Causal Relationship between Gut Microbiota and Gout: A Two-Sample Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Gut Microbiota Sample
2.3. Serum Urate Levels and Gout Samples
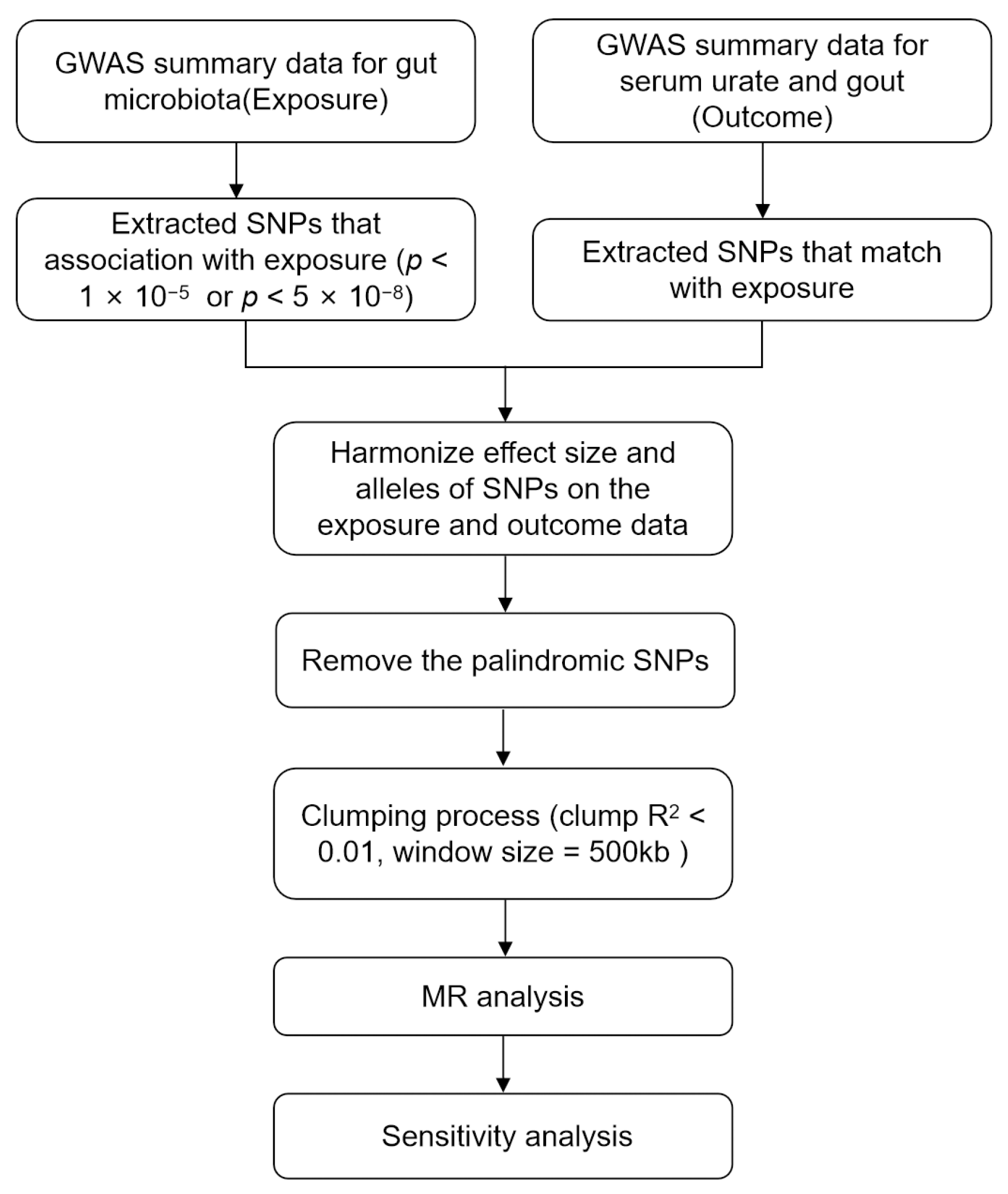
2.4. Selection of Instrumental Variables
2.5. Mendelian Randomization Analysis
2.6. Sensitivity Analysis
3. Results
3.1. Selection of Instrumental Variables
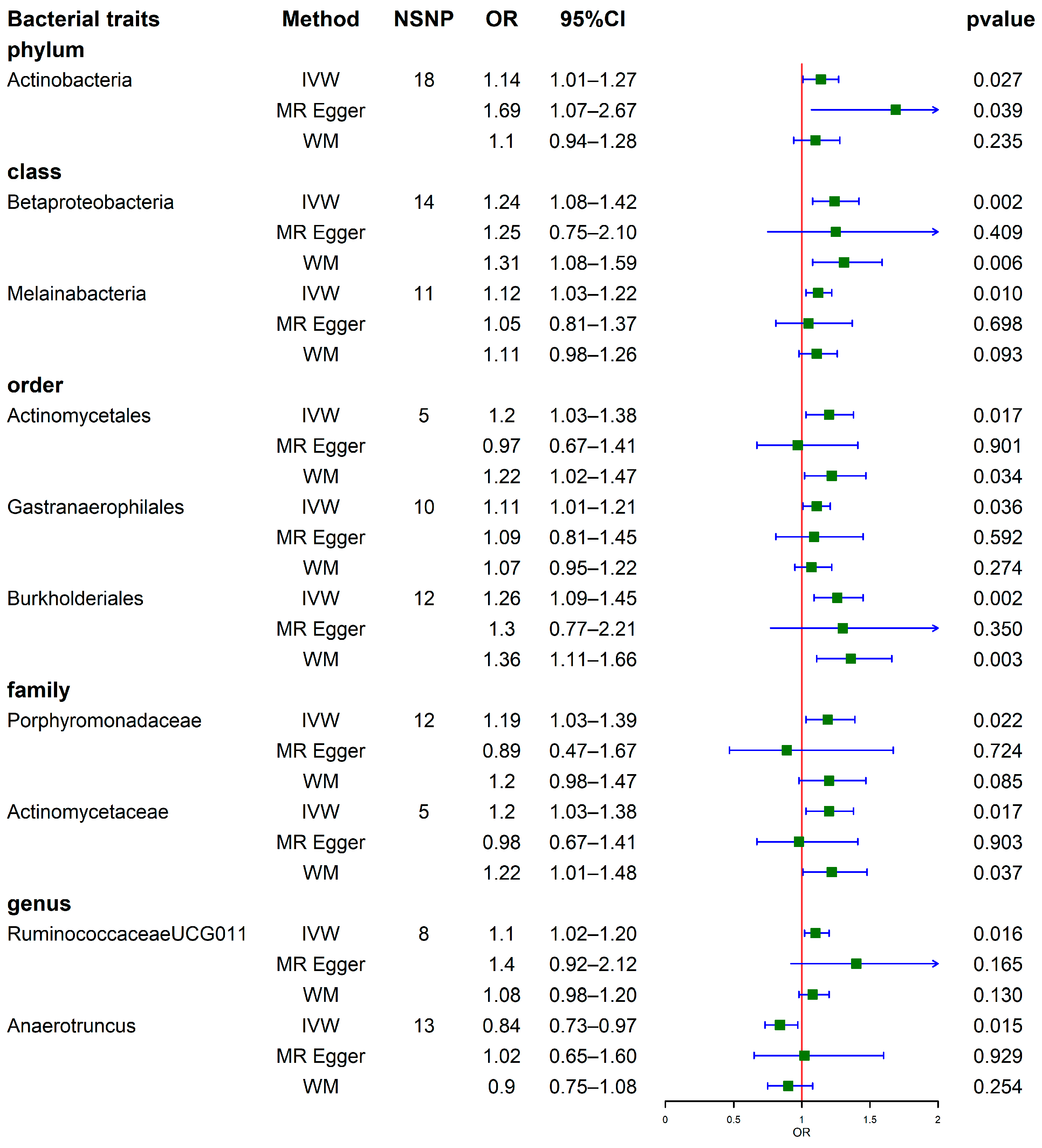
3.2. Results of MR Analysis (Locus-Wide Significance, p < 1 × 10−5)
3.2.1. Causal Effects of GM on SUA
3.2.2. Causal Effects of GM on Gout
3.3. Sensitivity Analysis
3.4. Results of MR Analysis (Genome-Wide Statistical Significance, p < 5 × 10−8)
3.4.1. Results of MR Analysis with GM as a Whole
3.4.2. Results of MR Analysis with 196 GM Taxa
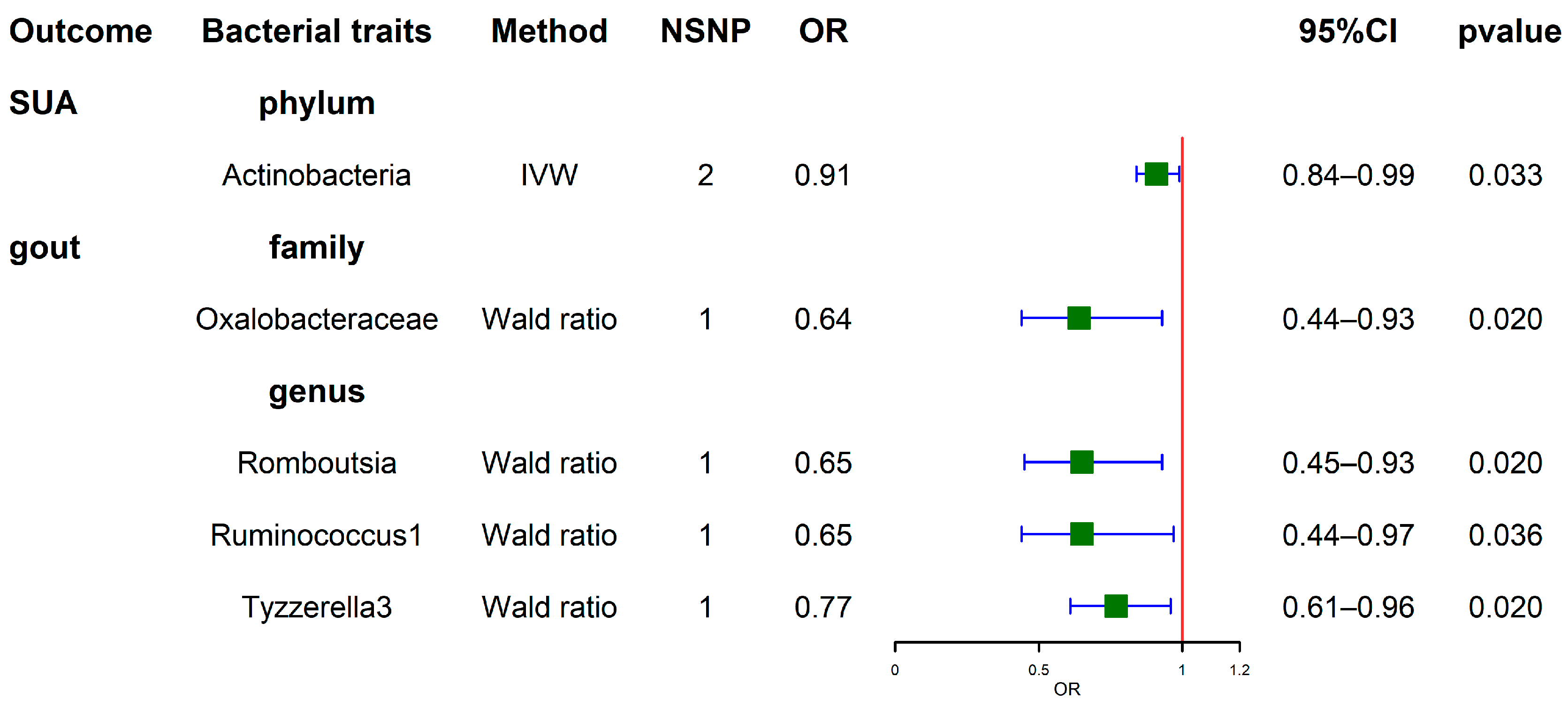
3.5. Results of the Reverse MR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; Doherty, M. Gout. Epidemiology of gout. Arthritis Res. Ther. 2010, 12, 223. [Google Scholar] [CrossRef]
- Roddy, E.; Choi, H.K. Epidemiology of gout. Rheum. Dis. Clin. N. Am. 2014, 40, 155–175. [Google Scholar] [CrossRef]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: A systematic review and meta-analysis. BioMed Res. Int. 2015, 2015, 762820. [Google Scholar] [CrossRef]
- Dehlin, M.; Drivelegka, P.; Sigurdardottir, V.; Svard, A.; Jacobsson, L.T. Incidence and prevalence of gout in Western Sweden. Arthritis Res. Ther. 2016, 18, 164. [Google Scholar] [CrossRef]
- Kim, J.W.; Kwak, S.G.; Lee, H.; Kim, S.K.; Choe, J.Y.; Park, S.H. Prevalence and incidence of gout in Korea: Data from the national health claims database 2007–2015. Rheumatol. Int. 2017, 37, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Gaffo, A. Gout epidemiology and comorbidities. Semin. Arthritis Rheum. 2020, 50, S11–S16. [Google Scholar] [CrossRef]
