular menses | 212 (24.5%) | 167 (33.2%) | 45 (17.8%) | <0.001 | |
| Parity | Nullipara | 616 (71.1%) | 541 (91.5%) | 75 (25.4%) | <0.001 |
| Previous labor | 270 (31.2%) | 50 (8.5%) | 220 (74.6%) | ||
| Family history of bone fracture | 101 (11.7%) | 75 (14.7%) | 26 (9.8%) | 0.053 | |
| Family history osteoporosis | 138 (15.9%) | 80 (15.7%) | 58 (21.8%) | <0.05 | |
| History of vitamin D deficiency | 388 (44.8%) | 253 (45%) | 135 (50.6%) | 0.882 | |
| History of vitamin D intake | 326 (37.6%) | 214 (41.6%) | 112 (42.3%) | 0.866 | |
| History of bone fracture | 26 (11.5%) | 15 (3.0%) | 11 (4.3%) | 0.334 | |
| BMI: Median (IQR) | 23.6 (6.9) | 22.9 (6.2) | 26.35 (6.5) | <0.001 | |
| Parathyroid hormone: pg/mL | 39.2 (32) | 37.7 (28.9) | 58.38 (53.69) | <0.01 | |
| Serum 25(OH) vitamin D: ng/mL | 22.8 (14.03) | 22.3 (16.2) | 25.67 (27.54) | 0.989 | |
| Insufficient vitamin D | 482 (77.2%) | 408 (77.6%) | 74 (75.5%) | 0.656 | |
| Sufficient vitamin D | 142 (22.8%) | 118 (22.4%) | 24 (24%) | ||
| Z-score: Median (IQR) | 0 (1.5) | 0 (1.5) | −0.2 (1.4) | 0.01 | |
| Age-matched Z-Score | 862 (97.3%) | 575 (97.3%) | 287 (97.3%) | 0.997 | |
| Non-age-matched Z-Score | 24 (2.7%) | 16 (2.7%) | 8 (2.7%) | ||
| T score: Median (IQR) | 0 (1.5) | 0 (1.5) | −0.3 (1.4) | 0.000 | |
| >−1 | 694 (78.3%) | 481 (81.4%) | 213 (72.2%) | 0.006 | |
| −1–2.4 | 175 (19.8%) | 99 (16.8%) | 76 (25.8%) | ||
| ≥−2.5 | 17 (1.9%) | 11 (1.9%) | 6 (2%) | ||
| Total N = 886 | Females ≤ 30 N = 605 | Females > 30 N = 281 | p Value | ||
|---|---|---|---|---|---|
| Weekly food frequency: median (IQR) | Legume seeds | 0 (1) | 0 (0.25) | 0.25 (1) | <0.001 |
| Dairy products | 8 (9) | 7 (10.5) | 7 (8.3) | 0.087 | |
| Adequate daily dairy intake | 149 (16.8%) | 95 (16.1%) | 54 (18.3%) | 0.731 | |
| Caffeinated drinks | 8.5 (7.2) | 8.25 (8.8) | 9.0 (7) | <0.05 | |
| Fresh vegetables/fruits | 7 (6.8) | 7 (6.8) | 7.25 (9) | <0.01 | |
| Salty food (pickles/junk) | 2 (6) | 2.1 (6) | 2.0 (1.5) | <0.001 | |
| Smoking | 276 (31.2%) | 85 (14.4%) | 191 (64.7%) | <0.001 | |
| TV/computer daily duration: median (IQR): HOURS | 3.0 (3) | 3.0 (2) | 2.0 (3) | <0.001 | |
| Sedentary life (>2 h daily) | 398 (44.9%) | 301 (59.8%) | 97 (40.1%) | <0.001 | |
| Total N = 886 | Females ≤ 30 Years N = 605 | Females > 30 Years N = 281 | p Value | ||||
|---|---|---|---|---|---|---|---|
| Physical Activity | N (%) | MET: Median (IQR) | N (%) | MET: Median (IQR) | N (%) | MET: Median (IQR) | |
| Walking exercise | 688 (77.7%) | 1.75 (6.2) | 467 (79%) | 2.31 (7.42) | 221 (74.9%) | 1.65 (4) | <0.01 |
| Ascending stairs | 684 (77.2%) | 32 (32) | 476 (80.5%) | 32.0 (48.0) | 208 (70.5%) | 16.0 (16) | <0.001 |
| Running | 216 (24.4%) | 0 (2.6) | 171 (28.9%) | 0.00 (2.64) | 45 (15.3%) | 0.00 (0) | <0.001 |
| Household activities | 513 (57.9%) | 1.16 (5.3) | 333 (56.3%) | 1.16 (3.5) | 180 (61%) | 2.31 (7) | <0.01 |
| Bicycling | 93 (10.5%) | 0 (0) | 76 (12.9%) | 0.00 (0) | 17 (5.8%) | 0.00 (0) | <0.01 |
| Swimming | 73 (8.2%) | 0 (0) | 55 (9.3%) | 0.00 (0) | 18 (6.1%) | 0.00 (0) | 0.171 |
| Moderate-intensity sport | 109 (12.3%) | 0 (0) | 87 (14.7%) | 0.00 (0) | 22 (7.5%) | 0.00 (0) | <0.01 |
| Vigorous-intensity sport | 81 (9.1%) | 0 (0) | 64 (12.8%) | 0.00 (0) | 17 (6.7%) | 0.00 (0) | <0.05 |
| Self-defense sport | 28 (3.2%) | 0 (0) | 25 (5%) | 0.00 (0) | 3 (1.2%) | 0.00 (0) | <0.05 |
| Bodybuilding | 112 (12.6%) | 0 (0) | 96 (19.1%) | 0.00 (0) | 16 (6.3%) | 0.00 (0) | <0.001 |
| Total | 622 (70.2%) | 45.2 (45.9) | 416 (70.4%) | 53.16 (48.3) | 206 (69.8%) | 35.38 (35.9) | <0.001 |
| All Premenopausal Women N = 886 | Premenopausal Women < 30 N = 605 | Premenopausal Women > 30 N = 281 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Odd Ratio | p Value | 95% Confidence Interval | Odd Ratio | p Value | 95% Confidence Interval | Odd Ratio | p Value | 95% Confidence Interval |
| Age of menarche | 0.044 | 0.400 | −0.039–0.044 | 0.063 | 0.256 | −0.032–0.119 | −0.235 | 0.176 | −0.296–0.057 |
| Regularity of menstruation | 0.060 | 0.252 | −0.106–0.402 | 0.075 | 0.176 | −0.082–0.447 | −0.250 | 0.172 | −1.601–0.302 |
| Parity | 0.034 | 0.523 | −0.301–0.034 | −0.004 | 0.937 | −0.595–0.550 | 0.340 | <0.05 | 0.028–1.450 |
| History of vitamin D deficiency | 0.043 | 0.438 | −0.155–0.043 | 0.026 | 0.665 | −0.214–0.334 | 0.352 | <0.05 | 0.007–1.437 |
| Family history of bone fracture | −0.014 | 0.799 | −0.375–−0.014 | −0.023 | 0.689 | −0.427–0.282 | 0.207 | 0.193 | −0.290–1.362 |
| Family history osteoporosis | −0.035 | 0.525 | −0.471–−0.035 | −0.067 | 0.242 | −0.636–0.161 | |||
| Weekly dairy products | −0.010 | 0.852 | −0.023–−0.010 | −0.007 | 0.909 | −0.024–0.021 | |||
| Fresh food | 0.070 | 0.186 | −0.009–0.070 | 0.075 | 0.176 | −0.009–0.049 | |||
| Salty food | −0.087 | 0.101 | −0.052–−0.087 | −0.087 | 0.122 | −0.054–0.006 | |||
| Natural food sources of vitamin D | −0.023 | 0.683 | −0.066–0.043 | −0.063 | 0.283 | −0.090–0.026 | 0.170 | 0.310 | −0.064–0.192 |
| Caffeinated drinks | 0.053 | 0.329 | −0.012–0.053 | 0.064 | 0.266 | −0.011–0.040 | |||
| Serum Parathyroid hormone | −0.018 | 0.743 | −0.006–−0.018 | −0.029 | 0.611 | −0.007–0.004 | −0.116 | 0.497 | −0.017–0.008 |
| Serum vitamin D | 0.004 | 0.944 | −0.009–0.004 | 0.026 | 0.668 | −0.008–0.013 | −0.339 | 0.072 | −0.050–0.002 |
| BMI | 0.179 | <0.01 | 0.016–0.179 | 0.167 | 0.003 | 0.013–0.063 | 0.497 | 0.019 | 0.018–0.186 |
| Total METs | 0.149 | <0.01 | 0.001–0.149 | 0.160 | 0.005 | 0.001–0.006 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, I.; Taha, I.; El Tarhouny, S.; Mohammed, R.A.; Allah, A.M.A.; Al Nozha, O.; Desouky, M.; Ghonimy, A.; Elmehallawy, Y.; Aldeeb, N.; et al. Determinants of Z-Score of Bone Mineral Density among Premenopausal Saudi Females in Different Age Groups: A Cross Sectional Study. Nutrients 2023, 15, 4280. https://doi.org/10.3390/nu15194280
Sultan I, Taha I, El Tarhouny S, Mohammed RA, Allah AMA, Al Nozha O, Desouky M, Ghonimy A, Elmehallawy Y, Aldeeb N, et al. Determinants of Z-Score of Bone Mineral Density among Premenopausal Saudi Females in Different Age Groups: A Cross Sectional Study. Nutrients. 2023; 15(19):4280. https://doi.org/10.3390/nu15194280
Chicago/Turabian StyleSultan, Intessar, Inass Taha, Shereen El Tarhouny, Rehab A. Mohammed, Azza M. Abdu Allah, Omar Al Nozha, Maha Desouky, Abdelrahman Ghonimy, Yara Elmehallawy, Nawaf Aldeeb, and et al. 2023. "Determinants of Z-Score of Bone Mineral Density among Premenopausal Saudi Females in Different Age Groups: A Cross Sectional Study" Nutrients 15, no. 19: 4280. https://doi.org/10.3390/nu15194280
APA StyleSultan, I., Taha, I., El Tarhouny, S., Mohammed, R. A., Allah, A. M. A., Al Nozha, O., Desouky, M., Ghonimy, A., Elmehallawy, Y., Aldeeb, N., & Iskandarani, Y. A. (2023). Determinants of Z-Score of Bone Mineral Density among Premenopausal Saudi Females in Different Age Groups: A Cross Sectional Study. Nutrients, 15(19), 4280. https://doi.org/10.3390/nu15194280