- Tang, Y.M.; Zhang, L.; Zhu, S.Z.; Pan, J.J.; Zhou, S.H.; He, T.J.; Li, Q. Gout in China, 1990–2017: The Global Burden of Disease Study 2017. Public Health 2021, 191, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.D.; Yang, X.K.; He, Y.S.; Ni, J.; Wang, J.; Yin, K.J.; Huang, J.X.; Chen, Y.; Feng, T.; Wang, P.; et al. Environmental factors and risk of gout. Environ. Res. 2022, 212 Pt C, 113377. [Google Scholar] [CrossRef]
- Taylor, W.J.; Fransen, J.; Jansen, T.L.; Dalbeth, N.; Schumacher, H.R.; Brown, M.; Louthrenoo, W.; Vazquez-Mellado, J.; Eliseev, M.; McCarthy, G.; et al. Study for updated gout classification criteria: Identification of features to classify gout. Arthritis Care Res. 2015, 67, 1304–1315. [Google Scholar] [CrossRef]
- Cipolletta, E.; Tata, L.J.; Nakafero, G.; Avery, A.J.; Mamas, M.A.; Abhishek, A. Association between gout flare and subsequent cardiovascular events among patients with gout. JAMA 2022, 328, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Lo, B.C.; Nunez, G. Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef]
- Džidić-Krivić, A.; Kusturica, J.; Sher, E.K.; Selak, N.; Osmančević, N.; Karahmet Farhat, E.; Sher, F. Effects of intestinal flora on pharmacokinetics and pharmacodynamics of drugs. Drug Metab. Rev. 2023, 55, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain axis in neuropsychiatric disorders: Pathophysiological Mechanisms and novel treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hang, Z.; Lei, T.; Du, H. Intestinal flora affect Alzheimer’s Disease by regulating endogenous hormones. Neurochem. Res. 2022, 47, 3565–3582. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Zhao, L.; Xiong, Q.; Stary, C.M.; Mahgoub, O.K.; Ye, Y.; Gu, L.; Xiong, X.; Zhu, S. Bidirectional gut-brain-microbiota axis as a potential link between inflammatory bowel disease and ischemic stroke. J. Neuroinflammation 2018, 15, 339. [Google Scholar] [CrossRef]
- Farhat, E.K.; Sher, E.K.; Džidić-Krivić, A.; Banjari, I.; Sher, F. Functional biotransformation of phytoestrogens by gut microbiota with impact on cancer treatment. J. Nutr. Biochem. 2023, 118, 109368. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, E.J.; Jeong, S.H.; Park, M.C. Distinct gut microbiota in patients with asymptomatic hyperuricemia: A potential protector against gout development. Yonsei Med. J. 2022, 63, 241–251. [Google Scholar] [CrossRef]
- Tong, S.; Zhang, P.; Cheng, Q.; Chen, M.; Chen, X.; Wang, Z.; Lu, X.; Wu, H. The role of gut microbiota in gout: Is gut microbiota a potential target for gout treatment. Front. Cell Infect. Microbiol. 2022, 12, 1051682. [Google Scholar] [CrossRef]
- Ul-Haq, A.; Lee, K.A.; Seo, H.; Kim, S.; Jo, S.; Ko, K.M.; Moon, S.J.; Kim, Y.S.; Choi, J.R.; Song, H.Y.; et al. Characteristic alterations of gut microbiota in uncontrolled gout. J. Microbiol. 2022, 60, 1178–1190. [Google Scholar] [CrossRef]
- Burgess, S.; Daniel, R.M.; Butterworth, A.S.; Thompson, S.G. Network Mendelian randomization: Using genetic variants as instrumental variables to investigate mediation in causal pathways. Int. J. Epidemiol. 2015, 44, 484–495. [Google Scholar] [CrossRef]
- Abdellaoui, A.; Yengo, L.; Verweij, K.J.H.; Visscher, P.M. 15 years of GWAS discovery: Realizing the promise. Am. J. Hum. Genet. 2023, 110, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 3979–3989. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization (STROBE-MR): Explanation and elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kurilshikov, A.; Radjabzadeh, D.; Turpin, W.; Croitoru, K.; Bonder, M.J.; Jackson, M.A.; Medina-Gomez, C.; Frost, F.; Homuth, G. Meta-analysis of human genome-microbiome association studies: The MiBioGen consortium initiative. Microbiome 2018, 6, 101. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef]
- Tin, A.; Marten, J.; Halperin Kuhns, V.L.; Li, Y.; Wuttke, M.; Kirsten, H.; Sieber, K.B.; Qiu, C.; Gorski, M.; Yu, Z.; et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat. Genet. 2019, 51, 1459–1474. [Google Scholar] [CrossRef]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef]
- Ni, J.J.; Xu, Q.; Yan, S.S.; Han, B.X.; Zhang, H.; Wei, X.T.; Feng, G.J.; Zhao, M.; Pei, Y.F.; Zhang, L. Gut microbiota and Psychiatric Disorders: A two-Sample Mendelian Randomization study. Front. Microbiol. 2021, 12, 737197. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Small, D.S.; Thompson, S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017, 26, 2333–2355. [Google Scholar] [CrossRef]
- Bowden, J.; Davey, S.G.; Haycock, P.C.; Burgess, S. Consistent estimation in Mendelian Randomization with some invalid instruments using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey, S.G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey, S.G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Ning, Y.; Yang, G.; Chen, Y.; Zhao, X.; Qian, H.; Liu, Y.; Chen, S.; Shi, G. Characteristics of the urinary microbiome from patients with gout: A prospective study. Front. Endocrinol. 2020, 11, 272. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Wang, Z.; Ang, K.Y.; Huang, S.; Hou, Q.; Su, X.; Qiao, J.; Zheng, Y.; Wang, L.; et al. Intestinal Microbiota distinguish gout patients from healthy humans. Sci. Rep. 2016, 6, 20602. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X. Altered gut microbiota in children with hyperuricemia. Front. Endocrinol. 2022, 13, 848715. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, W.; Geng, B.; You, B.; Wang, W.; Li, X. Intestinal dysbacteriosis leads to kidney stone disease. Mol. Med. Rep. 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yao, W.; Jiang, H. Short-chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J. Nutr. 2014, 144, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Sun, S.; Huang, Y.; Gao, Q.; Xie, X.; Wang, P.; Li, J.; Liang, L.; He, X.; Jiang, Y.; et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes 2021, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Shao, L.; Li, H.; Xie, Z.; He, Z.; Wen, C. Combined Signature of the Fecal Microbiome and Metabolome in Patients with Gout. Front. Microbiol. 2017, 8, 268. [Google Scholar] [CrossRef]
- Ferro, P.; Vaz-Moreira, I.; Manaia, C.M. Betaproteobacteria are predominant in drinking water: Are there reasons for concern? Crit. Rev. Microbiol. 2019, 45, 649–667. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Zheng, J.; Hu, G.; Wang, J.; Huang, C.; Lou, L.; Wang, X.; Zeng, Y. Mucosal adherent bacterial dysbiosis in patients with colorectal adenomas. Sci. Rep. 2016, 6, 26337. [Google Scholar] [CrossRef]
- Xiong, L.; Li, Y.; Li, J.; Yang, J.; Shang, L.; He, X.; Liu, L.; Luo, Y.; Xie, X. Intestinal microbiota profiles in infants with acute gastroenteritis caused by rotavirus and norovirus infection: A prospective cohort study. Int. J. Infect. Dis. 2021, 111, 76–84. [Google Scholar] [CrossRef]


| Outcome | Taxonomies | GM | Q | Q_p | Intercept | p |
|---|---|---|---|---|---|---|
| SUA | ||||||
| phylum | ||||||
| Actinobacteria | 25.78 | 0.060 | −0.002 | 0.755 | ||
| family | ||||||
| Family XIII | 8.62 | 0.570 | 0.007 | 0.161 | ||
| genus | ||||||
| Escherichia Shigella | 15.89 | 0.100 | 0.001 | 0.847 | ||
| Lachnospiraceae FCS020 group | 14.11 | 0.370 | 0.004 | 0.122 | ||
| Lachnospiraceae NC2004 group | 14.52 | 0.070 | −0.001 | 0.929 | ||
| gout | ||||||
| phylum | ||||||
| Actinobacteria | 9.92 | 0.871 | −0.025 | 0.098 | ||
| class | ||||||
| Betaproteobacteria | 12.42 | 0.412 | −0.001 | 0.967 | ||
| Melainabacteria | 9.62 | 0.382 | 0.007 | 0.640 | ||
| order | ||||||
| Actinomycetales | 2.89 | 0.409 | 0.022 | 0.326 | ||
| Gastranaerophilales | 10.00 | 0.265 | 0.002 | 0.895 | ||
| Burkholderiales | 11.25 | 0.338 | −0.002 | 0.893 | ||
| family | ||||||
| Porphyromonadaceae | 6.76 | 0.748 | 0.017 | 0.372 | ||
| Actinomycetaceae | 2.89 | 0.409 | 0.022 | 0.327 | ||
| genus | ||||||
| RuminococcaceaeUCG011 | 2.21 | 0.899 | −0.032 | 0.300 | ||
| Anaerotruncus | 12.52 | 0.326 | −0.014 | 0.377 |
| GM | Outcome | Method | NSNP | OR | 95% CI | p | Q | Q_p | Intercept | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | SUA | IVM | 12 | 0.98 | 0.96–1.00 | 0.099 | 11.18 | 0.428 | ||
| Total | SUA | MR Egger | 12 | 1.01 | 0.94–1.08 | 0.873 | 10.69 | 0.382 | −0.003 | 0.513 |
| Total | SUA | WM | 12 | 0.99 | 0.96–1.02 | 0.430 | ||||
| Total | gout | IVM | 12 | 0.96 | 0.86–1.06 | 0.436 | 18.60 | 0.069 | ||
| Total | gout | MR Egger | 12 | 1.00 | 0.70–1.44 | 0.997 | 18.49 | 0.047 | −0.005 | 0.816 |
| Total | gout | WM | 12 | 1.02 | 0.91–1.14 | 0.787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Fan, J.; Huang, Z.; Zhou, D.; Wang, X. Causal Relationship between Gut Microbiota and Gout: A Two-Sample Mendelian Randomization Study. Nutrients 2023, 15, 4260. https://doi.org/10.3390/nu15194260
Wang M, Fan J, Huang Z, Zhou D, Wang X. Causal Relationship between Gut Microbiota and Gout: A Two-Sample Mendelian Randomization Study. Nutrients. 2023; 15(19):4260. https://doi.org/10.3390/nu15194260
Chicago/Turabian StyleWang, Mengna, Jiayao Fan, Zhaohui Huang, Dan Zhou, and Xue Wang. 2023. "Causal Relationship between Gut Microbiota and Gout: A Two-Sample Mendelian Randomization Study" Nutrients 15, no. 19: 4260. https://doi.org/10.3390/nu15194260
APA StyleWang, M., Fan, J., Huang, Z., Zhou, D., & Wang, X. (2023). Causal Relationship between Gut Microbiota and Gout: A Two-Sample Mendelian Randomization Study. Nutrients, 15(19), 4260. https://doi.org/10.3390/nu15194260





